Recent Advances and Challenges in the Early Diagnosis and Treatment of Preterm Labor
Abstract
1. Introduction
2. PTB/PTL Risk Prediction
2.1. Physical Testing
2.2. Chemical Testing Method
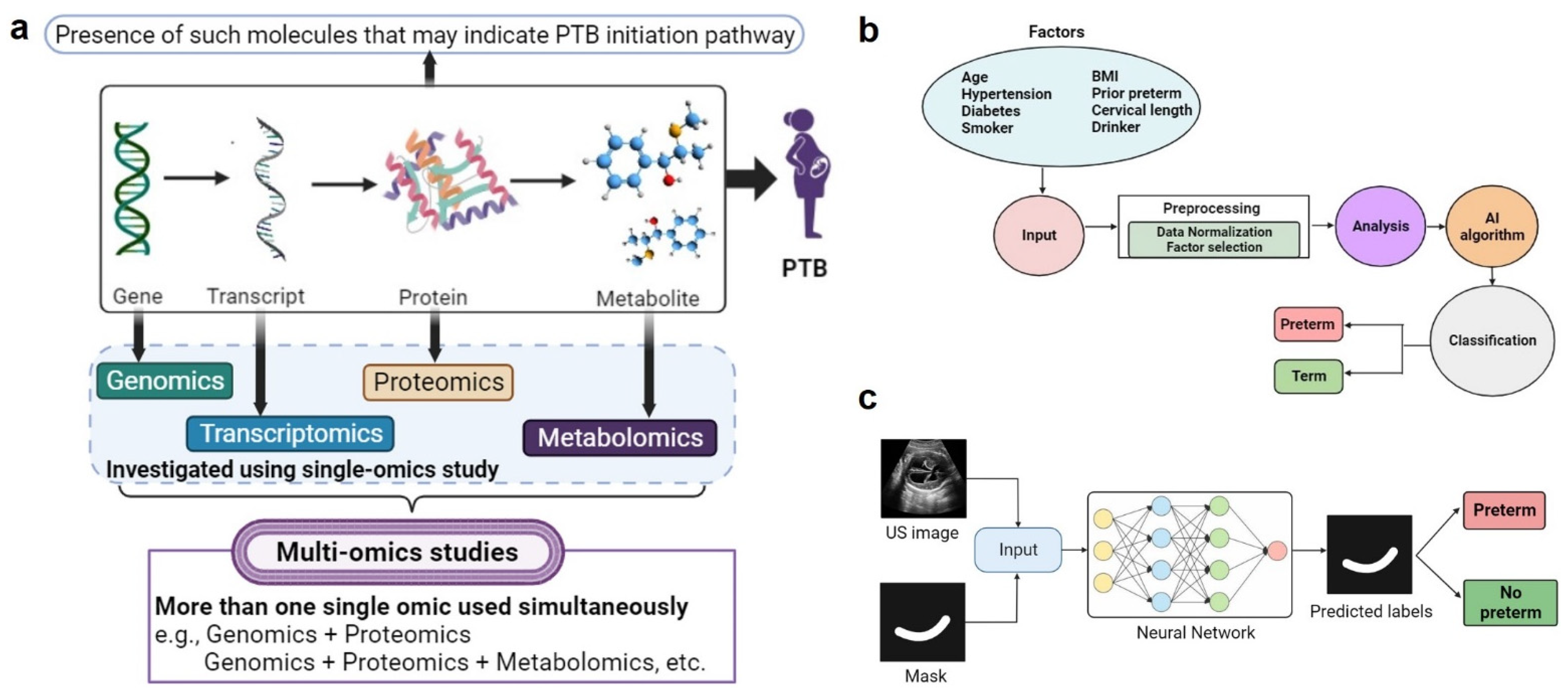
2.3. Multi-Omic Biomarker Studies
2.3.1. Genomic Biomarkers
2.3.2. Transcriptomic Biomarkers
2.3.3. Proteomic Biomarkers
| Sr. No. | Markers | Sample | Period (Weeks) | Detection Limit | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| I. Physical method | |||||||||
| 1. | Cervical length | NA | 22–24 | <25 mm | 47 | 89 | 37 | 93 | [86] |
| 2. | UCA | NA | 18–36 | ≥111° | 65.1 | 43.6 | 29.8 | 77.3 | [32] |
| 3. | Ferning test | NA | 34–37 | NA | 84.5 | 78.2 | 79.5 | 83.5 | [87] |
| II. Chemical method | |||||||||
| 1. | Nitrazine test | Amniotic fluid | 28–36 | NA | 87.3 | 80.9 | 82.1 | 86.4 | [87] |
| III. Biomarker-based method | |||||||||
| Specific biomarkers | |||||||||
| 1. | fFN | CVF | 23–34 | ≥50 µg/mL | 66.7 | 87.9 | 36.4 | 96.2 | [88] |
| 2. | PAMG-1 | CVF | 24–34 | ≥4 pg/mL | 90.0 | 93.8 | 78.3 | 97.4 | [89] |
| 66.7 | 98.6 | 75 | 97.9 | [90] | |||||
| 3. | IGFBP-1 | CVF | 20–35 | ≥30 µg/mL | 89.5 | 94.1 | 94.4 | 88.9 | [91] |
| 83.3 | 84.4 | 41.7 | 97.4 | [92] | |||||
| 70 | 74 | 48 | 88 | [93] | |||||
| Nonspecific biomarkers | |||||||||
| 1. | Ferritin | Serum | ≥37.5 ng/mL | 78.7 | 68.7 | 71.5 | 76.3 | [70] | |
| 2. | CRP | Serum | ≤20 | ≥5.27 mg/L | 75 | 86.1 | 37.5 | 96.87 | [76] |
| 3. | Prolactin | CVF | 24–36 | >7 ng/mL | 78 | 80 | 88.64 | 64.52 | [74] |
| 20–40 | 9.5 ng/L | 87.03 | 75 | 75.80 | 86.53 | [73] | |||
| 28–36 | 30 ng/L | 95 | 78 | 93 | 84 | [94] | |||
| 4. | Urocortin-1 | Amniotic fluid | 13–28 | ≥57.88 pg/mL | 81.8 | 40.0 | 40 | 82 | [95] |
| 5. | CRH | Serum | 24–36 | 10.45 pg/mL | 80 | 100 | 100 | 55.56 | [78] |
| 6. | ACTH | Serum | 24–36 | 14.65 pg/mL | 80 | 100 | 100 | 55.56 | [78] |
| 7. | MMP-8 | Amniotic fluid | 20 to 36 | >30 ng/mL | 82.4 | 78.0 | 36.0 | 97.7 | [96] |
2.3.4. Metabolomic Biomarkers
2.3.5. Multi-Omic Biomarkers
| Sr. No. | Identified Biomarkers | Phenotype | Ref. |
|---|---|---|---|
| Genomic biomarkers | |||
| 1 | ABCA13 | PTB | [46] |
| 2 | microRNAs (miRNA) and miR | PTB | [51,52,53] |
| 3 | TIMP2 | Inflammation and infection | [36,37] |
| 4 | COL4A3 | Inflammation and infection | [37,38] |
| 5 | TNF | Inflammation and infection | [39,40,41,42] |
| 6 | TNF1 and TNF2 | PTB | [42,47,48] |
| 7 | TNFRSF6 | PPROM | [38,41] |
| 8 | Toll-like receptor | PPROM | [43] |
| Transcriptomic biomarkers | |||
| 9 | miR-21, miR-142, miR-30e, miR-148b, miR-29b, and miR-223 | ↓ Gestational period | [53] |
| 10 | MIR4266, MIR1251, MIR601, and MIR3612 | ↑ sPTB risk | [57] |
| 11 | LINC00870 and LINC00094 | ↑ PTB risk | [57] |
| 12 | TLR4 | ↑ PTB risk | [58,59] |
| 13 | IL-6R | [60] | |
| Proteomic biomarkers | |||
| 14 | Lipocalin-type prostaglandin D2 synthase | ↑ PTB risk | [104] |
| 15 | ILs | ↑ PTB and PPROM risk | [62,63] |
| Metabolomics biomarkers | |||
| 16 | ↑ Glutamate, dulcitol, urocanic acid, N-acetyl glutamine, 1-methyladenine, salicylamide, oleic acid, diglyceride | ↑ PTB risk | [38,98,101] |
| ↓ Glutamine, pyruvate, inositol, alanine, pyroglutamic acid, glutamine, galactose, hexose clusters 5 and 3, inositol, urea, phosphatidylcholines, phosphatidylinositol, ceramides | ↑ PTB risk | [38,98,101] | |
| Multi-omics studies | |||
| 1 | Metabolomic (e.g., arabitol, xylitol, etc.), proteomic (e.g., VEGF 121, activin-A, MMPs, etc.), and immunome (e.g., CD56, INF-α, etc.) markers | Combine metabolome, proteome, and immunome | [102] |
| 2 | IL-6 polymorphisms and MMP-9 | Combine genomics and proteomics | [103] |
| 3 | TLR4 and TNF-α genes with TLR4 mRNA level | Combine transcriptomics and genetics | [59] |
2.4. AI/ML Methods
3. Principles of Biomarker Detection
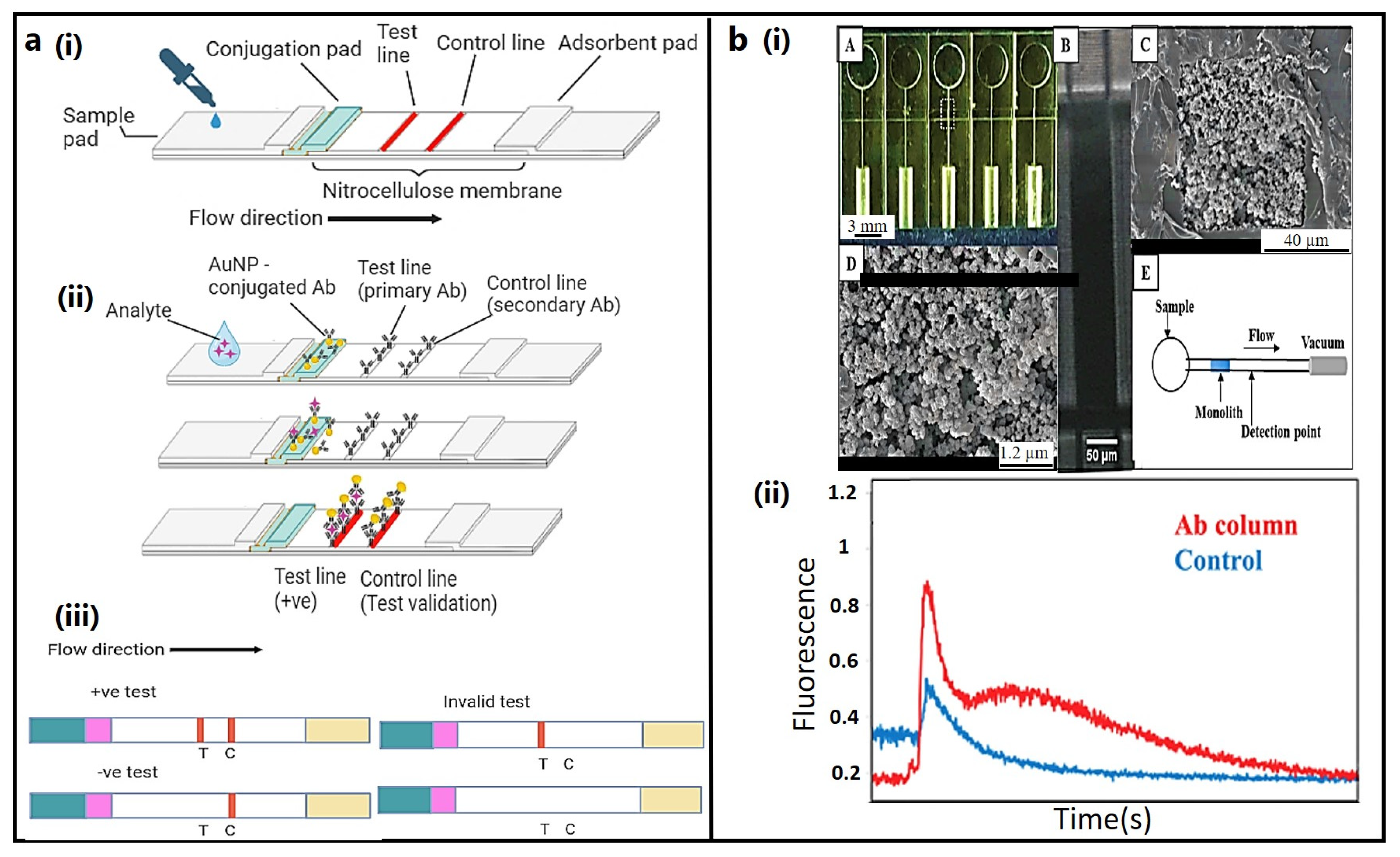
3.1. Lateral Flow Immunoassay (LFIA)
3.2. Microfluidic Devices
4. Point-of-Care Testing (POCT) Devices
4.1. PartoSure® Test
4.2. QuikCheck™ fFN
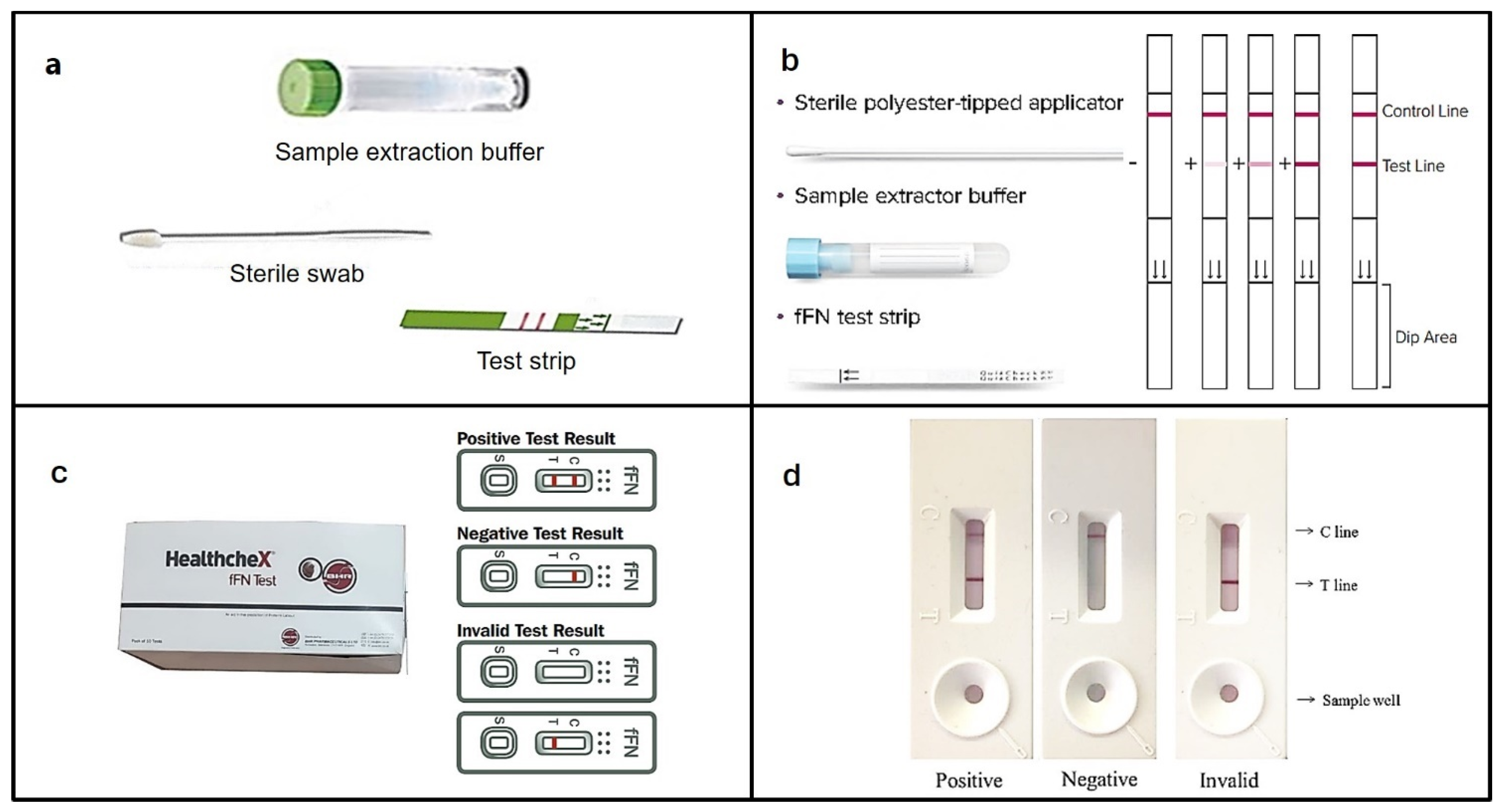
4.3. HealthcheX® Foetal Fibronectin (fFN) Test
4.4. Human Fetal Fibronectin XpressCard
4.5. Actim® Partus
4.6. Premaquick©
| Sr. No. | Device | Biomarker | Sample | LOD (ng/mL) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | PartoSure® test | PAMG-1 | CVS | 1.0 | 80 (<7 d) | 95 | 96 | 76 | - | [126] |
| 63 (<14 d) | 96 | 89 | 91 | - | ||||||
| 2. | Quikcheck fFN test | fFN | CVS | ≥ 50 | 94.5 | 89.1 | 89.7 | 94.2 | 91.8 | [87] |
| 3. | healthcheX fFN test | fFN | CVS | >50 | 98.1 | 98.7 | - | - | 98.4 | [128] |
| 4. | Antagen fFN XpressCard | fFN | Urine | 10 | - | - | - | - | - | [129] |
| 5. | Actim® Partus | ph IGFBP-1 | CVS | 10 | 60 | 67.7 | 23 | 91.3 | 66 | [131] |
| 95 | 92 | 86 | 97 | - | [133] | |||||
| 80 | 94 | 57 | 98 | - | [134] | |||||
| 6. | Premaquick© | IL-6/phIGFBP-1/IGFBP-1 | CVS | - | 95.1 | 97.5 | 97.5 | 95.2 | 96.3 | [132] |
5. Challenges
6. Treatment and Preventive Measures
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereyra, S.; Sosa, C.; Bertoni, B.; Sapiro, R. Transcriptomic analysis of fetal membranes reveals pathways involved in preterm birth. BMC Med. Genom. 2019, 12, 53. [Google Scholar] [CrossRef]
- Behrman, R.E.; Butler, A.S. Preterm Birth: Causes, Consequences, and Prevention; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- (WHO) W.H.O. Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 10 May 2023).
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000–2019: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- World Health Organization. Born too Soon: Decade of Action on Preterm Birth; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Griggs, K.M.; Hrelic, D.A.; Williams, N.; McEwen-Campbell, M.; Cypher, R. Preterm labor and birth: A clinical review. MCN Am. J. Matern. Child Nurs. 2020, 45, 328–337. [Google Scholar] [CrossRef]
- Nadeau, H.C.; Subramaniam, A.; Andrews, W.W. Infection and preterm birth. In Seminars in Fetal and Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 100–105. [Google Scholar]
- Couceiro, J.; Matos, I.; Mendes, J.J.; Baptista, P.V.; Fernandes, A.R.; Quintas, A. Inflammatory factors, genetic variants, and predisposition for preterm birth. Clin. Genet. 2021, 100, 357–367. [Google Scholar] [CrossRef]
- Sharami, S.H.; Darkhaneh, R.F.; Zahiri, Z.; Milani, F.; Asgharnia, M.; Shakiba, M.; Didar, Z. The relationship between vaginal bleeding in the first and second trimester of pregnancy and preterm labor. Iran. J. Reprod. Med. 2013, 11, 385. [Google Scholar]
- Waldorf, K.M.A.; Singh, N.; Mohan, A.R.; Young, R.C.; Ngo, L.; Das, A.; Tsai, J.; Bansal, A.; Paolella, L.; Herbert, B.R. Uterine overdistention induces preterm labor mediated by inflammation: Observations in pregnant women and nonhuman primates. Am. J. Obstet. Gynecol. 2015, 213, 830.e1–830.e19. [Google Scholar] [CrossRef]
- Hackney, D.N.; Glantz, J.C. Vaginal bleeding in early pregnancy and preterm birth: Systemic review and analysis of heterogeneity. J. Matern. Fetal Neonatal Med. 2011, 24, 778–786. [Google Scholar] [CrossRef][Green Version]
- Many, A.; Hill, L.M.; Lazebnik, N.; Martin, J.G. The association between polyhydramnios and preterm delivery. Obstet. Gynecol. 1995, 86, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Lilliecreutz, C.; Larén, J.; Sydsjö, G.; Josefsson, A. Effect of maternal stress during pregnancy on the risk for preterm birth. BMC Pregnancy Childbirth 2016, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scorza, W.E. Prelabor Rupture of Membranes at Term: Management; UpToDate: Waltham, MA, USA, 2021. [Google Scholar]
- Ikehara, S.; Kimura, T.; Kakigano, A.; Sato, T.; Iso, H.; Group, J.E.C.s.S.; Saito, H.; Kishi, R.; Yaegashi, N.; Hashimoto, K. Association between maternal alcohol consumption during pregnancy and risk of preterm delivery: The Japan Environment and Children’s Study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1448–1454. [Google Scholar] [CrossRef]
- Mercer, B.M.; Goldenberg, R.L.; Moawad, A.H.; Meis, P.J.; Iams, J.D.; Das, A.F.; Caritis, S.N.; Miodovnik, M.; Menard, M.K.; Thurnau, G.R. The preterm prediction study: Effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am. J. Obstet. Gynecol. 1999, 181, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Cobo, T.; Kacerovsky, M.; Jacobsson, B. Risk factors for spontaneous preterm delivery. Int. J. Gynecol. Obstet. 2020, 150, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Voltolini, C.; Torricelli, M.; Conti, N.; Vellucci, F.L.; Severi, F.M.; Petraglia, F. Understanding spontaneous preterm birth: From underlying mechanisms to predictive and preventive interventions. Reprod. Sci. 2013, 20, 1274–1292. [Google Scholar] [CrossRef] [PubMed]
- Crump, C. Preterm birth and mortality in adulthood: A systematic review. J. Perinatol. 2020, 40, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Chersich, M.F.; Pham, M.D.; Areal, A.; Haghighi, M.M.; Manyuchi, A.; Swift, C.P.; Wernecke, B.; Robinson, M.; Hetem, R.; Boeckmann, M. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: Systematic review and meta-analysis. BMJ 2020, 371, m3811. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Göpel, W.; Härtel, C.; German Neonatal Network; German Center for Lung Research; Priming Immunity at the Beginning of Life (PRIMAL) Consortium. Preterm birth and sustained inflammation: Consequences for the neonate. Semin. Immunopathol. 2020, 42, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Green, E.S.; Arck, P.C. Pathogenesis of preterm birth: Bidirectional inflammation in mother and fetus. Semin. Immunopathol. 2020, 42, 413–429. [Google Scholar] [CrossRef]
- Garg, A.; Jaiswal, A. Evaluation and Management of Premature Rupture of Membranes: A Review Article. Cureus 2023, 15, e36615. [Google Scholar] [CrossRef]
- Yild, C.; Tanir, H.; Sener, T. Comparison of conventional methods (nitrazine test, ferning test) and placental alphamicroglobulin-1 (PAMG-1) in cervicovaginal discharge for the diagnosis of rupture of membranes. Int. J. Gynaecol. Obs. 2009, 107, S530. [Google Scholar]
- Medley, N.; Poljak, B.; Mammarella, S.; Alfirevic, Z. Clinical guidelines for prevention and management of preterm birth: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1361–1369. [Google Scholar] [CrossRef]
- da Fonseca, E.B.; Damião, R.; Moreira, D.A. Preterm birth prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Dochez, V.; Ducarme, G.; Gueudry, P.; Joueidi, Y.; Boivin, M.; Boussamet, L.; Pelerin, H.; Le Thuaut, A.; Lamoureux, Z.; Riche, V.-P. Methods of detection and prevention of preterm labour and the PAMG-1 detection test: A review. J. Perinat. Med. 2021, 49, 119–126. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Prelabor rupture of membranes: ACOG practice bulletin, number 217. Obs. Gynecol 2020, 135, e80–e97. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, G.; Theodora, M.; Antsaklis, P.; Sindos, M.; Grigoriadis, T.; Antsaklis, A.; Papantoniou, N.; Loutradis, D.; Pergialiotis, V. Assessment of uterocervical angle width as a predictive factor of preterm birth: A systematic review of the literature. BioMed Res. Int. 2018, 2018, 1837478. [Google Scholar] [CrossRef] [PubMed]
- Luechathananon, S.; Songthamwat, M.; Chaiyarach, S. Uterocervical angle and cervical length as a tool to predict preterm birth in threatened preterm labor. Int. J. Women’s Health 2021, 13, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Olarinoye, A.O.; Olaomo, N.O.; Adesina, K.T.; Ezeoke, G.G.; Aboyeji, A.P. Comparative diagnosis of premature rupture of membrane by nitrazine test, urea, and creatinine estimation. Int. J. Health Sci. 2021, 15, 16. [Google Scholar]
- Dai, X.; Shen, L. Advances and trends in omics technology development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Alfirevic, A. Systematic review of preterm birth multi-omic biomarker studies. Expert Rev. Mol. Med. 2022, 24, e18. [Google Scholar] [CrossRef]
- Frey, H.A.; Stout, M.J.; Pearson, L.N.; Tuuli, M.G.; Cahill, A.G.; Strauss III, J.F.; Gomez, L.M.; Parry, S.; Allsworth, J.E.; Macones, G.A. Genetic variation associated with preterm birth in African-American women. Am. J. Obstet. Gynecol. 2016, 215, 235.e1–235.e8. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Edwards, D.R.V.; Kusanovic, J.P.; Hassan, S.S.; Mazaki-Tovi, S.; Vaisbuch, E.; Kim, C.J.; Chaiworapongsa, T.; Pearce, B.D.; Friel, L.A. Identification of fetal and maternal single nucleotide polymorphisms in candidate genes that predispose to spontaneous preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2010, 202, 431.e1–431.e34. [Google Scholar] [CrossRef]
- Romero, R.; Friel, L.A.; Edwards, D.R.V.; Kusanovic, J.P.; Hassan, S.S.; Mazaki-Tovi, S.; Vaisbuch, E.; Kim, C.J.; Erez, O.; Chaiworapongsa, T. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM). Am. J. Obstet. Gynecol. 2010, 203, 361.e1–361.e30. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, S.; Bruiners, N.; Hillermann, R. A novel exonic variant (221delT) in the LGALS13 gene encoding placental protein 13 (PP13) is associated with preterm labour in a low risk population. J. Reprod. Immunol. 2009, 82, 166–173. [Google Scholar] [CrossRef]
- Annells, M.F.; Hart, P.H.; Mullighan, C.G.; Heatley, S.L.; Robinson, J.S.; Bardy, P.; McDonald, H.M. Interleukins-1,-4,-6,-10, tumor necrosis factor, transforming growth factor-β, FAS, and mannose-binding protein C gene polymorphisms in Australian women: Risk of preterm birth. Am. J. Obstet. Gynecol. 2004, 191, 2056–2067. [Google Scholar] [CrossRef]
- Ramos, B.R.D.A.; Mendes, N.D.; Tanikawa, A.A.; Amador, M.A.T.; Santos, N.P.C.D.; Santos, S.E.B.D.; Castelli, E.C.; Witkin, S.S.; Silva, M.G.D. Ancestry informative markers and selected single nucleotide polymorphisms in immunoregulatory genes on preterm labor and preterm premature rupture of membranes: A case control study. BMC Pregnancy Childbirth 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, S.J.; Menon, R.; Velez, D.R.; Thorsen, P.; Williams, S.M. Racial disparity in maternal-fetal genetic epistasis in spontaneous preterm birth. Am. J. Obstet. Gynecol. 2008, 198, 666.e1–666.e10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Feenstra, B.; Bacelis, J.; Liu, X.; Muglia, L.M.; Juodakis, J.; Miller, D.E.; Litterman, N.; Jiang, P.-P.; Russell, L. Genetic associations with gestational duration and spontaneous preterm birth. N. Engl. J. Med. 2017, 377, 1156–1167. [Google Scholar] [CrossRef]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.-H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018, 9, 260. [Google Scholar] [CrossRef]
- Rappoport, N.; Toung, J.; Hadley, D.; Wong, R.J.; Fujioka, K.; Reuter, J.; Abbott, C.W.; Oh, S.; Hu, D.; Eng, C. A genome-wide association study identifies only two ancestry specific variants associated with spontaneous preterm birth. Sci. Rep. 2018, 8, 226. [Google Scholar] [CrossRef]
- Heng, Y.J.; Pennell, C.E.; McDonald, S.W.; Vinturache, A.E.; Xu, J.; Lee, M.W.; Briollais, L.; Lyon, A.W.; Slater, D.M.; Bocking, A.D. Maternal whole blood gene expression at 18 and 28 weeks of gestation associated with spontaneous preterm birth in asymptomatic women. PLoS ONE 2016, 11, e0155191. [Google Scholar] [CrossRef]
- Menon, R.; Velez, D.R.; Morgan, N.; Lombardi, S.J.; Fortunato, S.J.; Williams, S.M. Genetic regulation of amniotic fluid TNF-alpha and soluble TNF receptor concentrations affected by race and preterm birth. Hum. Genet. 2008, 124, 243–253. [Google Scholar] [CrossRef]
- Plunkett, J.; Muglia, L.J. Genetic contributions to preterm birth: Implications from epidemiological and genetic association studies. Ann. Med. 2008, 40, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kalish, R.B.; Nguyen, D.P.; Vardhana, S.; Gupta, M.; Perni, S.C.; Witkin, S.S. A single nucleotide A> G polymorphism at position− 670 in the Fas gene promoter: Relationship to preterm premature rupture of fetal membranes in multifetal pregnancies. Am. J. Obstet. Gynecol. 2005, 192, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.A.; Anton, L.; Bastek, J.; Brown, A.G. Can microRNA profiling in maternal blood identify women at risk for preterm birth? Am. J. Obstet. Gynecol. 2015, 212, 782.e1–782.e5. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Brown, A.G.; Anton, L.; Gilstrop, M.; Heiser, L.; Bastek, J. Distinct cervical microRNA profiles are present in women destined to have a preterm birth. Am. J. Obstet. Gynecol. 2014, 210, 221.e1–221.e11. [Google Scholar] [CrossRef]
- Sanders, A.P.; Burris, H.H.; Just, A.C.; Motta, V.; Svensson, K.; Mercado-Garcia, A.; Pantic, I.; Schwartz, J.; Tellez-Rojo, M.M.; Wright, R.O. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics 2015, 10, 221–228. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Gray, C.; McCowan, L.M.; Patel, R.; Taylor, R.S.; Vickers, M.H. Maternal plasma miRNAs as biomarkers during mid-pregnancy to predict later spontaneous preterm birth: A pilot study. Sci. Rep. 2017, 7, 815. [Google Scholar] [CrossRef]
- Cook, J.; Bennett, P.R.; Kim, S.H.; Teoh, T.G.; Sykes, L.; Kindinger, L.M.; Garrett, A.; Binkhamis, R.; MacIntyre, D.A.; Terzidou, V. First trimester circulating microRNA biomarkers predictive of subsequent preterm delivery and cervical shortening. Sci. Rep. 2019, 9, 5861. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Holzman, C.; Heng, Y.J.; Kibschull, M.; Lye, S.J. Maternal blood EBF1-based microRNA transcripts as biomarkers for detecting risk of spontaneous preterm birth: A nested case-control study. J. Matern.-Fetal Neonatal Med. 2022, 35, 1239–1247. [Google Scholar] [CrossRef]
- Zhou, G.; Holzman, C.; Chen, B.; Wang, P.; Heng, Y.J.; Kibschull, M.; Lye, S.J.; Kasten, E.P. EBF1-correlated long non-coding RNA transcript levels in 3rd trimester maternal blood and risk of spontaneous preterm birth. Reprod. Sci. 2021, 28, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Pandey, M. Association of TLR4 and TNF-α gene polymorphisms and TLR4 mRNA levels in preterm birth in a northern Indian population. Indian Pediatr. 2019, 56, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Buhimschi, I.A.; Dulay, A.T.; Ali, U.A.; Zhao, G.; Abdel-Razeq, S.S.; Bahtiyar, M.O.; Thung, S.F.; Funai, E.F.; Buhimschi, C.S. IL-6 trans-signaling system in intra-amniotic inflammation, preterm birth, and preterm premature rupture of the membranes. J. Immunol. 2011, 186, 3226–3236. [Google Scholar] [CrossRef] [PubMed]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 2021, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Gunko, V.; Pogorelova, T.; Linde, V. Proteomic profiling of the blood serum for prediction of premature delivery. Bull. Exp. Biol. Med. 2016, 161, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Liong, S.; Di Quinzio, M.K.; Heng, Y.J.; Fleming, G.; Permezel, M.; Rice, G.E.; Georgiou, H.M. Proteomic analysis of human cervicovaginal fluid collected before preterm premature rupture of the fetal membranes. Reproduction 2013, 145, 137–147. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Nowicki, B.J.; Izban, M.G.; Pratap, S.; Sashti, N.A.; Sanderson, M.; Nowicki, S. Spontaneous preterm labor is associated with an increase in the proinflammatory signal transducer TLR4 receptor on maternal blood monocytes. BMC Pregnancy Childbirth 2010, 10, 66. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Goepfert, A.R.; Ramsey, P.S. Biochemical markers for the prediction of preterm birth. Am. J. Obstet. Gynecol. 2005, 192, S36–S46. [Google Scholar] [CrossRef]
- Kolev, N.; Atanasova, T. COMBINED APPLICATION OF PLACENTAL ALPHA MICROGLOBULIN-1 (PAMG-1) AND INSULIN-LIKE GROWTH FACTOR IN THE DIAGNOSIS OF PRETERM BIRTH-OUR RESULTS. Know. Int. J. 2022, 52, 497–500. [Google Scholar]
- Leitich, H.; Kaider, A. Fetal fibronectin—How useful is it in the prediction of preterm birth? BJOG Int. J. Obstet. Gynaecol. 2003, 110, 66–70. [Google Scholar] [CrossRef]
- Chen, M.X.; Dansereau, J.; Hoag, G.N. Comparison of Fetal Fibronectin and Phosphorylated Insulin-Like Growth Factor Binding Protein-1 Testing to Predict Preterm Delivery in Symptomatic Women: A 10-Year Retrospective Study. J. Obstet. Gynaecol. Can. 2020, 42, 971–976. [Google Scholar] [CrossRef]
- Khambalia, A.Z.; Collins, C.E.; Roberts, C.L.; Morris, J.M.; Powell, K.L.; Tasevski, V.; Nassar, N. High maternal serum ferritin in early pregnancy and risk of spontaneous preterm birth. Br. J. Nutr. 2015, 114, 455–461. [Google Scholar] [CrossRef]
- Jahedbozorgan, T.; Yaghmaei, M.; Naserieh, M. Comparison of serum ferritin levels in pregnant women with preterm and term deliveries. Immunopathol. Persa 2020, 6, e25. [Google Scholar] [CrossRef]
- Shah, K.H.; Anjum, A.; Nair, P.; Bhat, P.; Bhat, R.G.; Bhat, S. Pregnancy associated plasma protein A: An indicator of adverse obstetric outcomes in a South India population. Turk. J. Obstet. Gynecol. 2020, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Laganà, A.S.; Rapisarda, A.M.C.; Scarale, M.G.; Corrado, F.; Cignini, P.; Butticè, S.; Rossetti, D. Role of urocortin in pregnancy: An update and future perspectives. World J. Clin. Cases 2016, 4, 165. [Google Scholar] [CrossRef] [PubMed]
- Kariman, N.; Hedayati, M.; Majd, S.A. The role of vaginal prolactin in diagnosis of premature rupture of membranes. Iran. Red Crescent Med. J. 2012, 14, 352. [Google Scholar]
- Mehrotra, S.; Solanki, V.; Natu, S.; Chauhan, S.; Sharma, R. Study of Prolactin in Cervicovaginal Secretion in Women with Preterm Labor and Normal Pregnancy. J. South Asian Fed. Obstet. Gynaecol. 2020, 12, 34–37. [Google Scholar]
- Vadillo-Ortega, F.; Estrada-Gutiérrez, G. Role of matrix metalloproteinases in preterm labour. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, R.; Moghadam, E.K.; Nasirkhani, Z. Maternal serum levels of C-reactive protein at early pregnancy to predict fetal growth restriction and preterm delivery: A prospective cohort study. Int. J. Reprod. Biomed. 2020, 18, 157. [Google Scholar] [CrossRef]
- Moghaddam Banaem, L.; Mohamadi, B.; Asghari Jaafarabadi, M.; Aliyan Moghadam, N. Maternal serum C-reactive protein in early pregnancy and occurrence of preterm premature rupture of membranes and preterm birth. J. Obstet. Gynaecol. Res. 2012, 38, 780–786. [Google Scholar] [CrossRef]
- Makrigiannakis, A.; Semmler, M.; Briese, V.; Eckerle, H.; Minas, V.; Mylonas, I.; Friese, K.; Jeschke, U. Maternal serum corticotropin-releasing hormone and ACTH levels as predictive markers of premature labor. Int. J. Gynecol. Obstet. 2007, 97, 115–119. [Google Scholar] [CrossRef]
- Beutler, B.; Greenwald, D.; Hulmes, J.; Chang, M.; Pan, Y.-C.; Mathison, J.; Ulevitch, R.; Cerami, A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 1985, 316, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Lombardi, S.J.; Fortunato, S.J. TNF-α promotes caspase activation and apoptosis in human fetal membranes. J. Assist. Reprod. Genet. 2002, 19, 201–204. [Google Scholar] [CrossRef]
- Pu, J.; Zeng, W.-Y. Relationship among TNF-alpha gene promoter-308 site polymorphism, the levels of maternal serum TNF-alpha, and the mRNA expression placental TNF-alpha in preterm labor. Sichuan Da Xue Xue Bao. Yi Xue Ban J. Sichuan Univ. Med. Sci. Ed. 2009, 40, 77–80. [Google Scholar]
- Wang, X.-J.; Li, L.; Cui, S.-H. Role of collagen III, CTGF and TNF-alpha in premature rupture of human fetal membranes. Sichuan Da Xue Xue Bao. Yi Xue Ban J. Sichuan Univ. Med. Sci. Ed. 2009, 40, 658–661, 675. [Google Scholar]
- Puchner, K.; Iavazzo, C.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Kouskouni, E.; Economou, E.; Malamitsi-Puchner, A.; Creatsas, G. The implication of second-trimester amniotic fluid TNF-alpha, cytochrome C and cell death nucleosomes in the prediction of preterm labor and/or premature rupture of membranes. Arch. Gynecol. Obstet. 2012, 285, 37–43. [Google Scholar] [CrossRef]
- McNamara, H.; Mallaiah, S. Managing coagulopathy following PPH. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 61, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.A.; Baron, J.; Phillippe, M. The role of thrombin in preterm parturition. Am. J. Obstet. Gynecol. 2001, 185, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Wildschut, H.I.; Weiner, C.P.; Peters, T.J. When to Screen in Obstetrics and Gynecology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Abdelazim, I.A. Fetal fibronectin (Quick Check fFN test®) for detection of premature rupture of fetal membranes. Arch. Gynecol. Obstet. 2013, 287, 205–210. [Google Scholar] [CrossRef]
- Liong, S.; Di Quinzio, M.; Fleming, G.; Permezel, M.; Rice, G.; Georgiou, H. New biomarkers for the prediction of spontaneous preterm labour in symptomatic pregnant women: A comparison with fetal fibronectin. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 370–379. [Google Scholar] [CrossRef]
- Nikolova, T.; Bayev, O.; Nikolova, N.; Di Renzo, G.C. Evaluation of a novel placental alpha microglobulin-1 (PAMG-1) test to predict spontaneous preterm delivery. J. Perinat. Med. 2014, 42, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, G.; Faraz, S.; Nasir, R.; Somini, S.; Abdeldayem, R.M.; Koratkar, R.; Alsawalhi, N.; Ammar, A. Comparison of the effectiveness of a PAMG-1 test and standard clinical assessment in the prediction of preterm birth and reduction of unnecessary hospital admissions. J. Matern.-Fetal Neonatal Med. 2019, 32, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Lembet, A.; Eroglu, D.; Ergin, T.; Kuscu, E.; Zeyneloglu, H.; Batioglu, S.; Haberal, A. New rapid bed-side test to predict preterm delivery: Phosphorylated insulin-like growth factor binding protein-1 in cervical secretions. Acta Obstet. Gynecol. Scand. 2002, 81, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, D.; Yanık, F.; Oktem, M.; Zeyneloglu, H.B.; Kuscu, E. Prediction of preterm delivery among women with threatened preterm labor. Gynecol. Obstet. Investig. 2007, 64, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tanir, H.M.; Sener, T.; Yildiz, Z. Cervical phosphorylated insulin-like growth factor binding proteın-1 for the prediction of preterm delivery in symptomatic cases with intact membranes. J. Obstet. Gynaecol. Res. 2009, 35, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Buyukbayrak, E.; Turan, C.; Unal, O.; Dansuk, R.; Cengizoğlu, B. Diagnostic power of the vaginal washing-fluid prolactin assay as an alternative method for the diagnosis of premature rupture of membranes. J. Matern.-Fetal Neonatal Med. 2004, 15, 120–125. [Google Scholar] [CrossRef]
- Karaer, A.; Celik, E.; Celik, O.; Simsek, O.Y.; Ozerol, İ.H.; Yılmaz, E.; Turkcuoglu, I.; Duz, S.A. Amniotic fluid urocortin-1 concentrations for the prediction of preterm delivery. J. Obstet. Gynaecol. Res. 2013, 39, 1236–1241. [Google Scholar] [CrossRef]
- Maymon, E.; Romero, R.; Chaiworapongsa, T.; Berman, S.; Conoscenti, G.; Gomez, R.; Edwin, S. Amniotic fluid matrix metalloproteinase–8 in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2001, 185, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Everett, J. NMR-based pharmacometabonomics: A new approach to personalised medicine. In NMR in Pharmaceutical Science; Wiley: Hoboken, NJ, USA, 2015; p. 359. [Google Scholar]
- Virgiliou, C.; Gika, H.G.; Witting, M.; Bletsou, A.A.; Athanasiadis, A.; Zafrakas, M.; Thomaidis, N.S.; Raikos, N.; Makrydimas, G.; Theodoridis, G.A. Amniotic fluid and maternal serum metabolic signatures in the second trimester associated with preterm delivery. J. Proteome Res. 2017, 16, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Considine, E.C.; Khashan, A.S.; Kenny, L.C. Screening for preterm birth: Potential for a metabolomics biomarker panel. Metabolites 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Ferguson, K.K.; Milne, G.L.; Rios-McConnell, R.; Vélez-Vega, C.; Rosario, Z.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Repeated measures of urinary oxidative stress biomarkers and preterm birth in Puerto Rico. Free Radic. Biol. Med. 2020, 146, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Morillon, A.-C.; Yakkundi, S.; Thomas, G.; Gethings, L.A.; Langridge, J.I.; Baker, P.N.; Kenny, L.C.; English, J.A.; McCarthy, F.P. Association between phospholipid metabolism in plasma and spontaneous preterm birth: A discovery lipidomic analysis in the cork pregnancy cohort. Metabolomics 2020, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, I.A.; Ghaemi, M.S.; Han, X.; Ando, K.; Hédou, J.J.; Feyaerts, D.; Peterson, L.S.; Rumer, K.K.; Tsai, E.S.; Ganio, E.A. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci. Transl. Med. 2021, 13, eabd9898. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Awasthi, S.; Baranwal, S. IL-6: An endogenous activator of MMP-9 in preterm birth. J. Reprod. Immunol. 2020, 141, 103147. [Google Scholar] [CrossRef]
- Kumar, S.; Palaia, T.; Hall, C.E.; Ragolia, L. Role of Lipocalin-type prostaglandin D2 synthase (L-PGDS) and its metabolite, prostaglandin D2, in preterm birth. Prostaglandins Other Lipid Mediat. 2015, 118, 28–33. [Google Scholar] [CrossRef]
- AlSaad, R.; Malluhi, Q.; Boughorbel, S. PredictPTB: An interpretable preterm birth prediction model using attention-based recurrent neural networks. BioData Min. 2022, 15, 6. [Google Scholar] [CrossRef]
- Prema, N.; Pushpalatha, M. Machine learning approach for preterm birth prediction based on maternal chronic conditions. In Proceedings of the Emerging Research in Electronics, Computer Science and Technology: Proceedings of International Conference, ICERECT 2018; Springer: Berlin/Heidelberg, Germany, 2019; pp. 581–588. [Google Scholar]
- Raja, R.; Mukherjee, I.; Sarkar, B.K. A machine learning-based prediction model for preterm birth in rural India. J. Healthc. Eng. 2021, 2021, 6665573. [Google Scholar] [CrossRef]
- Gu, Q.; Zhu, L.; Cai, Z. Evaluation measures of the classification performance of imbalanced data sets. In Proceedings of the Computational Intelligence and Intelligent Systems: 4th International Symposium, ISICA 2009, Huangshi, China, 23–25 October 2009; Proceedings 4. pp. 461–471. [Google Scholar]
- Mercer, B.; Goldenberg, R.; Das, A.; Moawad, A.; Iams, J.; Meis, P.; Copper, R.; Johnson, F.; Thom, E.; McNellis, D. The preterm prediction study: A clinical risk assessment system. Am. J. Obstet. Gynecol. 1996, 174, 1885–1895. [Google Scholar] [CrossRef]
- Mailath-Pokorny, M.; Polterauer, S.; Kohl, M.; Kueronyai, V.; Worda, K.; Heinze, G.; Langer, M. Individualized assessment of preterm birth risk using two modified prediction models. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 186, 42–48. [Google Scholar] [CrossRef]
- Gao, C.; Osmundson, S.; Edwards, D.R.V.; Jackson, G.P.; Malin, B.A.; Chen, Y. Deep learning predicts extreme preterm birth from electronic health records. J. Biomed. Inform. 2019, 100, 103334. [Google Scholar] [CrossRef]
- Elaveyini, U.; Devi, S.P.; Rao, K.S. Neural networks prediction of preterm delivery with first trimester bleeding. Arch. Gynecol. Obstet. 2011, 283, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, L.K.; Iannacchione, M.A.; Hammond, W.E.; Crockett, P.; Maher, S.; Schlitz, K. Data mining methods find demographic predictors of preterm birth. Nurs. Res. 2001, 50, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Darmstadt, G.L.; Gruber, S.; Foeller, M.E.; Carmichael, S.L.; Stevenson, D.K.; Shaw, G.M. Application of machine-learning to predict early spontaneous preterm birth among nulliparous non-Hispanic black and white women. Ann. Epidemiol. 2018, 28, 783–789.e1. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Miller, E.S. Predicting preterm birth: Cervical length and fetal fibronectin. Semin. Perinatol. 2017, 41, 445–451. [Google Scholar] [CrossRef]
- Włodarczyk, T.; Płotka, S.; Rokita, P.; Sochacki-Wójcicka, N.; Wójcicki, J.; Lipa, M.; Trzciński, T. Spontaneous preterm birth prediction using convolutional neural networks. In Proceedings of the Medical Ultrasound, and Preterm, Perinatal and Paediatric Image Analysis: First International Workshop, ASMUS 2020, and 5th International Workshop, PIPPI 2020, Held in Conjunction with MICCAI 2020, Lima, Peru, 4–8 October 2020; Proceedings 1. pp. 274–283. [Google Scholar]
- Włodarczyk, T.; Płotka, S.; Szczepański, T.; Rokita, P.; Sochacki-Wojcicka, N.; Wojcicki, J.; Lipa, M.; Trzciński, T. Machine learning methods for preterm birth prediction: A review. Electronics 2021, 10, 586. [Google Scholar] [CrossRef]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten years of lateral flow immunoassay technique applications: Trends, challenges and future perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar]
- Wong, R.; Tse, H. Lateral Flow Immunoassay; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Mitchell, K.R.; Esene, J.E.; Woolley, A.T. Advances in multiplex electrical and optical detection of biomarkers using microfluidic devices. Anal. Bioanal. Chem. 2022, 414, 167–180. [Google Scholar] [CrossRef]
- Esplin, M.S.; Merrell, K.; Goldenberg, R.; Lai, Y.; Iams, J.D.; Mercer, B.; Spong, C.Y.; Miodovnik, M.; Simhan, H.N.; Van Dorsten, P. Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. Am. J. Obstet. Gynecol. 2011, 204, 391.e1–391.e8. [Google Scholar] [CrossRef]
- Sahore, V.; Kumar, S.; Rogers, C.; Jensen, J.; Sonker, M.; Woolley, A. Pressure-actuated microfluidic devices for electrophoretic separation of pre-term birth biomarkers. Anal. Bioanal. Chem. 2016, 408, 599–607. [Google Scholar] [CrossRef]
- Almughamsi, H.M.; Howell, M.K.; Parry, S.R.; Esene, J.E.; Nielsen, J.B.; Nordin, G.P.; Woolley, A.T. Immunoaffinity monoliths for multiplexed extraction of preterm birth biomarkers from human blood serum in 3D printed microfluidic devices. Analyst 2022, 147, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Nielsen, A.V.; Carson, R.H.; Lin, H.J.L.; Hanson, R.L.; Sonker, M.; Mortensen, D.N.; Price, J.C.; Woolley, A.T. Analysis of thrombin-antithrombin complex formation using microchip electrophoresis and mass spectrometry. Electrophoresis 2019, 40, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Rouholamin, S.; Razavi, M.; Rezaeinejad, M.; Sepidarkish, M. A diagnostic profile on the PartoSure test. Expert Rev. Mol. Diagn. 2020, 20, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, I.A.; Abdelrazak, K.M.; Al-Kadi, M.; Yehia, A.H.; Abdulkareem, A.F. Fetal fibronectin (Quick Check fFN test) versus placental alpha microglobulin-1 (AmniSure test) for detection of premature rupture of fetal membranes. Arch. Gynecol. Obstet. 2014, 290, 457–464. [Google Scholar] [CrossRef] [PubMed]
- BHR Pharmaceuticals Ltd. Healthchex fFN Test Instructions. Available online: https://professional.bhr.co.uk/pub/media/wysiwyg/ffn-userguide.pdf (accessed on 21 December 2023).
- Antagen Pharmaceuticals, Inc. Human Fetal Fibronectin XpressCard. Available online: https://www.antagen.net/wp-content/uploads/2015/05/fFN-XpressCard-Antagen.pdf (accessed on 21 December 2023).
- Varley-Campbell, J.; Mújica-Mota, R.; Coelho, H.; Ocean, N.; Barnish, M.; Packman, D.; Dodman, S.; Cooper, C.; Snowsill, T.; Kay, T. Biomarker Tests to Help Diagnose Preterm Labour in Women with Intact Membranes; University of Exeter: Exeter, UK, 2018. [Google Scholar]
- Tenoudji-Cohen Couka, L.; Donato, X.-C.; Glowaczower, E.; Squercioni-Aumont, A.; Katsogiannou, M.; Desbriere, R. Does Assessment of Cervical Phosphorylated Insulin-like Growth Factor Binding Protein-1 by Bedside Vaginal Swab Test Really Predict Preterm Birth? Reprod. Sci. 2021, 28, 2006–2011. [Google Scholar] [CrossRef]
- Abdelazim, I.A.; Amer, O.O.; Shikanova, S.; Karimova, B. Diagnostic accuracy of PremaQuick in detection of preterm labor in symptomatic women. Ginekol. Pol. 2022, 93, 121–125. [Google Scholar] [CrossRef]
- Tripathi, R.; Tyagi, S.; Mala, Y.M.; Singh, N.; Pandey, N.B.; Yadav, P. Comparison of rapid bedside tests for phosphorylated insulin-like growth factor-binding protein 1 and fetal fibronectin to predict preterm birth. Int. J. Gynecol. Obstet. 2016, 135, 47–50. [Google Scholar] [CrossRef]
- Azlin, M.N.; Bang, H.K.; An, L.J.; Mohamad, S.; Mansor, N.; Yee, B.S.; Zulkifli, N.; Tamil, A.M. Role of phIGFBP-1 and ultrasound cervical length in predicting pre-term labour. J. Obstet. Gynaecol. 2010, 30, 456–459. [Google Scholar] [CrossRef]
- QIAGEN. PartoSure Test. Available online: https://herqiagen.com/partosure/ (accessed on 21 December 2023).
- Hologic, Inc. QuikCheck fFN Test Kit Instructions. Available online: https://www.hologic.com/sites/default/files/2018-05/AW-05842-002_004_02.pdf (accessed on 21 December 2023).
- Bruijn, M.M.; Kamphuis, E.I.; Hoesli, I.M.; de Tejada, B.M.; Loccufier, A.R.; Kühnert, M.; Helmer, H.; Franz, M.; Porath, M.M.; Oudijk, M.A. The predictive value of quantitative fibronectin testing in combination with cervical length measurement in symptomatic women. Am. J. Obstet. Gynecol. 2016, 215, 793.e1–793.e8. [Google Scholar] [CrossRef]
- Faron, G.; Balepa, L.; Parra, J.; Fils, J.-F.; Gucciardo, L. The fetal fibronectin test: 25 years after its development, what is the evidence regarding its clinical utility? A systematic review and meta-analysis. J. Matern.-Fetal Neonatal Med. 2020, 33, 493–523. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R. Cervical phosphorylated insulin-like growth factor binding protein-1 test for the prediction of preterm birth: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2016, 214, 57–73. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Kim, S.Y.; England, L.; Wilson, H.G.; Bish, C.; Satten, G.A.; Dietz, P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am. J. Public Health 2010, 100, 1047–1052. [Google Scholar] [CrossRef]
- Han, Z.; Mulla, S.; Beyene, J.; Liao, G.; McDonald, S.D. Maternal underweight and the risk of preterm birth and low birth weight: A systematic review and meta-analyses. Int. J. Epidemiol. 2011, 40, 65–101. [Google Scholar] [CrossRef] [PubMed]
- Ferré, C.; Callaghan, W.; Olson, C.; Sharma, A.; Barfield, W. Effects of maternal age and age-specific preterm birth rates on overall preterm birth rates—United States, 2007 and 2014. Morb. Mortal. Wkly. Rep. 2016, 65, 1181–1184. [Google Scholar] [CrossRef]
- Backes, E.P.; Scrimshaw, S.C.; National Academies of Sciences, Engineering, and Medicine. Epidemiology of Clinical Risks in Pregnancy and Childbirth. In Birth Settings in America: Outcomes, Quality, Access, and Choice; National Academies Press (US): Cambridge, MA, USA, 2020. [Google Scholar]
- Bovbjerg, M.L.; Cheyney, M.; Brown, J.; Cox, K.J.; Leeman, L. Perspectives on risk: Assessment of risk profiles and outcomes among women planning community birth in the United States. Birth 2017, 44, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Hubinont, C.; Debiève, F. Prevention of preterm labour: 2011 update on tocolysis. J. Pregnancy 2011, 2011, 941057. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. WHO recommendations on antenatal corticosteroids for improving preterm birth outcomes. In WHO Recommendations on Antenatal Corticosteroids for Improving Preterm Birth Outcomes; World Health Organization: Geneva, Switzerland, 2022; p. 40. [Google Scholar]
- Patel, S.S.; Ludmir, J. Drugs for the treatment and prevention of preterm labor. Clin. Perinatol. 2019, 46, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Romero, R.; Vidyadhari, D.; Fusey, S.; Baxter, J.; Khandelwal, M.; Vijayaraghavan, J.; Trivedi, Y.; Soma-Pillay, P.; Sambarey, P. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: A multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet. Gynecol. 2011, 38, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.F. Magnesium sulfate: The first-line tocolytic. Obstet. Gynecol. Clin. 2005, 32, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Simhan, H.N.; Caritis, S.N. Prevention of preterm delivery. N. Engl. J. Med. 2007, 357, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.N.; Crowther, C.A.; Wilkinson, D.; Bain, E. Magnesium sulphate for women at term for neuroprotection of the fetus. Cochrane Database Syst. Rev. 2013, CD009395. [Google Scholar] [CrossRef]
- Chollat, C.; Sentilhes, L.; Marret, S. Fetal neuroprotection by magnesium sulfate: From translational research to clinical application. Front. Neurol. 2018, 9, 247. [Google Scholar] [CrossRef]
- Younger, J.D.; Reitman, E.; Gallos, G. Tocolysis: Present and future treatment options. Semin. Perinatol. 2017, 41, 493–504. [Google Scholar] [CrossRef]
- Pang, Q.; Jia, X.; Chen, L. Perinatal outcomes after emergency cervical cerclage for cervical insufficiency with prolapsed membranes. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4202. [Google Scholar] [CrossRef] [PubMed]
- Garfield, L.; Chin, E. Pharmacology for preterm labor. J. Perinat. Neonatal Nurs. 2020, 34, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Suzuki, H.; Saito, K.; Swa, T.; Namba, F.; Vogel, J.P.; Ramson, J.A.; Cao, J.; Tina, L.; Ota, E. Tocolytic Therapy Inhibiting Preterm Birth in High-Risk Populations: A Systematic Review and Meta-Analysis. Children 2023, 10, 443. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gondane, P.; Kumbhakarn, S.; Maity, P.; Kapat, K. Recent Advances and Challenges in the Early Diagnosis and Treatment of Preterm Labor. Bioengineering 2024, 11, 161. https://doi.org/10.3390/bioengineering11020161
Gondane P, Kumbhakarn S, Maity P, Kapat K. Recent Advances and Challenges in the Early Diagnosis and Treatment of Preterm Labor. Bioengineering. 2024; 11(2):161. https://doi.org/10.3390/bioengineering11020161
Chicago/Turabian StyleGondane, Prashil, Sakshi Kumbhakarn, Pritiprasanna Maity, and Kausik Kapat. 2024. "Recent Advances and Challenges in the Early Diagnosis and Treatment of Preterm Labor" Bioengineering 11, no. 2: 161. https://doi.org/10.3390/bioengineering11020161
APA StyleGondane, P., Kumbhakarn, S., Maity, P., & Kapat, K. (2024). Recent Advances and Challenges in the Early Diagnosis and Treatment of Preterm Labor. Bioengineering, 11(2), 161. https://doi.org/10.3390/bioengineering11020161