Abstract
Peri-implant diseases, such as peri-implant mucositis and peri-implantitis, are induced by dysbiotic microbiota resulting in the inflammatory destruction of peri-implant tissue. Nonetheless, there has yet to be an established protocol for the treatment of these diseases in a predictable manner, although many clinicians and researchers have proposed various treatment modalities for their management. With the increase in the number of reports evaluating the efficacy of various treatment modalities and new materials, the use of multiple decontamination methods to clean infected implant surfaces is recommended; moreover, the use of hard tissue laser and/or air abrasion techniques may prove advantageous in the future. Limited evidence supports additional effects on clinical improvement in antimicrobial administration for treating peri-implantitis. Implantoplasty may be justified for decontaminating the implant surfaces in the supracrestal area. Surgical treatment is employed for advanced peri-implantitis, and appropriate surgical methods, such as resection therapy or combination therapy, should be selected based on bone defect configuration. This review presents recent clinical advances in debridement methods for contaminated implant surfaces and regenerative materials for treating peri-implant bone defects. It also proposes a new flowchart to guide the treatment decisions for peri-implant disease.
1. Introduction
In recent decades, the incidence of biological complications associated with dental implants has increased due to their increased use to replace missing or damaged teeth. Implant-related complications, specifically, can be divided into four main categories: biological, mechanical, and iatrogenic failures and inadequate adaptation. Biological failure can be further categorized as early failure due to unsuccessful osseointegration and late failure due to unsuccessful maintenance of the achieved osseointegration [1]. Early failure is mainly attributed to insufficient primary stability, surgical trauma, and the occurrence of infections. Conversely, late failure is more frequently associated with occlusal overload and peri-implantitis induced by poor oral hygiene [1]. Recently, titanium particles have been suggested as a possible cause of implant failure [2].
Peri-implant mucositis and peri-implantitis, categorized as late failures, are inflammatory conditions that are caused by bacterial plaque and affect the tissues around dental implants [3]. According to a recent meta-analysis, the prevalences of peri-implant mucositis and peri-implantitis were 42.9% and 21.7%, respectively [4]. These peri-implant diseases often cause rapid destruction of the tissues around an implant and require prompt and thorough therapeutic interventions [5], as treatment success depends on continual bacterial elimination from the contaminated implant surface and arrest of inflammatory processes [6]. Various mechanical and chemical decontamination methods have been suggested, either alone or in combination [7,8,9], for the elimination of bacteria from the micro- and macro-complex topography of implant surfaces. Although peri-implantitis is often treated using non-surgical treatment methods followed by surgical management, the success rate is low [10]; the non-surgical therapeutic approach alone is considered particularly inadequate in managing this condition [11]. During surgical treatment, soft tissue management (resection or grafting) is occasionally performed in conjunction with plaque debridement. Additionally, regenerative approaches are often used adjunctively to achieve bone regeneration and re-osseointegration around the implant, and various regenerative materials are currently being used for these purposes. The efficacy of surgical treatments for peri-implantitis has been evaluated in several studies, but factors such as the size and morphology of peri-implant bone defects, treatment procedures, and observation periods vary considerably across these studies, and comparing the effectiveness of each surgical treatment is challenging. Moreover, the results are often inconsistent even in studies with similar study designs. Currently, no clinical guidelines have been established for the treatment of peri-implant mucositis or peri-implantitis, yet the number of randomized controlled trials (RCTs) on these conditions has only been increasing. Therefore, reliable and practical clinical guidelines for the management of peri-implant mucositis and peri-implantitis are urgently required.
This narrative review summarizes the current knowledge regarding the diagnostic methods for evaluating the risk and status assessment of peri-implant diseases as well as decision-making protocols for their non-surgical and surgical treatments. We also propose a new flowchart to guide treatment decisions for peri-implant diseases and further recommend employing the clinical parameter of bleeding on probing (BOP) for initial screening of peri-implant status along with additional analyses such as bone defect configuration and keratinized mucosa (KM) thickness and width assessments.
2. Methods
The Medline/PubMed and Google Scholar databases were searched for relevant articles on each addressed topic published until September 2023. All papers listed in the table are extracted from RCTs. The search strategy for RCT used for Medline/PubMed was as follows: ((“peri-implantitis”[All Fields] OR “peri-implant mucositis”[All Fields]) AND “non-surgical”[All Fields] AND “randomized controlled trial”[Publication Type]) AND (randomizedcontrolledtrial[Filter]) for papers about the treatment of peri-implant diseases using non-surgical therapy at August 2023 and (((“peri implantitis”[MeSH Terms] OR “peri implantitis”[All Fields] OR (“peri”[All Fields] AND “implantitis”[All Fields]) OR “peri implantitis”[All Fields]) AND (“surgical procedures, operative”[MeSH Terms] OR (“surgical”[All Fields] AND “procedures”[All Fields] AND “operative”[All Fields]) OR “operative surgical procedures”[All Fields] OR “surgical”[All Fields] OR “surgically”[All Fields] OR “surgicals”[All Fields]) AND (“surgical flaps”[MeSH Terms] OR (“surgical”[All Fields] AND “flaps”[All Fields]) OR “surgical flaps”[All Fields] OR “flap”[All Fields])) NOT (“review”[Publication Type] OR “review literature as topic”[MeSH Terms] OR “review”[All Fields])) AND (randomizedcontrolledtrial[Filter]) for papers about the treatment of peri-implant diseases using surgical therapy at September 2023. Papers that were not published in English or were not considered relevant based on the title and abstract were excluded. Full-text reviews were performed as the next step to further exclude articles that did not fit into the scope of this review.
3. Non-Surgical Treatment
3.1. Debridement Methods in Non-Surgical Treatment
Non-surgical debridement has been widely employed for managing peri-implant mucositis and peri-implantitis. It is less invasive than surgical procedures and can result in reduced patient discomfort, shorter recovery periods, and lower incidences of post-treatment complications. In addition, non-surgical debridement allows for timely intervention, which may be preferable for both patients and clinicians and has proved effective for peri-implant mucositis [12]. Currently, manual instruments and ultrasonic devices are the main tools and devices employed for non-surgical mechanical debridement of contaminated implant surfaces [13,14,15,16,17]. Several RCTs have examined the efficacy of these tools and devices in managing peri-implant complications. These mechanical debridement methods have been shown to reduce peri-implant inflammation. Renvert et al. compared two non-surgical approaches (treated with either titanium manual instruments or ultrasonic devices) for managing peri-implantitis and observed a noticeable reduction in both the plaque and bleeding scores. However, no impact on the probing depth (PD) was observed [15]. In addition, no significant difference was observed in treatment outcomes between the manual and ultrasonic instruments groups. Thus, although both instruments are therapeutically effective to some extent, neither is superior in promoting healing. Furthermore, upon comparison between repetitive treatments using oscillating chitosan brushes and titanium curettes, significant reductions in the PD and bleeding index were observed in both the treatment groups after 6 and 12 months compared to those at baseline [18,19]. Thus, these studies support the opinion that non-surgical interventions, albeit limited, can indeed exert a positive influence in controlling peri-implant inflammation.
The clinical effectiveness of ultrasonic devices and air abrasives in peri-implant therapy has been compared in several studies. Air-abrasive decontamination using agents such as amino acid glycine powder and erythritol was introduced for non-surgical decontamination of implant surfaces [20,21,22]. Hentenaar et al. demonstrated comparable effectiveness of both ultrasonic devices and air abrasives in the treatment of peri-implantitis in an RCT [21]. Similarly, studies comparing air abrasives and erbium-doped yttrium aluminum garnet (Er:YAG) laser debridement have shown similar clinical improvement [23,24]. However, the incorporation of air abrasives in conventional non-surgical techniques offers minimal additional benefit, thus limiting its clinical significance [20,25]. On the other hand, an RCT comparing air-abrasive and chemical disinfection using chlorhexidine (CHX) demonstrated a greater reduction in the BOP with air abrasive disinfection [26]. Although evidence suggests that air abrasives are beneficial in treating peri-implantitis, determining their superiority remains challenging. Thus, continuous assessment of the appropriate positioning and applicability of air abrasives amidst evolving treatment modalities is necessary.
Lasers are a reasonable treatment option when considering the intricate surface morphology of implant fixtures [27,28,29]. A study emphasized that laser decontamination is often not employed as a stand-alone application but rather as an adjunct to standard mechanical debridement, and suggested the supplementary advantages of diode lasers in non-surgical treatments to be small [27]. However, adjunctive beneficial effects of diode lasers have also been reported [29]. Another study reported that non-surgical mechanical debridement with adjunctive antimicrobial photodynamic therapy (aPDT) was as effective as adjunctive topical minocycline microspheres in reducing mucosal inflammation for up to 12 months [30]. Comparative research delineating the differences between various lasers is sparse, emphasizing the urgent need for clear guidelines for optimal laser selection and treatment protocols. The contents of this section are summarized in Table 1.

Table 1.
List of randomized control studies comparing non-surgical debridement methods.
3.2. Effectiveness of Local and Systemic Antibiotics and Antiseptics in Non-Surgical Treatment
Administrations of various antibiotics, including amoxicillin, metronidazole, and azithromycin, have been investigated as supplementary treatments for the management of peri-implantitis in RCTs. However, reaching definitive conclusions regarding the overall effectiveness of systemic antibiotics in non-surgical treatment [31,32,33,34] is challenging. Moreover, Blanco et al. [35] reported beneficial effects of systemic administration of metronidazole in combination with non-surgical treatments. They observed significant improvements in the clinical parameters, such as PD, clinical attachment loss (CAL), and bone regeneration, after 12 months of treatment compared with those treated with placebo. Nevertheless, it is worth noting that the success rate of these interventions remained relatively low; only 56.3% of patients bore the success criteria. Although certain antibiotics such as metronidazole have demonstrated successful outcomes in specific cases, the use of antibiotics in peri-implantitis may be considered as part of individualized treatment approaches.
Daily use of antiseptics is a common plaque control measure for preventing periodontal and peri-implant diseases. A recent study by Alqutub et al. demonstrated the efficacy of post non-surgical treatment maintenance using CHX, NaCl, and herbal mouthwashes for short-term relief from peri-implant mucositis [36]. Another study also highlighted the potential complementary benefits of herbal-based and 0.12% CHX oral rinses to non-surgical mechanical debridement [37]. This is further supported by studies emphasizing the positive outcomes of integrating hypochlorite-based formulas with conventional treatments [38]. However, some reports could not successfully demonstrate the effectiveness of these antiseptics. A few studies have found limited to no additional benefit upon implementation of 0.12% CHX mouthwash in the treatment regimen [39]. Similarly, the additional application of CHX or chloramine gel after mechanical cleaning did not yield significant benefits [40,41]. Additionally, although the use of various mouth rinsing agents has demonstrated significant reductions in certain inflammatory markers, determining the type of mouthwash most appropriate and effective for peri-implantitis treatment remains challenging [42].
In summary, the use of antiseptic mouthwashes as an adjunct to non-surgical mechanical debridement may be helpful in suppressing peri-implant mucositis and peri-implantitis. However, considering the potential variability in the outcomes based on specific agents and individual patient conditions, a tailored and comprehensive approach is warranted. The contents of this section are summarized in Table 2.

Table 2.
List of randomized control studies on non-surgical treatment using local and systemic antibiotics, rinses, and gels.
3.3. Type of Materials Considered for the Non-Surgical Treatment of Peri-Implant Diseases
Some pioneering techniques for non-surgical management of peri-implantitis are gaining attention owing to the increasing demand for effective and minimally invasive approaches. The use of enamel matrix derivatives (EMD) for non-surgical treatment has emerged as a promising strategy [43]. Kashefimehr et al. highlighted the potential of EMD, particularly when combined with mechanical debridement therapy, for treating peri-implant mucositis. They conducted a double-blind clinical trial in patients with peri-implant mucositis and demonstrated that the adjunctive use of an EMD with mechanical debridement led to significant improvements in BOP and PD after three months, compared to the debridement alone. Furthermore, inflammation was significantly curtailed by EMD application, as evidenced by the reduced levels of interleukin (IL)-6 and IL-17 in the peri-implant crevicular fluid [43].
Furthermore, the use of probiotics is emerging as an intriguing strategy for the management of peri-implant diseases [44,45]. In one RCT, the administration of Lactobacillus reuteri as an oral probiotic along with non-surgical mechanical therapy resulted in significant improvements in the clinical parameters of implants with peri-implant mucositis or peri-implantitis. Specifically, improvements were observed in the patient-wide BOP and PD values at the implant sites over a 90-day period [44]. However, this approach had limited effect on the microbiota in peri-implant tissue; the only parameter that showed a significant decrease was the Porphyromonas gingivalis count in peri-implant mucositis. Thus, the microbiological effects seemed limited despite the presence of immediate benefits of this combination therapy. Studies investigating such innovative treatment approaches may change non-surgical interventions for peri-implant diseases. The contents of this section are summarized in Table 3.

Table 3.
Innovative approaches to non-surgical treatment of peri-implant diseases.
4. Surgical Treatment
4.1. Decontamination Methods of the Implant Surface for Surgical Treatment of Peri-Implantitis
Surgical approaches have been recommended for treating peri-implantitis [46]. However, the improvement rate of peri-implantitis after surgical debridement with conventional surgical procedures employed in periodontitis remains challenging, as less than 50% of affected implants can be recovered after surgical treatment in most cases [17,47]. Various therapeutic approaches have been used to enhance the efficacy of surgical treatments for peri-implantitis.
Although implant surface decontamination methods, such as Er:YAG laser, plastic curettes, cotton pellets, and gauze, have been compared, the results have been inconsistent. Some studies have demonstrated no superiority for any specific debridement technique [48,49,50,51,52]. In contrast, several RCTs have reported that certain debridement methods are more effective than others [53,54,55,56]. Pranno et al. evaluated the efficiency of bacterial removal from contaminated implant surfaces in patients with severe peri-implantitis by using mechanical debridement with air-powder abrasion, chemical decontamination with hydrogen peroxide and CHX gluconate, or a combination of mechanical-chemical decontamination. The amount of residual bacteria on the implant surfaces after mechanical debridement alone or combined mechanical-chemical decontamination was significantly lower than that after untreated or chemical decontamination alone, indicating the superiority of mechanical debridement to chemical decontamination [53]. Bombeccari et al. reported that the aPDT after the decontamination with plastic scalers and irrigation with a 0.2% CHX digluconate solution for 1 min in open flap debridement (OFD) significantly reduced the proinflammatory index of peri-implantitis at 24 weeks of follow-up compared to that of the control group [54] Regarding the difference in the mechanical debridement methods, the adjunctive use of Er:YAG laser in regenerative therapy significantly improved the PD compared to the control group; however, no significant difference was observed in terms of improvement in radiographic findings [55]. Romeo et al. compared the clinical outcomes with and without modification of the surface topography of the contaminated implant surfaces (implantoplasty) in the surgical treatment of peri-implantitis. They demonstrated that additional implantoplasty at the supra-crestal area as a means of decontamination during resective therapy could improve the PD, probing attachment level, and modified bleeding index compared to those of the control group at 24 months; however, the recession index in the control group was significantly lower than that in the experimental group. The cumulative survival rate of the implants in the implantoplasty with resective surgery group in this study was found to be 100% after 3 years [56].
At present, it is difficult to conclude which debridement method is superior; furthermore, complete decontamination of the infected implant surface is challenging even following application of these methods. Multiple decontamination methods have been recommended for cleaning peri-implant surfaces. The contents of this section are summarized in Table 4.

Table 4.
List of randomized control studies comparing debridement methods during surgical therapy.
4.2. Use of Antibacterial Agents in Surgical Treatment
Some randomized control studies have suggested that adjunctive local and systemic antibiotic and antiseptic administration during OFD for peri-implantitis did not provide clinical benefits compared to OFD alone [57,58]. Teughels et al. reported the efficacy of choline-stabilized orthosilicic acid (CS-OSA) in patients who underwent OFD for the treatment of peri-implantitis. CS-OSA has been known to stimulate bone collagen formation in osteopenia, and oral administration of CS-OSA twice daily for one year prevented further bone loss after OFD for peri-implantitis in a patient [59]. Limited evidence exists regarding the use of antibacterial agents and decontamination methods during surgical treatment of peri-implantitis, thus warranting further research. The contents of this section are summarized in Table 5.

Table 5.
List of randomized control studies with and without the use of antimicrobial and supplement during surgical therapy.
4.3. Types of Regeneration Material Used in Surgical Treatment of Peri-Implantitis
Many RCTs have compared the efficacy of various regenerative materials and their combinations in surgical procedures for treating peri-implantitis [60,61,62,63,64,65,66,67,68,69]. A few studies have reported no improvement in the clinical parameters upon the use of regenerative materials in peri-implant surgical treatments [60,61]. Wohlfahrt et al. performed OFD with or without porous titanium granules (PTGs) and compared the 12-month post-treatment outcomes [60]. They showed that both the surgical interventions induced a reduction in the PD and biomarker levels involved in extracellular matrix degradation, regulation of bone resorption, and bone formation ( i.e., matrix metalloproteinase (MMP)-8, IL-6, insulin, and osteoprotegerin) in the peri-implant sulcal fluid; however, no significant differences were observed between the two groups. Isehed et al. also showed no additional improvement in the bone level or PD, even when EMD was used in combination with OFD [61]. However, some RCTs have shown that the combination of OFD and regenerative materials is more effective than OFD alone [62,63,64,65]. Derks et al. reported that OFD with cancellous bone granules plus 10% highly purified porcine collagen demonstrated statistically less buccal gingival recession than OFD alone, although OFD with graft material did not improve the reduction in the PD or BOP at 12 months after surgery [62]. Jepsen et al. also reported that significantly enhanced radiographic defect filling was observed in the treatment of OFD with PTGs compared to that with OFD alone, even though no significant differences were observed in the PD and BOP between the two groups 12 months after surgery [63]. Another comparative study examining OFD with and without PTGs reported similar results [64]. Emanuel et al. administrated D-PLEX500 (a biodegradable prolonged release local doxycycline formulated with β-tricalcium phosphate bone graft) in the surgical treatment of peri-implantitis. At 12 months after surgery, the D-PLEX500 group showed more significant improvements in clinical parameters such as PD, CAL, radiographic bone levels, and BOP than the OFD-alone group; however, there was no significant difference in CAL between groups [65]. As for the improvement in the soft tissue morphology around implants, Solonko et al. compared the efficacy of an apically positioned flap with an autologous free gingival graft (FGG) or a collagen matrix. Both treatments resulted in a significant increase in peri-implant KM at 12 months after treatment, whereas the increase in the KM was significantly greater with FGG. No significant differences were observed in the PD, BOP, or vestibular depth (VD) in the study [66]. A few comparative randomized controlled studies employed regenerative substitutes in both the test and control groups [67,68,69]. The application of a natural bone mineral in combination with a collagen membrane (NBM + CM) showed higher mean PPD reduction than nanocrystalline hydroxyapatite (NHA) (NBM + CM: 2.4 ± 0.8 mm vs. NHA: 1.5 ± 0.6 mm at 24 months after surgery and NBM + CM: 2.5 ± 0.9 mm vs. NHA: 1.1 ± 0.3 mm at 48 months after surgery) and CAL gains (NBM + CM: 2.0 ± 0.8 mm vs. NHA: 1.0 ± 0.4 mm at 24 months after surgery and NBM + CM: 2.0 ± 1.0 mm vs. NHA: 0.6 ± 0.5 mm at 48 months after surgery), indicating the application of NBM + CM to have superior clinical outcomes compared to those with NHA; however, statistical analysis was not performed in these studies [67,68]. Schwarz et al. evaluated the efficacy of NHA application and a bovine-derived xenograft combined with a collagen membrane (BDX + CM) in the surgical treatment of peri-implantitis. No significant difference was observed in the PD and CAL between the two groups 6 months after surgery owing to the significant decrease in the PD and increase in the CAL in both groups [69].
At present, only a limited number of RCTs have been conducted on regenerative treatment of peri-implantitis; thus, determining which material is superior is challenging. Radiographic improvement of the bone would indeed be further enhanced with the use of regenerative materials. However, we should cautiously interpret whether radiographic improvement actually leads to clinical improvement of the peri-implant condition [67]. The contents of this section are summarized in Table 6.

Table 6.
List of randomized control studies on the use of regenerative materials during surgical therapy.
5. Clinical Parameters for Evaluating the Peri-Implant Condition
5.1. Probing Depth
The depth of the peri-implant sulcus in healthy implants is generally 3–4 mm [70], whereas diseased implant sites (peri-implant mucositis and peri-implantitis) are generally slightly deeper PD (4–6 mm) [71], and a 6 mm PD has been suggested as the threshold for progressive bone loss [72]. The discrepancy between the anatomical peri-implant sulcus and PD is slight in the absence of inflammatory changes but increases with the exacerbation of inflammation around the teeth [73]. As with natural teeth, the tip of periodontal probe can identify the apical border of the epithelium barrier with an error of approximately 0.2 mm for both healthy and peri-implant mucositis sites. However, at the sites of peri-implantitis, the measurement error is much greater at 1.5 mm [74]. Additionally, Serino et al. reported a discrepancy in the PD with or without a superstructure at the implants, and PD after prosthesis removal is highly correlated with the bone level of implants assessed at surgery [75]. Clinicians should also be aware of the fact that the presence or form of the implant superstructure can interfere with the PD measurements. Peri-implantitis sites exhibit increased PD and clinical signs of inflammation compared to those at baseline [76]. In cases where patients can receive maintenance care at the clinic where their implants were placed, repeated assessment of PD is very useful for evaluating the peri-implant condition. Conversely, a diagnosis of peri-implantitis can be considered in cases of a PD of ≥6 mm when information about the baseline PD is not available [3].
5.2. Bleeding on Probing
BOP, which is a sign of inflammation of the periodontal sulcus, is considered a valid predictor of future loss of attachment around teeth [77]. Moreover, the absence of BOP is a reliable indicator of periodontal tissue stability [78]. An examination of the BOP to monitor attachment loss around dental implants would be superior to that of natural teeth when a probing force of 0.25 N is used [79]. A meta-analysis revealed that the incidence of peri-implantitis in implants with BOP was 24.1%, with high variability among studies [80]. Although the positive rate of BOP seems to be low, it is considered reasonable because peri-implant mucositis is also included in BOP-positive implants. BOP with additional microbiological tests on natural teeth and implants significantly enhanced the diagnostic accuracy for attachment loss compared with BOP alone [79]. BOP is a reliable indicator of peri-implant inflammatory complications, although it should be used in combination with other parameters for the diagnosis of peri-implant diseases.
5.3. Radiographic Interpretation
Regular inspection of the marginal bone level around the implant site is important for maintaining implant health and early detection of peri-implant lesions. An annual marginal bone loss of less than 0.2 mm one year after implant placement has been regarded as one of the criteria for implant success [81]. Orthopantomograms and periapical radiographs are convenient and conventionally used as low-dose radiation tools to assess marginal bone loss around implants. On the other hand, visualizing buccal and lingual bone resorption using this method is challenging. Additionally, changes in the crestal bone morphology may only be noticeable when the resorptive lesions are of significant size and shape [82]. Serino et al. reported that the measurement of peri-implant bone loss based on periapical radiographs differed from that of clinical bone loss during surgery [83]. Accurate assessment of the extent [84] and configuration of the bone defect around the implant is important during decision making, such as whether regenerative therapy could be possible, in the treatment for peri-implantitis [85]. The recent advances in three-dimensional (3D) imaging using cone-beam computed tomography (CBCT) are remarkable, and the size and types of periodontal bone defects can be easily predicted using this technique [86,87]. However, X-ray artifacts can seriously decrease the quality of CBCT images [88] and sometimes make it impossible to assess bone resorption around dental implants. In addition, the quality of CBCT images is affected by various factors, including the device, field of view, X-ray beam quality, and image reconstruction parameters [89]. Color flow ultrasonography is a recently introduced non-invasive method for instantaneous assessment of dynamic tissue performance and degree of clinical inflammation at the implant site [90]. The combination of radiographic interpretation and ultrasound could facilitate observing changes in the marginal bone and determining bone defect configurations around implants in the future.
5.4. Bone Sounding
Christiaens et al. measured the bone levels at periodontal disease sites using clinical measurements, radiographs (analog and digital intraoral radiographs), and bone sounding (BS) without a flap elevation, and compared these values with the true bone levels measured when the flap was elevated [91]. BS was the most accurate, with all the other evaluation methods underestimating the true bone level [91]. Shan et al. compared the bone levels around the teeth among conventional methods using a 3D radiographic technique, BS, and direct measurements under open-flapped conditions. They showed high agreement between the BS measurements and true bone level, whereas the results of the 3D radiographic technique were weakly correlated [92]. Artifacts cannot be avoided when using CBCT and X-ray imaging. Many studies have used BS to determine the actual bone defect morphology around implants [50,93,94,95]; although BS is somewhat invasive, it shows high predictability of the peri-implant bone levels.
Using multiple clinical parameters is necessary for diagnosing peri-implant mucositis and peri-implantitis. Among the clinical parameters, BOP can be considered a useful tool for differentiating between healthy and diseased states. The removal of the superstructure is recommended for accurate PD measurements. BS should be used to select appropriate treatment methods when identification of the bone defects on radiographic images is challenging.
6. Management of Peri-Implant Health and Treatment Protocol of Peri-Implant Disease
It is now clear that peri-implant disease is more complex and challenging to treat than diseases involving natural teeth. Therefore, management of the health of the tissues surrounding functioning dental implants is essential. We propose a new management flowchart for peri-implant health and disease (Figure 1). The treatment plan for peri-implant disease is decided based on the mobility, BOP, presence of dental plaque, width of the KM and VD, PD, presence of bone loss, amount of bone defect configuration, and thickness of the KM. The BOP is considered the initial examination parameter to assess the peri-implant health condition, except for mobility, in the process of clinical screening. Microbiological tests as additional evaluation items may be helpful in assessing the risk of peri-implant diseases when BOP is observed in the soft tissue around the dental implant [79]. In addition, several clinical parameters, such as PD, plaque deposition, and radiographic examination, should be evaluated. Complete understanding of the peri-implant conditions is critical for planning treatments and supportive procedures. BS is sometimes useful when the morphology of the bone defect is not clearly understood on radiography or CBCT [91]. In addition, it is important to evaluate the KM and VD widths, KM thickness, and bone defect configuration to determine the appropriate treatment strategies for advanced peri-implant disease.
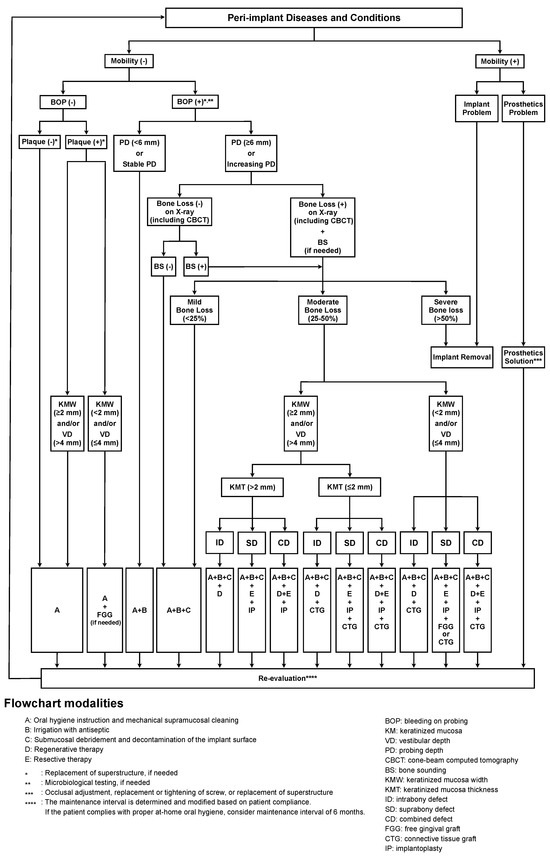
Figure 1.
Proposed flowchart for management of peri-implant diseases (FMPD). The bleeding on probing (BOP) is used as an initial examination parameter in this protocol, except for mobility. Treatment options cumulatively increase depending on the severity of the peri-implant disease. An improperly shaped suprastructure that accelerates plaque accumulation should be converted into an appropriate suprastructure. The adjunct use of microbiological tests is sometimes recommended for the assessment of the peri-implant conditions when BOP is observed around the dental implant. If bone defects and their configuration cannot be evaluated by radiographic examination alone, bone sounding (BS) may be additionally recommended to accurately analyze the bone defect. Moreover, regular reexamination is required to evaluate treatment efficacy. This flowchart can be used at every patient visit, including a follow-up visit. The recommended interval for reexamination is at least once every 6 months. Implants with loss of osseointegration must be promptly removed.
Similar to natural teeth, the need for a minimum amount of KM to maintain peri-implant tissue health remains controversial [96,97,98,99,100]. Some studies have suggested that implants with a reduced KM width of <2 mm are more susceptible to plaque accumulation and marginal inflammation [101,102,103,104]. The thickness of the preoperative gingival tissue also appears to affect the changes in the marginal bone around the implant. The crestal bone loss 1 year after implant placement in the sites with KM thickness ≤2.0 mm is less than in the sites with thickness >2.0 mm [105]. Halperin–Sternfeld et al. reported that sites with a shallow VD around dental implants presented with a lower KM width than sites with adequate VD in a 6-year retrospective longitudinal study [106]. Sites with a shallow VD (≤4 mm) around dental implants are associated with higher mucosal recession, increased relative attachment loss, and enhanced relative bone loss compared to sites with an adequate VD (>4 mm) [106]. Thus, minimum levels of KM and VD may be desirable to prevent bone resorption and maintain the soft tissue around the implant.
The surgical modality performed to improve the residual pocket after non-surgical treatment is also determined by the configuration of the bone defect. Schwarz et al. characterized the bone-defect configuration and distinguished between Class I (infrabony) and Class II (suprabony) defects in the crestal implant insertion area, and further subdivided Class I into five different configurations. Circular bone resorption (Class Ie) was most frequently observed at peri-implantitis sites [107]. Resective therapy has been proposed for Class II bone resorption because improving deficient bone conditions is challenging even with the application of regenerative therapy. In contrast, in Class I defects, circular bone resorption, such as Class Ie, has great potential for regeneration [85]. A systematic review by Daugela et al. assessed the efficacy of regeneration therapy for peri-implantitis and reported that the average radiographic marginal bone level fill was 1.97 mm, and the PD and BOP were reduced by 2.78 mm and 52.5%, respectively [108]; therefore, regenerative therapy can be recommended in cases of Class I bone resorption.
Combined defects (Class I and Class II) are often observed in daily clinical practice, and both regenerative and resective therapies may be required simultaneously or in stages. In cases of peri-implantitis with buccal deficiency and/or supracrestal and intrabony defects, combined surgical treatment with implantoplasty using a peri-implant regenerative approach is effective [48,109,110]. The combination of surgical treatment of peri-implantitis and soft tissue grafting might also be effective in controlling severe peri-implantitis lesions [111].
The major treatment elements in our flowchart are as follows: (A) oral hygiene instruction and mechanical cleaning; (B) irrigation with antiseptics; (C) debridement at the submucosal area and decontamination at the implant surface; (D) regenerative therapy; and (E) resective therapy. Treatment options increase cumulatively depending on the disease severity, similar to cumulative interceptive supportive therapy [112]. If plaque deposits are always observed at a site with inadequate widths of the KM and VD (<2 mm and ≤4 mm, respectively), protocol A plus FGG may be recommended to establish a cleanable oral environment. If the implant is diagnosed as peri-implant mucositis and peri-implantitis with mild bone loss (<25%), non-surgical treatments (protocols A, B, and C) are recommended [113,114]. For debridement and decontamination (protocol C), simultaneous use of multiple devices (such as ultrasonic scalers, titanium curettes, plastic curettes, Er:YAG laser, diode laser, air abrasion, etc.) is recommended as much as possible. In the case of moderate bone loss (25–50%) around an implant, surgical therapies (protocols D and E) can be applied based on the configuration of the bone defect. If the width of the KM and VD (<2 mm and ≤4 mm, respectively) and the thickness of the KM are inadequate (≤2 mm) in the case of moderate bone loss around an implant, surgical therapy comprising flap elevation with FGG or connective tissue graft is recommended [66,114]. For implants with severe bone loss (>50%) or mobility owing to implant fracture, the removal is recommended [114,115]. When retreatment with new implants is required after implant removal, transmucosal implants with a convergent hyperbolic design might be recommended based on a result of complication-free stability of marginal bone loss and BOP for up to 3 years [116]. Additionally, convergent collar implants with crowns fabricated using the biologically oriented preparation technique (BOPT) showed a significant increase in soft tissue volume at the papillae and the buccal margin around implants [117]. Finally, patients who were enrolled in a recall system and maintained a high standard of oral hygiene were reported to be able to keep a stable peri-implant condition for at least 5 years following peri-implant surgery [118].
It must be noted that this review did have some limitations. It only included articles published in English; hence, some pertinent articles published in other languages may have been excluded. Some relevant studies could have been overlooked because only titles and abstracts were used for screening. In addition, meta-analysis was not available because the experimental methods were not homogeneous in RCTs.
7. Conclusions
BOP is considered a useful screening parameter for signs of inflammation. Therefore, we propose that BOP should be the first parameter assessed after mobility evaluation for successful management of peri-implant health. If signs of inflammation or tissue destruction are observed, the condition of the peri-implant tissue must be carefully evaluated for treatment planning. Non-surgical treatment using multiple decontamination methods should be used initially to improve the diseased peri-implant condition. For advanced lesions, surgical treatment modalities should be considered based on the bone defect configuration and the condition of surrounding soft tissues. Surgical procedures, including those that involve the use of regenerative materials, should be considered in cases of infrabony defects. The newly proposed flowchart serves as an excellent and reproducible guideline for peri-implant health management and treatment planning for peri-implant diseases.
Author Contributions
Study design and conceptualization: T.S., K.K. and Y.T. (Yasuo Takeuchi); reviewed previously published related articles: T.S. and K.K.; writing–initial draft of the manuscript: T.S., K.K. and Y.T. (Yasuo Takeuchi); and writing–final draft and revision of the manuscript: T.K., Y.T. (Yoichi Taniguchi), T.T., S.M. (Shogo Maekawa), T.N., R.K., S.M. (Shunsuke Matsumura), S.K., Y.I., A.A. and T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding by the Japan Society for the Promotion of Science KAKENHI (grant numbers 21K16987 to T.S., 22K17089 to K.K., 20K09934 to Y.T., 22K10031 to T.K., and 21K10152 to Y.I.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Hugo Song for English language editing and Mariko Shiba for modifying our flowchart.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implant. Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-implant Diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J.; Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Charalampakis, G.; Abrahamsson, I.; Wennstrom, J.; Berglundh, T. Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Peri-implantitis: A Randomized Controlled Clinical Trial. J. Dent. Res. 2016, 95, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Gustafsson, A.; Berglundh, T. A systematic review of the effect of anti-infective therapy in the treatment of peri-implantitis. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 213–225; discussion 232–233. [Google Scholar] [CrossRef] [PubMed]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: A systematic review. Clin. Oral Implant. Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Salvi, G.E.; Mombelli, A.; Loup, P.J.; Heitz, F.; Kruger, E.; Lang, N.P. Supportive peri-implant therapy following anti-infective surgical peri-implantitis treatment: 5-year survival and success. Clin. Oral Implant. Res. 2018, 29, 1–6. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I. Treatment of pathologic peri-implant pockets. Periodontol. 2000 2018, 76, 180–190. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansaker, A.M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 305–315. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Blasi, A.; Isola, G.; Sculean, A.; Salvi, G.E.; Ramaglia, L. Resolution of peri-implant mucositis at tissue- and bone-level implants: A 6-month prospective controlled clinical trial. Clin. Oral Implant. Res. 2023, 34, 450–462. [Google Scholar] [CrossRef]
- Persson, G.R.; Samuelsson, E.; Lindahl, C.; Renvert, S. Mechanical non-surgical treatment of peri-implantitis: A single-blinded randomized longitudinal clinical study. II. Microbiological results. J. Clin. Periodontol. 2010, 37, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Samuelsson, E.; Lindahl, C.; Persson, G.R. Mechanical non-surgical treatment of peri-implantitis: A double-blind randomized longitudinal clinical study. I: Clinical results. J. Clin. Periodontol. 2009, 36, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Laforí, A.; Pedrinaci, I.; Baima, G.; Ferrarotti, F.; Lima, C.; Paternó Holtzman, L.; Aimetti, M.; Cordaro, L.; Sanz, M. Effect of sub-marginal instrumentation before surgical treatment of peri-implantitis: A multi-centre randomized clinical trial. J. Clin. Periodontol. 2022, 49, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.P.; Pires, P.R.; Rios, F.S.; de Oliveira, J.A.P.; Costa, R.; Cunha, K.F.; Silveira, H.L.D.; Pimentel, S.; Casati, M.Z.; Rosing, C.K.; et al. Surgical and non-surgical debridement for the treatment of peri-implantitis: A two-center 12-month randomized trial. Clin. Oral Investig. 2021, 25, 5723–5733. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Koldsland, O.C.; Roos-Jansåker, A.M.; Wohlfahrt, J.C.; Verket, A.; Mdala, I.; Magnusson, A.; Salvesen, E.; Hjortsjö, C. Non-surgical treatment of mild to moderate peri-implantitis using an oscillating chitosan brush or a titanium curette-A randomized multicentre controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Koldsland, O.C.; Roos-Jansaker, A.M.; Wohlfahrt, J.C.; Verket, A.; Mdala, I.; Magnusson, A.; Salvesen, E.; Hjortsjo, C. Non-surgical treatment of mild to moderate peri-implantitis with an oscillating chitosan brush or a titanium curette-12-month follow-up of a multicenter randomized clinical trial. Clin. Oral Implant. Res. 2023, 34, 684–697. [Google Scholar] [CrossRef]
- Ji, Y.J.; Tang, Z.H.; Wang, R.; Cao, J.; Cao, C.F.; Jin, L.J. Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: A pilot clinical trial. Clin. Oral Implant. Res. 2014, 25, 683–689. [Google Scholar] [CrossRef]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Stewart, R.E.; Van Winkelhoff, A.J.; Meijer, H.J.A.; Raghoebar, G.M. Erythritol airpolishing in the non-surgical treatment of peri-implantitis: A randomized controlled trial. Clin. Oral Implant. Res. 2021, 32, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Riben-Grundstrom, C.; Norderyd, O.; André, U.; Renvert, S. Treatment of peri-implant mucositis using a glycine powder air-polishing or ultrasonic device: A randomized clinical trial. J. Clin. Periodontol. 2015, 42, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Roos-Jansåker, A.M.; Lindahl, C.; Renvert, S. Microbiologic results after non-surgical erbium-doped:yttrium, aluminum, and garnet laser or air-abrasive treatment of peri-implantitis: A randomized clinical trial. J. Periodontol. 2011, 82, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Lindahl, C.; Roos Jansåker, A.M.; Persson, G.R. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: A randomized clinical trial. J. Clin. Periodontol. 2011, 38, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Selimović, A.; Bunæs, D.F.; Lie, S.A.; Lobekk, M.A.; Leknes, K.N. Non-surgical treatment of peri-implantitis with and without erythritol air-polishing a 12-month randomized controlled trial. BMC Oral Health 2023, 23, 240. [Google Scholar] [CrossRef] [PubMed]
- Sahm, N.; Becker, J.; Santel, T.; Schwarz, F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: A prospective, randomized, controlled clinical study. J. Clin. Periodontol. 2011, 38, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Alpaslan Yayli, N.Z.; Talmac, A.C.; Keskin Tunc, S.; Akbal, D.; Altindal, D.; Ertugrul, A.S. Erbium, chromium-doped: Yttrium, scandium, gallium, garnet and diode lasers in the treatment of peri-implantitis: Clinical and biochemical outcomes in a randomized-controlled clinical trial. Lasers Med. Sci. 2022, 37, 665–674. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Klossner, S.; Stähli, A.; Imber, J.C.; Eick, S.; Sculean, A.; Salvi, G.E. Non-surgical mechanical therapy of peri-implantitis with or without repeated adjunctive diode laser application. A 6-month double-blinded randomized clinical trial. Clin. Oral Implant. Res. 2022, 33, 900–912. [Google Scholar] [CrossRef]
- Tenore, G.; Montori, A.; Mohsen, A.; Mattarelli, G.; Palaia, G.; Romeo, U. Evaluation of adjunctive efficacy of diode laser in the treatment of peri-implant mucositis: A randomized clinical trial. Lasers Med. Sci. 2020, 35, 1411–1417. [Google Scholar] [CrossRef]
- Bassetti, M.; Schär, D.; Wicki, B.; Eick, S.; Ramseier, C.A.; Arweiler, N.B.; Sculean, A.; Salvi, G.E. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin. Oral Implant. Res. 2014, 25, 279–287. [Google Scholar] [CrossRef]
- De Waal, Y.C.M.; Vangsted, T.E.; Van Winkelhoff, A.J. Systemic antibiotic therapy as an adjunct to non-surgical peri-implantitis treatment: A single-blind RCT. J. Clin. Periodontol. 2021, 48, 996–1006. [Google Scholar] [CrossRef]
- Hallström, H.; Persson, G.R.; Lindgren, S.; Olofsson, M.; Renvert, S. Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. J. Clin. Periodontol. 2012, 39, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Polymeri, A.; van der Horst, J.; Anssari Moin, D.; Wismeijer, D.; Loos, B.G.; Laine, M.L. Non-surgical peri-implantitis treatment with or without systemic antibiotics: A randomized controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Ferrari, D.S.; Siroma, R.S.; Figueiredo, L.C.; Faveri, M.; Feres, M. Microbiological and clinical effects of adjunctive systemic metronidazole and amoxicillin in the non-surgical treatment of peri-implantitis: 1 year follow-up. Braz. Oral Res. 2019, 33 (Suppl. S1), e080. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Pico, A.; Dopico, J.; Gándara, P.; Blanco, J.; Liñares, A. Adjunctive benefits of systemic metronidazole on non-surgical treatment of peri-implantitis. A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2022, 49, 15–27. [Google Scholar] [CrossRef]
- Alqutub, M.N.; Alhumaidan, A.A.; Alali, Y.; Al-Aali, K.A.; Javed, F.; Vohra, F.; Abduljabbar, T. Comparison of the postoperative anti-inflammatory efficacy of chlorhexidine, saline rinses and herbal mouthwashes after mechanical debridement in patients with peri-implant mucositis: A randomized controlled trial. Int. J. Dent. Hyg. 2023, 21, 203–210. [Google Scholar] [CrossRef]
- Alzoman, H.; Alojaym, T.G.; Chalikkandy, S.N.; Mehmood, A.; Rashed, F.; Divakar, D.D. Comparison of an Herbal- and a 0.12% Chlorhexidine-based Oral Rinse as Adjuncts to Nonsurgical Mechanical Debridement in the Management of Peri-implant Mucositis: A Randomised Controlled Trial. Oral Health Prev. Dent. 2020, 18, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Cosola, S.; Oldoini, G.; Giammarinaro, E.; Covani, U.; Genovesi, A.; Marconcini, S. The effectiveness of the information-motivation model and domestic brushing with a hypochlorite-based formula on peri-implant mucositis: A randomized clinical study. Clin. Exp. Dent. Res. 2022, 8, 350–358. [Google Scholar] [CrossRef]
- Menezes, K.M.; Fernandes-Costa, A.N.; Silva-Neto, R.D.; Calderon, P.S.; Gurgel, B.C. Efficacy of 0.12% Chlorhexidine Gluconate for Non-Surgical Treatment of Peri-Implant Mucositis. J. Periodontol. 2016, 87, 1305–1313. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Salvi, G.E.; Botticelli, D.; Mombelli, A.; Faddy, M.; Lang, N.P.; Implant Complication Research Group. Anti-infective treatment of peri-implant mucositis: A randomised controlled clinical trial. Clin. Oral Implant. Res. 2011, 22, 237–241. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.M.; Almhöjd, U.S.; Jansson, H. Treatment of peri-implantitis: Clinical outcome of chloramine as an adjunctive to non-surgical therapy, a randomized clinical trial. Clin. Oral Implant. Res. 2017, 28, 43–48. [Google Scholar] [CrossRef]
- Philip, J.; Laine, M.L.; Wismeijer, D. Adjunctive effect of mouthrinse on treatment of peri-implant mucositis using mechanical debridement: A randomized clinical trial. J. Clin. Periodontol. 2020, 47, 883–891. [Google Scholar] [CrossRef]
- Kashefimehr, A.; Pourabbas, R.; Faramarzi, M.; Zarandi, A.; Moradi, A.; Tenenbaum, H.C.; Azarpazhooh, A. Effects of enamel matrix derivative on non-surgical management of peri-implant mucositis: A double-blind randomized clinical trial. Clin. Oral Investig. 2017, 21, 2379–2388. [Google Scholar] [CrossRef]
- Galofré, M.; Palao, D.; Vicario, M.; Nart, J.; Violant, D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. J. Periodontal. Res. 2018, 53, 378–390. [Google Scholar] [CrossRef]
- Laleman, I.; Pauwels, M.; Quirynen, M.; Teughels, W. The usage of a lactobacilli probiotic in the non-surgical therapy of peri-implantitis: A randomized pilot study. Clin. Oral Implant. Res. 2020, 31, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Keeve, P.L.; Ramanauskaite, A.; Schwarz, F.; Koo, K.-T.; Sculean, A.; Romanos, G. Surgical treatment of peri-implantitis—Consensus report of working group 4. Int. Dent. J. 2019, 69 (Suppl. S2), 18–22. [Google Scholar] [CrossRef] [PubMed]
- De Waal, Y.C.; Raghoebar, G.M.; Meijer, H.J.; Winkel, E.G.; van Winkelhoff, A.J. Prognostic indicators for surgical peri-implantitis treatment. Clin. Oral Implant. Res. 2016, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Iglhaut, G.; Becker, J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: A randomized controlled clinical study. J. Clin. Periodontol. 2011, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; John, G.; Mainusch, S.; Sahm, N.; Becker, J. Combined surgical therapy of peri-implantitis evaluating two methods of surface debridement and decontamination. A two-year clinical follow up report. J. Clin. Periodontol. 2012, 39, 789–797. [Google Scholar] [CrossRef]
- Schwarz, F.; Hegewald, A.; John, G.; Sahm, N.; Becker, J. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J. Clin. Periodontol. 2013, 40, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.A.; Vouros, I.; Menexes, G.; Konstantinidis, A. The utilization of a diode laser in the surgical treatment of peri-implantitis. A randomized clinical trial. Clin. Oral Investig. 2015, 19, 1851–1860. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; De Angelis, N.; Camurati, A.; Campailla, M.; Felice, P. The adjunctive use of light-activated disinfection (LAD) with FotoSan is ineffective in the treatment of peri-implantitis: 1-year results from a multicentre pragmatic randomised controlled trial. Eur. J. Oral Implantol. 2013, 6, 109–119. [Google Scholar]
- Pranno, N.; Cristalli, M.P.; Mengoni, F.; Sauzullo, I.; Annibali, S.; Polimeni, A.; La Monaca, G. Comparison of the effects of air-powder abrasion, chemical decontamination, or their combination in open-flap surface decontamination of implants failed for peri-implantitis: An ex vivo study. Clin. Oral Investig. 2021, 25, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Bombeccari, G.P.; Guzzi, G.; Gualini, F.; Gualini, S.; Santoro, F.; Spadari, F. Photodynamic therapy to treat periimplantitis. Implant Dent. 2013, 22, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Ashnagar, S.; Gianfilippo, R.D.; Arnett, M.; Kinney, J.; Wang, H.L. Laser-assisted regenerative surgical therapy for peri-implantitis: A randomized controlled clinical trial. J. Periodontol. 2021, 92, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Ghisolfi, M.; Murgolo, N.; Chiapasco, M.; Lops, D.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: Clinical outcome. Clin. Oral Implant. Res. 2005, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hallstrom, H.; Persson, G.R.; Lindgren, S.; Renvert, S. Open flap debridement of peri-implantitis with or without adjunctive systemic antibiotics: A randomized clinical trial. J. Clin. Periodontol. 2017, 44, 1285–1293. [Google Scholar] [CrossRef]
- De Waal, Y.C.; Raghoebar, G.M.; Huddleston Slater, J.J.; Meijer, H.J.; Winkel, E.G.; van Winkelhoff, A.J. Implant decontamination during surgical peri-implantitis treatment: A randomized, double-blind, placebo-controlled trial. J. Clin. Periodontol. 2013, 40, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Celik, G.U.; Tarce, M.; De Cock, I.; Persyn, S.M.; Haytac, M.C. The effect of choline-stabilized orthosilicic acid in patients with peri-implantitis: An exploratory randomized, double-blind, placebo controlled study. BMC Oral Health 2021, 21, 485. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, J.C.; Aass, A.M.; Granfeldt, F.; Lyngstadaas, S.P.; Reseland, J.E. Sulcus fluid bone marker levels and the outcome of surgical treatment of peri-implantitis. J. Clin. Periodontol. 2014, 41, 424–431. [Google Scholar] [CrossRef]
- Isehed, C.; Holmlund, A.; Renvert, S.; Svenson, B.; Johansson, I.; Lundberg, P. Effectiveness of enamel matrix derivative on the clinical and microbiological outcomes following surgical regenerative treatment of peri-implantitis. A randomized controlled trial. J. Clin. Periodontol. 2016, 43, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Ortiz-Vigon, A.; Guerrero, A.; Donati, M.; Bressan, E.; Ghensi, P.; Schaller, D.; Tomasi, C.; Karlsson, K.; Abrahamsson, I.; et al. Reconstructive surgical therapy of peri-implantitis: A multicenter randomized controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 921–944. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.; Jepsen, S.; Laine, M.L.; Anssari Moin, D.; Pilloni, A.; Zeza, B.; Sanz, M.; Ortiz-Vigon, A.; Roos-Jansaker, A.M.; Renvert, S. Reconstruction of Peri-implant Osseous Defects: A Multicenter Randomized Trial. J. Dent. Res. 2016, 95, 58–66. [Google Scholar] [CrossRef]
- Wohlfahrt, J.C.; Lyngstadaas, S.P.; Ronold, H.J.; Saxegaard, E.; Ellingsen, J.E.; Karlsson, S.; Aass, A.M. Porous titanium granules in the surgical treatment of peri-implant osseous defects: A randomized clinical trial. Int. J. Oral Maxillofac. Implant. 2012, 27, 401–410. [Google Scholar]
- Emanuel, N.; Machtei, E.E.; Reichart, M.; Shapira, L. D-PLEX500: A local biodegradable prolonged release doxycycline-formulated bone graft for the treatment for peri-implantitis. A randomized controlled clinical study. Quintessence Int. 2020, 51, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Solonko, M.; Regidor, E.; Ortiz-Vigón, A.; Montero, E.; Vilchez, B.; Sanz, M. Efficacy of keratinized mucosal augmentation with a collagen matrix concomitant to the surgical treatment of peri-implantitis: A dual-center randomized clinical trial. Clin. Oral Implant. Res. 2022, 33, 105–119. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Bieling, K.; Becker, J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: A four-year clinical follow-up report. J. Clin. Periodontol. 2009, 36, 807–814. [Google Scholar] [CrossRef]
- Schwarz, F.; Sculean, A.; Bieling, K.; Ferrari, D.; Rothamel, D.; Becker, J. Two-year clinical results following treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane. J. Clin. Periodontol. 2008, 35, 80–87. [Google Scholar] [CrossRef]
- Schwarz, F.; Bieling, K.; Latz, T.; Nuesry, E.; Becker, J. Healing of intrabony peri-implantitis defects following application of a nanocrystalline hydroxyapatite (Ostim) or a bovine-derived xenograft (Bio-Oss) in combination with a collagen membrane (Bio-Gide). A case series. J. Clin. Periodontol. 2006, 33, 491–499. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S230–S236. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Sahm, N.; Horstkemper, T.; Rousi, K.; Becker, J. The prevalence of peri-implant diseases for two-piece implants with an internal tube-in-tube connection: A cross-sectional analysis of 512 implants. Clin. Oral Implant. Res. 2017, 28, 24–28. [Google Scholar] [CrossRef]
- Fransson, C.; Wennstrom, J.; Berglundh, T. Clinical characteristics at implants with a history of progressive bone loss. Clin. Oral Implant. Res. 2008, 19, 142–147. [Google Scholar] [CrossRef]
- Listgarten, M.A. Periodontal probing: What does it mean? J. Clin. Periodontol. 1980, 7, 165–176. [Google Scholar] [CrossRef]
- Lang, N.P.; Wetzel, A.C.; Stich, H.; Caffesse, R.G. Histologic probe penetration in healthy and inflamed peri-implant tissues. Clin. Oral Implant. Res. 1994, 5, 191–201. [Google Scholar] [CrossRef]
- Serino, G.; Turri, A.; Lang, N.P. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin. Oral Implant. Res. 2013, 24, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Joss, A.; Orsanic, T.; Gusberti, F.A.; Siegrist, B.E. Bleeding on probing. A predictor for the progression of periodontal disease? J. Clin. Periodontol. 1986, 13, 590–596. [Google Scholar] [CrossRef]
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing. An indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721. [Google Scholar] [CrossRef]
- Luterbacher, S.; Mayfield, L.; Bragger, U.; Lang, N.P. Diagnostic characteristics of clinical and microbiological tests for monitoring periodontal and peri-implant mucosal tissue conditions during supportive periodontal therapy (SPT). Clin. Oral Implant. Res. 2000, 11, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Cionca, N.; Combescure, C.; Mombelli, A. The diagnosis of peri-implantitis: A systematic review on the predictive value of bleeding on probing. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 276–293. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Lang, N.P.; Hill, R.W. Radiographs in periodontics. J. Clin. Periodontol. 1977, 4, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Sato, H.; Holmes, P.; Turri, A. Intra-surgical vs. radiographic bone level assessments in measuring peri-implant bone loss. Clin. Oral Implant. Res. 2017, 28, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Tsitoura, E.; Tucker, R.; Suvan, J.; Laurell, L.; Cortellini, P.; Tonetti, M. Baseline radiographic defect angle of the intrabony defect as a prognostic indicator in regenerative periodontal surgery with enamel matrix derivative. J. Clin. Periodontol. 2004, 31, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Schwarz, K.; Becker, J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J. Clin. Periodontol. 2010, 37, 449–455. [Google Scholar] [CrossRef]
- Li, F.; Jia, P.Y.; Ouyang, X.Y. Comparison of Measurements on Cone Beam Computed Tomography for Periodontal Intrabony Defect with Intra-surgical Measurements. Chin. J. Dent. Res. 2015, 18, 171–176. [Google Scholar]
- Misch, K.A.; Yi, E.S.; Sarment, D.P. Accuracy of cone beam computed tomography for periodontal defect measurements. J. Periodontol. 2006, 77, 1261–1266. [Google Scholar] [CrossRef]
- Draenert, F.G.; Coppenrath, E.; Herzog, P.; Muller, S.; Mueller-Lisse, U.G. Beam hardening artefacts occur in dental implant scans with the NewTom cone beam CT but not with the dental 4-row multidetector CT. Dentomaxillofac. Radiol. 2007, 36, 198–203. [Google Scholar] [CrossRef]
- Miracle, A.C.; Mukherji, S.K. Conebeam CT of the head and neck, part 1: Physical principles. AJNR Am. J. Neuroradiol. 2009, 30, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Barootchi, S.; Tavelli, L.; Majzoub, J.; Chan, H.L.; Wang, H.L.; Kripfgans, O.D. Ultrasonographic Tissue Perfusion in Peri-implant Health and Disease. J. Dent. Res. 2022, 101, 278–285. [Google Scholar] [CrossRef]
- Christiaens, V.; Jacobs, R.; Dierens, M.; Vervaeke, S.; De Bruyn, H.; Koole, S.; Cosyn, J. Intraoral radiography lacks accuracy for the assessment of peri-implant bone level—A controlled clinical study. Eur. J. Oral Implantol. 2017, 10, 435–441. [Google Scholar]
- Shah, M.A.; Shah, S.S.; Dave, D. Dentascan—Is the investment worth the hype? J. Clin. Diagn. Res. 2013, 7, 3039–3043. [Google Scholar] [CrossRef]
- Trombelli, L.; Severi, M.; Farina, R.; Simonelli, A. Sub-Periosteal Peri-Implant Augmented Layer Technique to Treat Peri-Implantitis Lesions. Clin. Adv. Periodontics 2020, 10, 169–174. [Google Scholar] [CrossRef]
- Froum, S.J.; Froum, S.H.; Rosen, P.S. Successful management of peri-implantitis with a regenerative approach: A consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int. J. Periodontics Restor. Dent. 2012, 32, 11–20. [Google Scholar]
- Schlee, M.; Rathe, F.; Brodbeck, U.; Ratka, C.; Weigl, P.; Zipprich, H. Treatment of Peri-implantitis-Electrolytic Cleaning Versus Mechanical and Electrolytic Cleaning-A Randomized Controlled Clinical Trial-Six-Month Results. J. Clin. Med. 2019, 8, 1909. [Google Scholar] [CrossRef]
- Brito, C.; Tenenbaum, H.C.; Wong, B.K.; Schmitt, C.; Nogueira-Filho, G. Is keratinized mucosa indispensable to maintain peri-implant health? A systematic review of the literature. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 643–650. [Google Scholar] [CrossRef]
- Gobbato, L.; Avila-Ortiz, G.; Sohrabi, K.; Wang, C.W.; Karimbux, N. The effect of keratinized mucosa width on peri-implant health: A systematic review. Int. J. Oral Maxillofac. Implant. 2013, 28, 1536–1545. [Google Scholar] [CrossRef]
- Lin, G.H.; Chan, H.L.; Wang, H.L. The significance of keratinized mucosa on implant health: A systematic review. J. Periodontol. 2013, 84, 1755–1767. [Google Scholar] [CrossRef]
- Thoma, D.S.; Muhlemann, S.; Jung, R.E. Critical soft-tissue dimensions with dental implants and treatment concepts. Periodontol. 2000 2014, 66, 106–118. [Google Scholar] [CrossRef]
- Wennstrom, J.L.; Derks, J. Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 136–146. [Google Scholar] [CrossRef]
- Bouri, A., Jr.; Bissada, N.; Al-Zahrani, M.S.; Faddoul, F.; Nouneh, I. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int. J. Oral Maxillofac. Implant. 2008, 23, 323–326. [Google Scholar]
- Boynuegri, D.; Nemli, S.K.; Kasko, Y.A. Significance of keratinized mucosa around dental implants: A prospective comparative study. Clin. Oral Implant. Res. 2013, 24, 928–933. [Google Scholar] [CrossRef]
- Chung, D.M.; Oh, T.J.; Shotwell, J.L.; Misch, C.E.; Wang, H.L. Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J. Periodontol. 2006, 77, 1410–1420. [Google Scholar] [CrossRef]
- Schrott, A.R.; Jimenez, M.; Hwang, J.W.; Fiorellini, J.; Weber, H.P. Five-year evaluation of the influence of keratinized mucosa on peri-implant soft-tissue health and stability around implants supporting full-arch mandibular fixed prostheses. Clin. Oral Implant. Res. 2009, 20, 1170–1177. [Google Scholar] [CrossRef]
- Linkevicius, T.; Puisys, A.; Steigmann, M.; Vindasiute, E.; Linkeviciene, L. Influence of Vertical Soft Tissue Thickness on Crestal Bone Changes around Implants with Platform Switching: A Comparative Clinical Study. Clin. Implant Dent. Relat. Res. 2015, 17, 1228–1236. [Google Scholar] [CrossRef]
- Halperin-Sternfeld, M.; Zigdon-Giladi, H.; Machtei, E.E. The association between shallow vestibular depth and peri-implant parameters: A retrospective 6 years longitudinal study. J. Clin. Periodontol. 2016, 43, 305–310. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Sager, M.; Bieling, K.; Sculean, A.; Becker, J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin. Oral Implant. Res. 2007, 18, 161–170. [Google Scholar] [CrossRef]
- Daugela, P.; Cicciu, M.; Saulacic, N. Surgical Regenerative Treatments for Peri-Implantitis: Meta-analysis of Recent Findings in a Systematic Literature Review. J. Oral Maxillofac. Res. 2016, 7, e15. [Google Scholar] [CrossRef]
- Suh, J.J.; Simon, Z.; Jeon, Y.S.; Choi, B.G.; Kim, C.K. The use of implantoplasty and guided bone regeneration in the treatment of peri-implantitis: Two case reports. Implant Dent. 2003, 12, 277–282. [Google Scholar] [CrossRef][Green Version]
- Schwarz, F.; John, G.; Schmucker, A.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: A 7-year follow-up observation. J. Clin. Periodontol. 2017, 44, 337–342. [Google Scholar] [CrossRef]
- Schwarz, F.; John, G.; Sahm, N.; Becker, J. Combined surgical resective and regenerative therapy for advanced peri-implantitis with concomitant soft tissue volume augmentation: A case report. Int. J. Periodontics Restor. Dent. 2014, 34, 489–495. [Google Scholar] [CrossRef]
- Mombelli, A.; Lang, N.P. The diagnosis and treatment of peri-implantitis. Periodontol. 2000 1998, 17, 63–76. [Google Scholar] [CrossRef]
- Renvert, S.; Hirooka, H.; Polyzois, I.; Kelekis-Cholakis, A.; Wang, H.L.; Working, G. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants—Consensus report of working group 3. Int. Dent. J. 2019, 69 (Suppl. S2), 12–17. [Google Scholar] [CrossRef]
- Sinjab, K.; Garaicoa-Pazmino, C.; Wang, H.L. Decision Making for Management of Periimplant Diseases. Implant Dent. 2018, 27, 276–281. [Google Scholar] [CrossRef]
- Okayasu, K.; Wang, H.L. Decision tree for the management of periimplant diseases. Implant Dent. 2011, 20, 256–261. [Google Scholar] [CrossRef]
- Prati, C.; Zamparini, F.; Canullo, L.; Pirani, C.; Botticelli, D.; Gandolfi, M.G. Factors Affecting Soft and Hard Tissues around Two-Piece Transmucosal Implants: A 3-Year Prospective Cohort Study. Int. J. Oral Maxillofac. Implant. 2020, 35, 1022–1036. [Google Scholar] [CrossRef]
- Cabanes-Gumbau, G.; Pascual-Moscardó, A.; Peñarrocha-Oltra, D.; García-Mira, B.; Aizcorbe-Vicente, J.; Peñarrocha-Diago, M.A. Volumetric variation of peri-implant soft tissues in convergent collar implants and crowns using the biologically oriented preparation technique (BOPT). Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e643–e651. [Google Scholar] [CrossRef]
- Serino, G.; Turri, A.; Lang, N.P. Maintenance therapy in patients following the surgical treatment of peri-implantitis: A 5-year follow-up study. Clin. Oral Implant. Res. 2015, 26, 950–956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).