Full-Field Strain Measurements of the Muscle-Tendon Junction Using X-ray Computed Tomography and Digital Volume Correlation
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation and Phosphotungstic Acid (PTA) Staining
2.2. Tensile Testing Using Bose ElectroForce
2.3. In Situ X-ray Computed Tomography (XCT) Mechanical Testing
2.4. Image Postprocessing
2.5. Digital Volume Correlation and Statistical Analysis
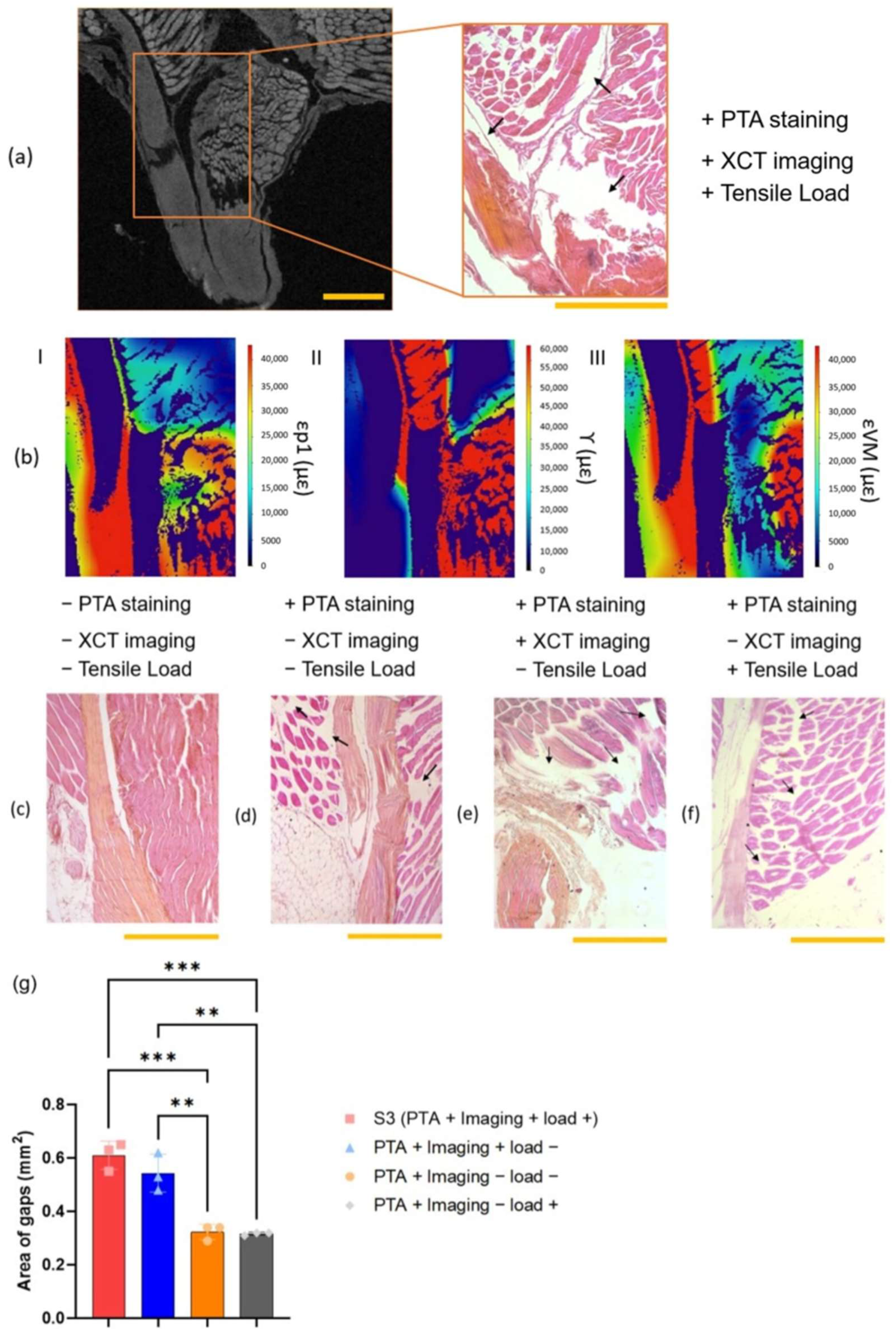
2.6. Histology
3. Results
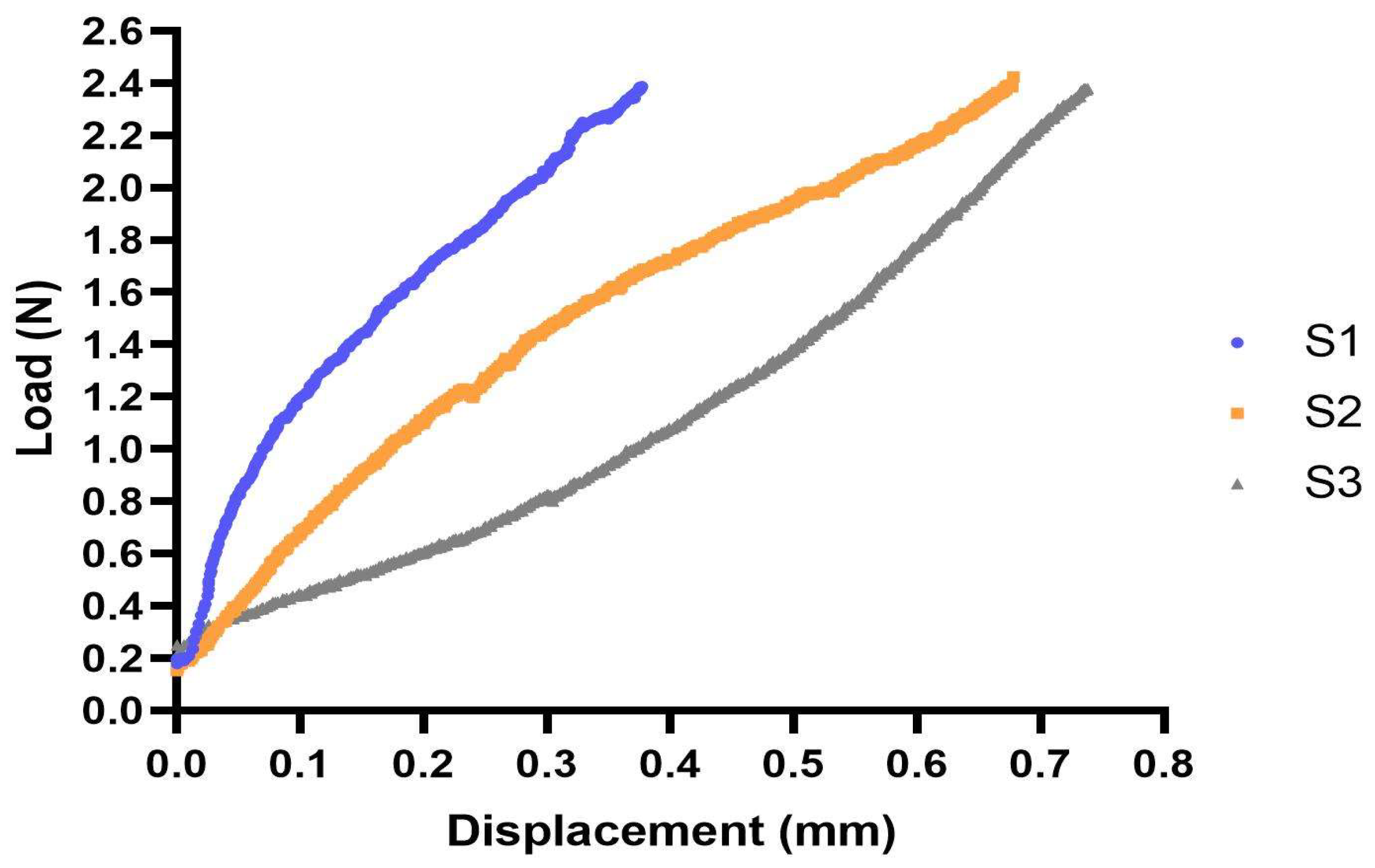
3.1. Tensile Testing
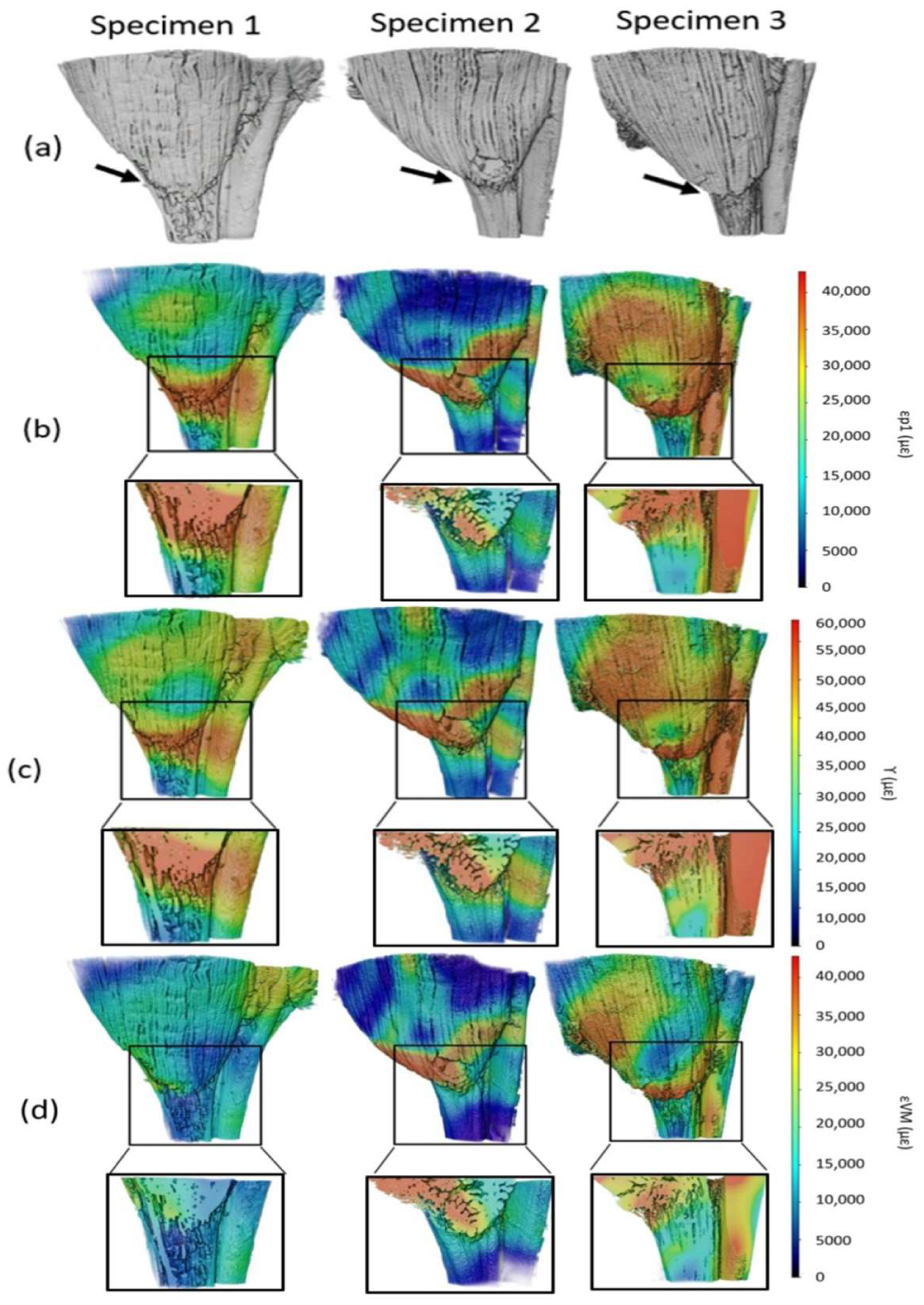
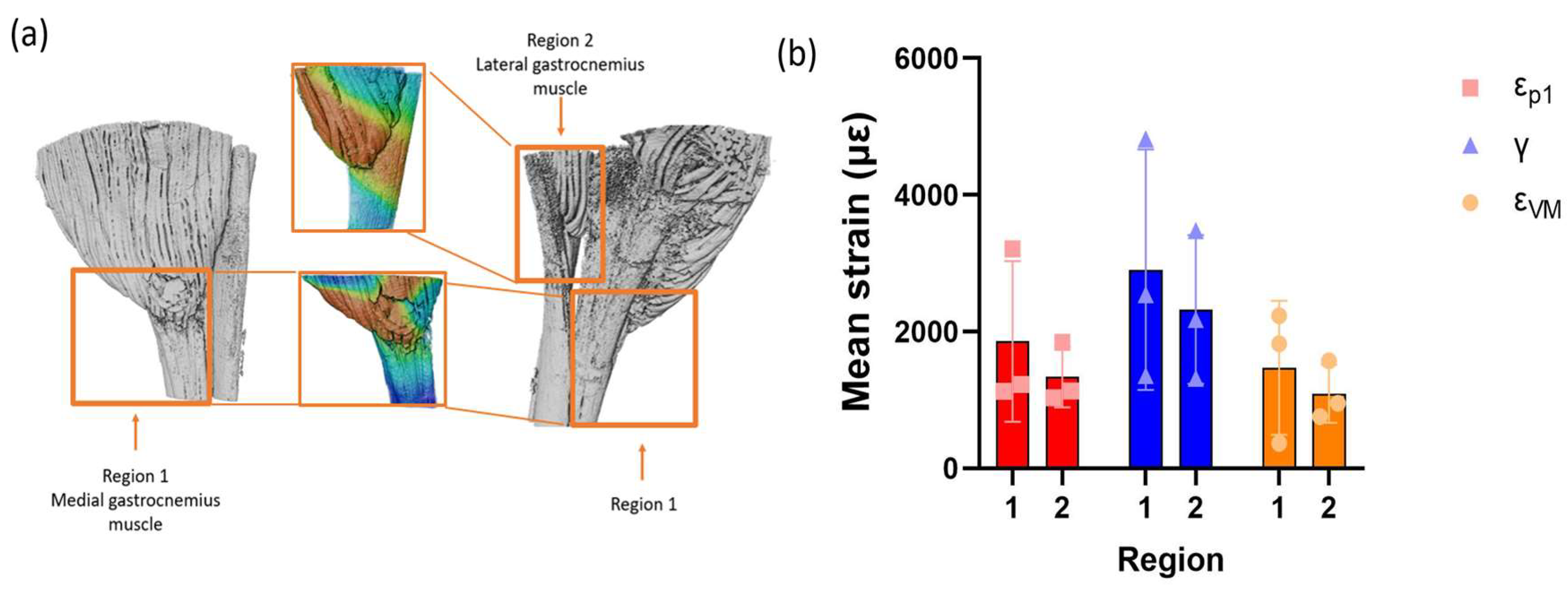
3.2. Strain Maps
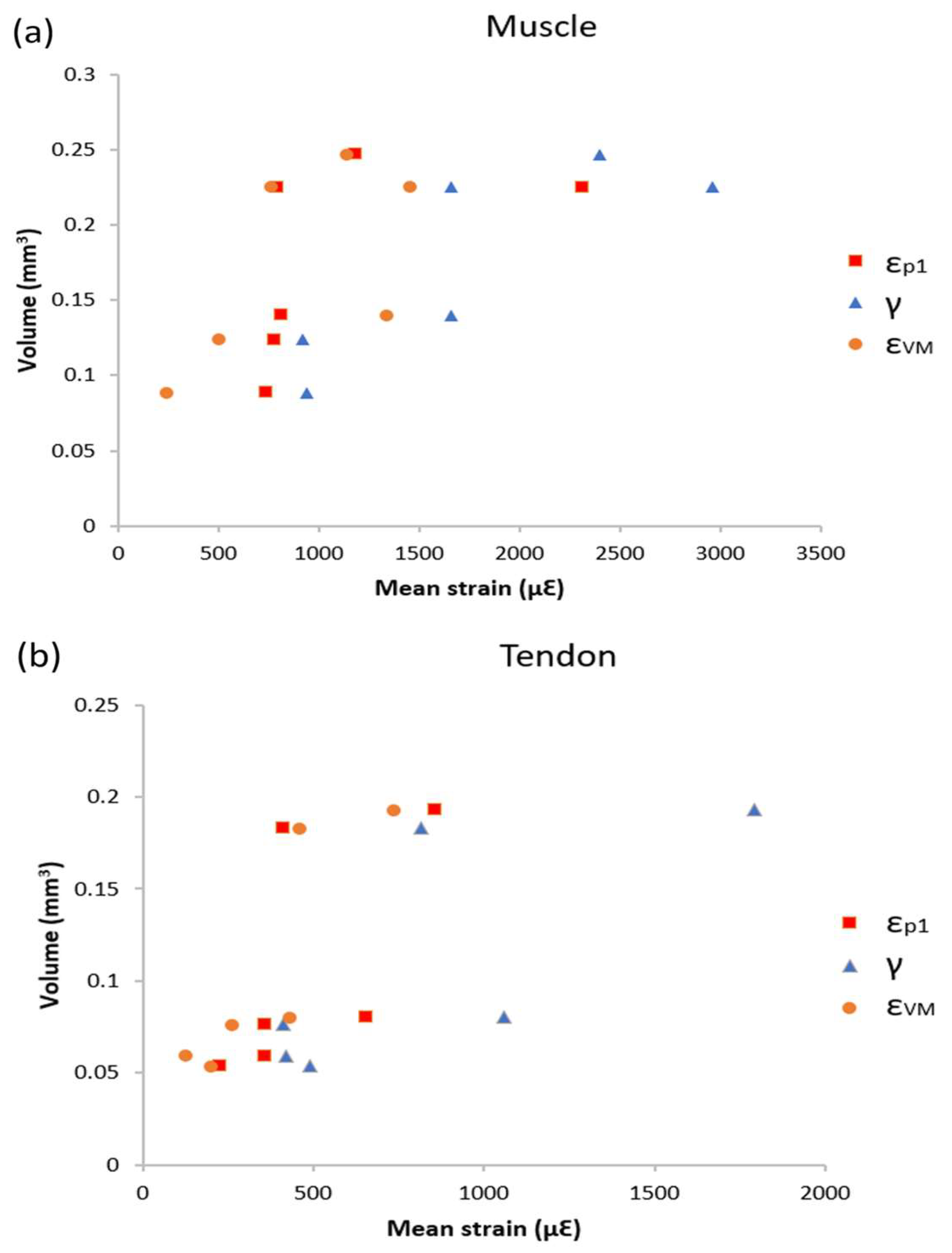
3.3. Volume Strain Correlation
3.4. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huijing, P.A. Muscle as a collagen fiber reinforced composite: A review of force transmission in muscle and whole limb. J. Biomech. 1999, 32, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Del Buono, A.; Oliva, F.; Via, A.G.; Frizziero, A.; Barazzuol, M.; Brancaccio, P.; Freschi, M.; Galletti, S.; Lisitano, G.; et al. Muscle Injuries: A Brief Guide to Classification and Management. Transl. Med. UniSa. 2015, 12, 14–18. [Google Scholar] [PubMed]
- Smoak, M.M.; Mikos, A.G. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater. Today Bio 2020, 7, 100069. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Juncosa, N.; Dressler, M.R. Functional Efficacy of Tendon Repair Processes. Annu. Rev. Biomed. Eng. 2004, 6, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Pouca, M.C.P.V.; Parente, M.P.L.; Jorge, R.M.N.; Ashton-Miller, J.A. Injuries in Muscle-Tendon-Bone Units: A Systematic Review Considering the Role of Passive Tissue Fatigue. Orthop. J. Sports Med. 2021, 9, 23259671211020731. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, S.; Wang, G.; Su, M.; Song, L.; Chen, J.; Fan, S.; Lin, X. Preparation of decellularized biphasic hierarchical myotendinous junction extracellular matrix for muscle regeneration. Acta Biomater. 2018, 68, 15–28. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-Induced Muscle Damage in Humans. Am. J. Phys. Med. Rehabil. 2002, 81 (Suppl. 11), S52–S69. [Google Scholar] [CrossRef]
- Yan, Z.; Yin, H.; Nerlich, M.; Pfeifer, C.G.; Docheva, D. Boosting tendon repair: Interplay of cells, growth factors and scaffold-free and gel-based carriers. J. Exp. Orthop. 2018, 5, 1. [Google Scholar] [CrossRef]
- Garrett, W.E.; Faherty, M.S. Muscle-Tendon Junction Injury. In Muscle and Tendon Injuries; Springer: Berlin/Heidelberg, Germany, 2017; pp. 51–60. [Google Scholar] [CrossRef]
- Myer, C.; Fowler, J.R. Flexor Tendon Repair. Orthop. Clin. N. Am. 2016, 47, 219–226. [Google Scholar] [CrossRef]
- Arruda, E.M.; Calve, S.; Dennis, R.G.; Mundy, K.; Baar, K. Regional variation of tibialis anterior tendon mechanics is lost following denervation. J. Appl. Physiol. 2006, 101, 1113–1117. [Google Scholar] [CrossRef]
- van Vijven, M.; van Groningen, B.; Janssen, R.; van der Steen, M.; van Doeselaar, M.; Stefanoska, D.; van Donkelaar, C.; Ito, K.; Foolen, J. Local variations in mechanical properties of human hamstring tendon autografts for anterior cruciate ligament reconstruction do not translate to a mechanically inferior strand. J. Mech. Behav. Biomed. Mater. 2021, 126, 105010. [Google Scholar] [CrossRef]
- Kellis, E.; Patsika, G.; Karagiannidis, E. Karagiannidis. Strain and elongation of the human semitendinosus muscle—Tendon unit. J. Electromyogr. Kinesiol. 2013, 23, 1384–1390. [Google Scholar] [CrossRef]
- Maganaris, C.N.; Paul, J.P. Load-elongation characteristics of in vivo human tendon and aponeurosis. J. Exp. Biol. 2000, 203, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.; Stafilidis, S.; DeMonte, G.; Karamanidis, K.; Morey-Klapsing, G.; Brüggemann, G. Strain and elongation of the human gastrocnemius tendon and aponeurosis during maximal plantarflexion effort. J. Biomech. 2005, 38, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Leonard, M.E.; Brown, C.G.; Trestik, C.L. Frog semitendinosis tendon load-strain and stress-strain properties during passive loading. Am. J. Physiol. Physiol. 1991, 261, C86–C92. [Google Scholar] [CrossRef] [PubMed]
- Finni, T.; Hodgson, J.A.; Lai, A.M.; Edgerton, V.R.; Sinha, S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J. Appl. Physiol. 2003, 95, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.P.; Hansen, P.; Aagaard, P.; Brønd, J.; Kjaer, M.; Dyhre-Poulsen, P.; Bojsen-Moller, J. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol. Scand. 2003, 177, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, D.; Ouyang, H.; Chen, N.; Chang, B.; Zhu, Q.; Li, J. Ultrasound Assessment of Muscle Injury Associated with Closed Limb Fracture. BioMed Res. Int. 2019, 2019, 9365291. [Google Scholar] [CrossRef] [PubMed]
- Affagard, J.S.; Feissel, P.; Bensamoun, S. Measurement of the quadriceps muscle displacement and strain fields with ultrasound and Digital Image Correlation (DIC) techniques. IRBM 2015, 36, 170–177. [Google Scholar] [CrossRef]
- Okotie, G.; Duenwald-Kuehl, S.; Kobayashi, H.; Wu, M.-J.; Vanderby, R. Tendon Strain Measurements with Dynamic Ultrasound Images: Evaluation of Digital Image Correlation. J. Biomech. Eng. 2012, 134, 024504. [Google Scholar] [CrossRef]
- Palanca, M.; Tozzi, G.; Cristofolini, L. The use of digital image correlation in the biomechanical area: A review. Int. Biomech. 2015, 3, 1–21. [Google Scholar] [CrossRef]
- Holak, K.; Kohut, P.; Ekiert, M.; Tomaszewski, K.; Uhl, T. The Use of Digital Image Correlation in the Study of Achilles Tendon Strain Field. Mech. Control. 2015, 34, 19. [Google Scholar] [CrossRef]
- Tang, C.Y.; Zhang, G.; Tsui, C.P. A 3D skeletal muscle model coupled with active contraction of muscle fibres and hyperelastic behaviour. J. Biomech. 2009, 42, 865–872. [Google Scholar] [CrossRef]
- Rehorn, M.R.; Blemker, S.S. The effects of aponeurosis geometry on strain injury susceptibility explored with a 3D muscle model. J. Biomech. 2010, 43, 2574–2581. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hosseinnejad, S.H.; Jabran, A.; Baltzopoulos, V.; Ren, L.; Qian, Z. A biomechanical analysis of 3D stress and strain patterns in patellar tendon during knee flexion. Int. J. Numer. Methods Biomed. Eng. 2020, 36, e3379. [Google Scholar] [CrossRef] [PubMed]
- Blemker, S.S.; Pinsky, P.M.; Delp, S.L. DelA 3D model of muscle reveals the causes of nonuniform strains in the biceps brachii. J. Biomech. 2005, 38, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.R.; Morrow, D.A.; Felmlee, J.P.; Murthy, N.S.; Kaufman, K.R. Method of quantifying 3D strain distribution in skeletal muscle using cine phase contrast MRI. Physiol. Meas. 2015, 36, N135–N146. [Google Scholar] [CrossRef] [PubMed]
- Bay, B.K.; Smith, T.S.; Fyhrie, D.P.; Saad, M. Digital volume correlation: Three-dimensional strain mapping using x-ray tomography. Exp. Mech. 1999, 39, 217–226. [Google Scholar] [CrossRef]
- Karali, A.; Kao, A.P.; Zekonyte, J.; Blunn, G.; Tozzi, G. Micromechanical evaluation of cortical bone using in situ XCT indentation and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2021, 115, 104298. [Google Scholar] [CrossRef]
- Ranganath, S.K.; Schlund, M.; Delattre, J.; Ferri, J.; Chai, F. Bilateral double site (calvarial and mandibular) critical-size bone defect model in rabbits for evaluation of a craniofacial tissue engineering constructs. Mater. Today Bio 2022, 14, 100267. [Google Scholar] [CrossRef]
- Fung, A.K.; Paredes, J.; Andarawis-Puri, N. Novel image analysis methods for quantification of in situ 3-D tendon cell and matrix strain. J. Biomech. 2017, 67, 184–189. [Google Scholar] [CrossRef]
- Davis, S.; Karali, A.; Zekonyte, J.; Roldo, M.; Blunn, G. Development of a method to investigate strain distribution across the cartilage-bone interface in guinea pig model of spontaneous osteoarthritis using lab-based contrast enhanced X-ray-computed tomography and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2023, 144, 105999. [Google Scholar] [CrossRef] [PubMed]
- Gillard, F.; Boardman, R.; Mavrogordato, M.; Hollis, D.; Sinclair, I.; Pierron, F.; Browne, M. The application of digital volume correlation (DVC) to study the microstructural behaviour of trabecular bone during compression. J. Mech. Behav. Biomed. Mater. 2014, 29, 480–499. [Google Scholar] [CrossRef]
- Disney, C.; Eckersley, A.; McConnell, J.; Geng, H.; Bodey, A.; Hoyland, J.; Lee, P.; Sherratt, M.; Bay, B. Synchrotron tomography of intervertebral disc deformation quantified by digital volume correlation reveals microstructural influence on strain patterns. Acta Biomater. 2019, 92, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Tavana, S.; Clark, J.; Prior, J.; Baxan, N.; Masouros, S.; Newell, N.; Hansen, U. Quantifying deformations and strains in human intervertebral discs using Digital Volume Correlation combined with MRI (DVC-MRI). J. Biomech. 2020, 102, 109604. [Google Scholar] [CrossRef] [PubMed]
- Kastner, J.; Harrer, B.; Requena, G.; Brunke, O. A comparative study of high resolution cone beam X-ray tomography and synchrotron tomography applied to Fe- and Al-alloys. NDT E Int. 2010, 43, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.P.; Kao, A.P.; Bonithon, R.; Howells, D.; Bodey, A.J.; Wanelik, K.; Witte, F.; Johnston, R.; Arora, H.; Tozzi, G. Time-resolved in situ synchrotron-microCT: 4D deformation of bone and bone analogues using digital volume correlation. Acta Biomater. 2021, 131, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Schulz, G.; Weitkamp, T.; Zanette, I.; Pfeiffer, F.; Beckmann, F.; David, C.; Rutishauser, S.; Reznikova, E.; Müller, B. High-resolution tomographic imaging of a human cerebellum: Comparison of absorption and grating-based phase contrast. J. R. Soc. Interface 2010, 7, 1665–1676. [Google Scholar] [CrossRef]
- Brunet, J.; Walsh, C.L.; Wagner, W.L.; Bellier, A.; Werlein, C.; Marussi, S.; Jonigk, D.D.; Verleden, S.E.; Ackermann, M.; Lee, P.D.; et al. Preparation of large biological samples for high-resolution, hierarchical, synchrotron phase-contrast tomography with multimodal imaging compatibility. Nat. Protoc. 2023, 18, 1441–1461. [Google Scholar] [CrossRef]
- Lwin, T.-T.; Yoneyama, A.; Maruyama, H.; Takeda, T. Visualization Ability of Phase-Contrast Synchrotron-Based X-Ray Imaging Using an X-Ray Interferometer in Soft Tissue Tumors. Technol. Cancer Res. Treat. 2021, 20, 15330338211010121. [Google Scholar] [CrossRef]
- Huang, L.; Korhonen, R.K.; Turunen, M.J.; Finnilä, M.A. Experimental mechanical strain measurement of tissues. PeerJ 2019, 7, e6545. [Google Scholar] [CrossRef] [PubMed]
- Naresh, K.; Khan, K.; Umer, R.; Cantwell, W. The use of X-ray computed tomography for design and process modeling of aerospace composites: A review. Mater. Des. 2020, 190, 108553. [Google Scholar] [CrossRef]
- Rawson, S.D.; Maksimcuka, J.; Withers, P.J.; Cartmell, S.H. X-ray computed tomography in life sciences. BMC Biol. 2020, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Lowe, T.; Shearer, T. Optimal contrast agent staining of ligaments and tendons for X-ray computed tomography. PLoS ONE 2016, 11, e0153552. [Google Scholar] [CrossRef] [PubMed]
- Cnudde, V.; Boone, M.N. High-resolution X-ray computed tomography in geosciences: A review of the current technology and applications. Earth-Sci. Rev. 2013, 123, 1–17. [Google Scholar] [CrossRef]
- Lin, E.; Alessio, A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J. Cardiovasc. Comput. Tomogr. 2009, 3, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Fabiszewska, E.; Pasicz, K.; Grabska, I.; Skrzyński, W.; Ślusarczyk-Kacprzyk, W.; Bulski, W. Evaluation of Imaging Parameters of Ultrasound Scanners: Baseline for Future Testing. Pol. J. Radiol. 2017, 82, 773–782. [Google Scholar] [CrossRef]
- Junn, J.C.; Soderlund, K.A.; Glastonbury, C.M. Imaging of Head and Neck Cancer With CT, MRI, and US. Semin. Nucl. Med. 2021, 51, 3–12. [Google Scholar] [CrossRef]
- Wu, P.; Yu, Y.; McGhee, C.E.; Tan, L.H.; Lu, Y. Applications of Synchrotron-Based Spectroscopic Techniques in Studying Nucleic Acids and Nucleic Acid-Functionalized Nanomaterials. Adv. Mater. 2014, 26, 7849–7872. [Google Scholar] [CrossRef]
- Chappard, C.; Basillais, A.; Benhamou, L.; Bonassie, A.; Brunet-Imbault, B.; Bonnet, N.; Peyrin, F. Comparison of synchrotron radiation and conventional X-ray microcomputed tomography for assessing trabecular bone microarchitecture of human femoral heads. Med Phys. 2006, 33, 3568–35777. [Google Scholar] [CrossRef]
- Disney, C.; Vo, N.; Bodey, A.; Bay, B.; Lee, P. Image quality and scan time optimisation for in situ phase contrast x-ray tomography of the intervertebral disc. J. Mech. Behav. Biomed. Mater. 2023, 138, 105579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ji, D.; Li, Y.; Zhao, X.; Lv, W.; Xin, X.; Han, S.; Hu, C. Three-dimensional visualization of microvasculature from few-projection data using a novel CT reconstruction algorithm for propagation-based X-ray phase-contrast imaging. Biomed. Opt. Express 2019, 11, 364–387. [Google Scholar] [CrossRef] [PubMed]
- Karali, A.; Dall’ara, E.; Zekonyte, J.; Kao, A.P.; Blunn, G.; Tozzi, G. Effect of radiation-induced damage of trabecular bone tissue evaluated using indentation and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2023, 138, 105636. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.H.; Winter, D.A. Internal forces at chronic running injury sites. Med. Sci. Sports Exerc. 1990, 22, 357–369. [Google Scholar] [CrossRef]
- Almonroeder, T.; Willson, J.D.; Kernozek, T.W. The Effect of Foot Strike Pattern on Achilles Tendon Load during Running. Ann. Biomed. Eng. 2013, 41, 1758–1766. [Google Scholar] [CrossRef]
- Giddings, V.L.; Beaupré, G.S.; Whalen, R.T.; Carter, D.R. Calcaneal loading during walking and running. Med. Sci. Sports Exerc. 2000, 32, 627–634. [Google Scholar] [CrossRef]
- Liu, L.; Morgan, E.F. Accuracy and precision of digital volume correlation in quantifying displacements and strains in trabecular bone. J. Biomech. 2007, 40, 3516–3520. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, R. Adaptive nonlinear filters for simultaneous removal of different kinds of noise in images. IEEE Trans. Circuits Syst. 1987, 34, 1275–1291. [Google Scholar] [CrossRef]
- Dall’ara, E.; Peña-Fernández, M.; Palanca, M.; Giorgi, M.; Cristofolini, L.; Tozzi, G. Precision of Digital Volume Correlation Approaches for Strain Analysis in Bone Imaged with Micro-Computed Tomography at Different Dimensional Levels. Front. Mater. 2017, 4, 31. [Google Scholar] [CrossRef]
- Fernández, M.P.; Barber, A.; Blunn, G.; Tozzi, G. Optimization of digital volume correlation computation in SR-microCT images of trabecular bone and bone-biomaterial systems. J. Microsc. 2018, 272, 213–228. [Google Scholar] [CrossRef]
- Pappas, G.P.; Asakawa, D.S.; Delp, S.L.; Zajac, F.E.; Drace, J.E. Nonuniform shortening in the biceps brachii during elbow flexion. J. Appl. Physiol. 2002, 92, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Guan, Y.; Kay, M.D.; Cummings, J.F.; Feder, S.M.; Levy, M.S. Location-dependent variations in the material properties of the anterior cruciate ligament. J. Biomech. 1992, 25, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Galbusera, F.; Freutel, M.; Dürselen, L.; D’Aiuto, M.; Croce, D.; Villa, T.; Sansone, V.; Innocenti, B. Material Models and Properties in the Finite Element Analysis of Knee Ligaments: A Literature Review. Front. Bioeng. Biotechnol. 2014, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Premakumar, Y.; Seifalian, A.; Butler, P.E.; Szarko, M. Biomechanical Characterization of Human Soft Tissues Using Indentation and Tensile Testing. J. Vis. Exp. 2016, 118, e54872. [Google Scholar] [CrossRef]
- Fiorentino, N.M.; Blemker, S.S. Musculotendon variability influences tissue strains experienced by the biceps femoris long head muscle during high-speed running. J. Biomech. 2014, 47, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Arts, T.; Costa, K.D.; Covell, J.W.; McCulloch, A.D.; Rossi, A.C.; Pluijmert, M.; Bovendeerd, P.H.M.; Kroon, W.; Delhaas, T.; LeGrice, I.J.; et al. Relating myocardial laminar architecture to shear strain and muscle fiber orientation. Am. J. Physiol. Circ. Physiol. 2001, 280, H2222–H2229. [Google Scholar] [CrossRef] [PubMed]
- LeGrice, I.J.; Takayama, Y.; Covell, J.W. Transverse Shear Along Myocardial Cleavage Planes Provides a Mechanism for Normal Systolic Wall Thickening. Circ. Res. 1995, 77, 182–193. [Google Scholar] [CrossRef]
- Kharazi, M.; Bohm, S.; Theodorakis, C.; Mersmann, F.; Arampatzis, A. Quantifying mechanical loading and elastic strain energy of the human Achilles tendon during walking and running. Sci. Rep. 2021, 11, 5830. [Google Scholar] [CrossRef]
- Boyer, M.I.; Meunier, M.J.; Lescheid, J.; Burns, M.E.; Gelberman, R.H.; Silva, M.J. The influence of cross-sectional area on the tensile properties of flexor tendons. J. Hand Surg. 2001, 26, 828–832. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Kemp, A.D.; Harding, C.C.; Cabral, W.A.; Marini, J.C.; Wallace, J.M. Effects of tissue hydration on nanoscale structural morphology and mechanics of individual Type I collagen fibrils in the Brtl mouse model of Osteogenesis Imperfecta. J. Struct. Biol. 2012, 180, 428–438. [Google Scholar] [CrossRef]
- McDaniel, D.P.; Shaw, G.A.; Elliott, J.T.; Bhadriraju, K.; Meuse, C.; Chung, K.-H.; Plant, A.L. The Stiffness of Collagen Fibrils Influences Vascular Smooth Muscle Cell Phenotype. Biophys. J. 2007, 92, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Metscher, B.D. MicroCT for developmental biology: A versatile tool for high-contrast 3D imaging at histological resolutions. Dev. Dyn. 2009, 238, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Garman, E.F.; Weik, M. Radiation damage to biological macromolecules∗. Curr. Opin. Struct. Biol. 2023, 82, 102662. [Google Scholar] [CrossRef] [PubMed]
- de la Mora, E.; Coquelle, N.; Bury, C.S.; Rosenthal, M.; Holton, J.M.; Carmichael, I.; Garman, E.F.; Burghammer, M.; Colletier, J.-P.; Weik, M. Radiation damage and dose limits in serial synchrotron crystallography at cryo- and room temperatures. Proc. Natl. Acad. Sci. USA 2020, 117, 4142–4151. [Google Scholar] [CrossRef] [PubMed]
- Horng, A.; Brun, E.; Mittone, A.; Gasilov, S.; Weber, L.; Geith, T.; Adam-Neumair, S.; Auweter, S.D.; Bravin, A.; Reiser, M.F.; et al. Cartilage and Soft Tissue Imaging Using X-rays Propagation-Based Phase-Contrast Computed Tomography of the Human Knee in Comparison with Clinical Imaging Techniques and Histology. Investig. Biol. 2014, 49, 627–634. [Google Scholar]
- Bravin, A.; Coan, P.; Suortti, P. X-ray phase-contrast imaging: From pre-clinical applications towards clinics. Phys. Med. Biol. 2012, 58, R1–R35. [Google Scholar] [CrossRef] [PubMed]
- Villarraga-Gómez, H.; Smith, S.T. Effect of the number of projections on dimensional measurements with X-ray computed tomography. Precis. Eng. 2020, 66, 445–456. [Google Scholar] [CrossRef]

| Strain in Tendon | Strain in Aponeurosis | Load Applied | Subject | Imaging Technique | Reference |
|---|---|---|---|---|---|
| 0.8 to 2.5% | 2.1 to 7% | five series of stimulated contractions at 20, 40, 60, 80, and 100% of maximum isometric dorsiflexion moment | human tibialis anterior tendon | Ultrasound | [14] |
| 4.72 ± 1.85% | 5.12 ± 2.07% | isometric maximal voluntary contractions | human Achilles tendon | Ultrasound | [15] |
| 1.95 ± 1.49% | 7.95 ± 7.47% | passive loading to a tension equal to maximum tetanic contraction | frog semitendinosis tenodn | He-Ne laser | [16] |
| 2.8% and 4.7% respectively | 1.2% and 2.2% respectively | 20 and 40% of maximal voluntary contraction | human Achilles tendon | Magnetic resonance scanner | [17] |
| 8.0 ± 1.2% | 1.4 ± 0.4% | voluntary isometric contraction | human Achilles tendon | Ultrasound | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, N.; Karali, A.; Roldo, M.; Blunn, G. Full-Field Strain Measurements of the Muscle-Tendon Junction Using X-ray Computed Tomography and Digital Volume Correlation. Bioengineering 2024, 11, 162. https://doi.org/10.3390/bioengineering11020162
Iwasaki N, Karali A, Roldo M, Blunn G. Full-Field Strain Measurements of the Muscle-Tendon Junction Using X-ray Computed Tomography and Digital Volume Correlation. Bioengineering. 2024; 11(2):162. https://doi.org/10.3390/bioengineering11020162
Chicago/Turabian StyleIwasaki, Nodoka, Aikaterina Karali, Marta Roldo, and Gordon Blunn. 2024. "Full-Field Strain Measurements of the Muscle-Tendon Junction Using X-ray Computed Tomography and Digital Volume Correlation" Bioengineering 11, no. 2: 162. https://doi.org/10.3390/bioengineering11020162
APA StyleIwasaki, N., Karali, A., Roldo, M., & Blunn, G. (2024). Full-Field Strain Measurements of the Muscle-Tendon Junction Using X-ray Computed Tomography and Digital Volume Correlation. Bioengineering, 11(2), 162. https://doi.org/10.3390/bioengineering11020162








