Population and Co-Occurrence Characteristics of Diagnoses and Comorbidities in Coronary Artery Disease Patients: A Case Study from a Hospital in Guangxi, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Study Population
2.3. Data Normalization
2.4. Statistical Analysis
2.5. Network Analysis
3. Results
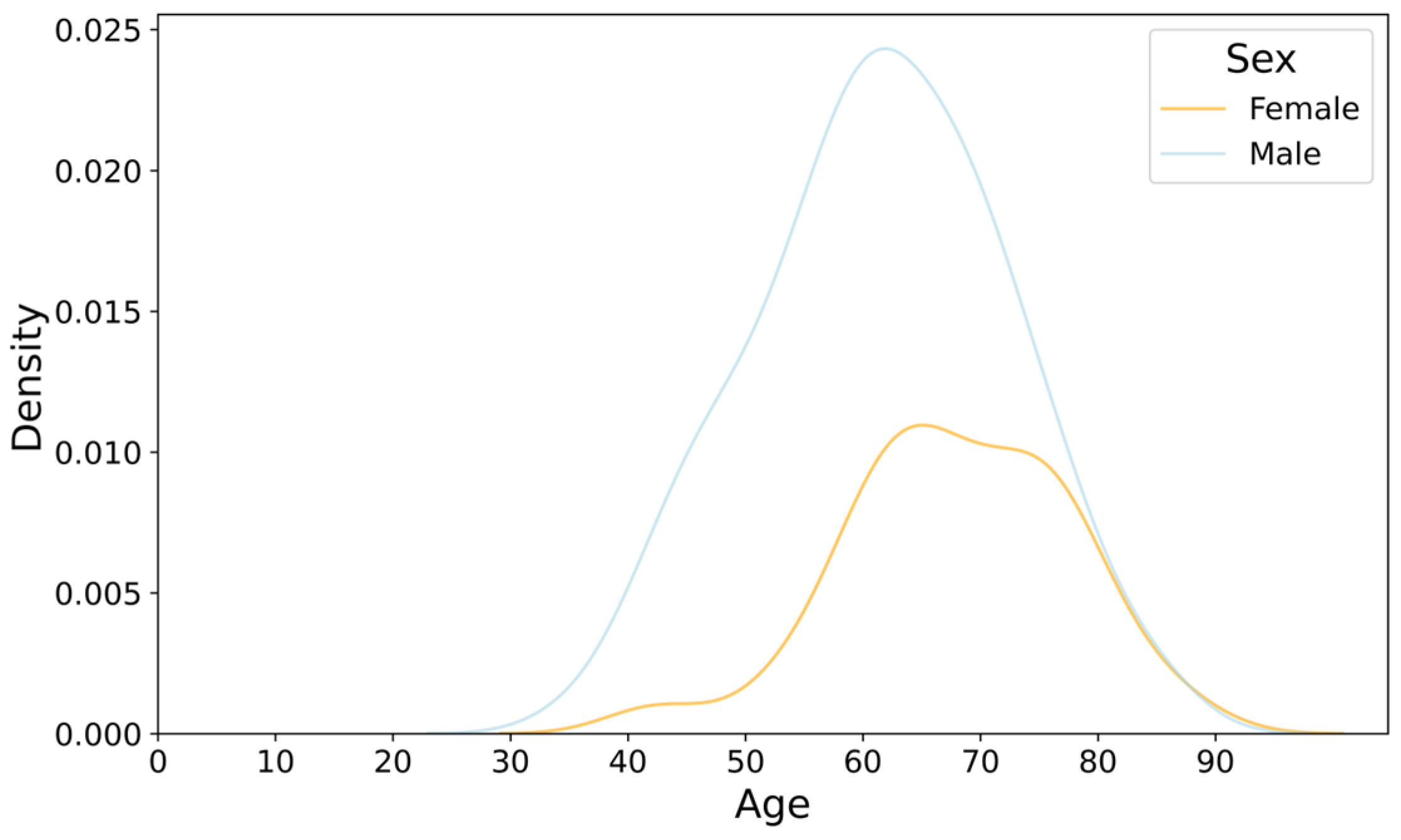
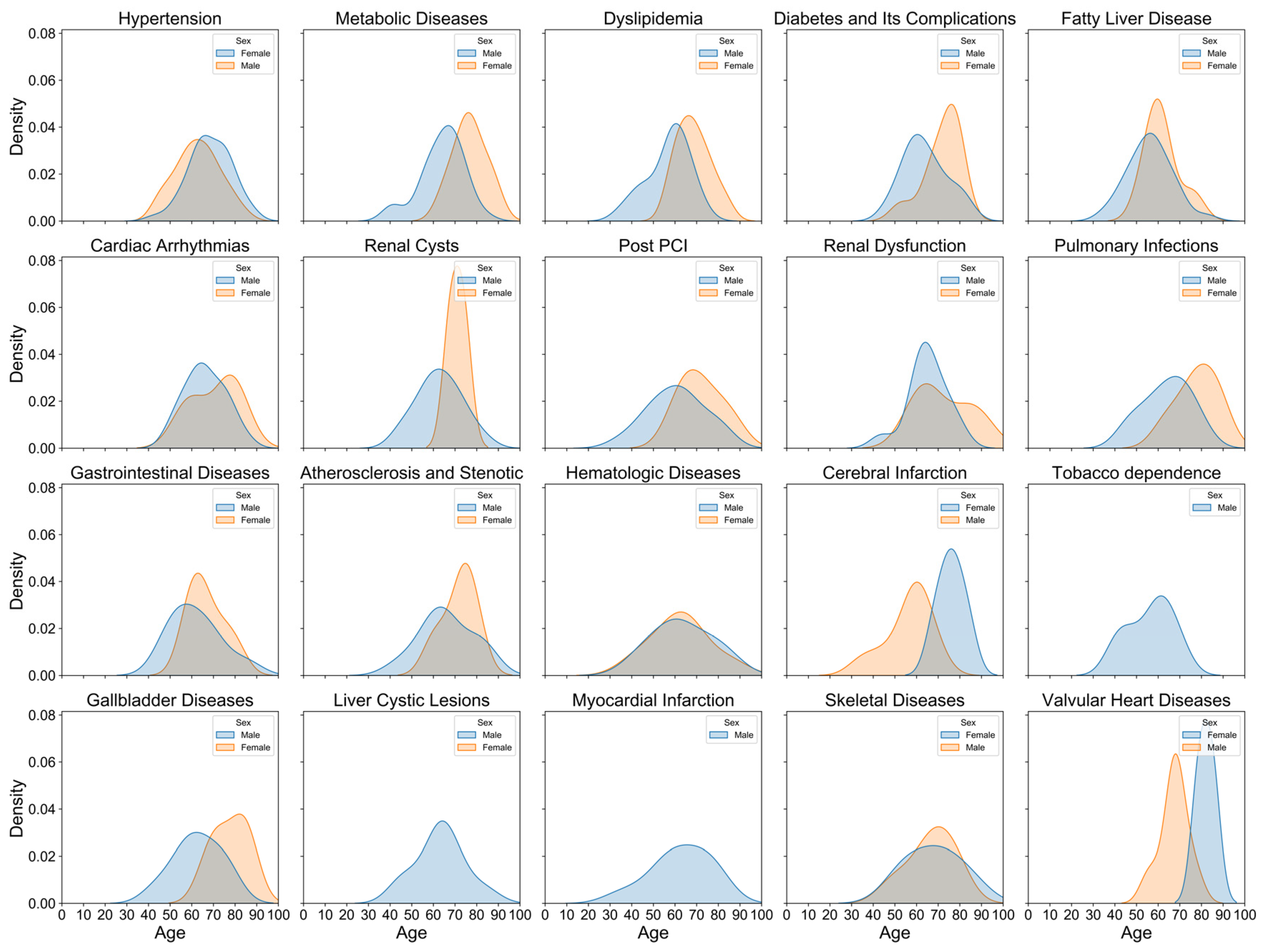
3.1. Age and Sex Distribution of CAD Patients
3.2. Detection Rates of Top Diagnoses and Comorbidities
3.3. Top Five Diagnoses and Comorbidities Across Demographics
3.3.1. Top Five Diagnoses of CAD Across Age Groups, Sexes, and Admission Years
3.3.2. Top Five Comorbidities of CAD Across Age Groups, Sexes, and Admission Years
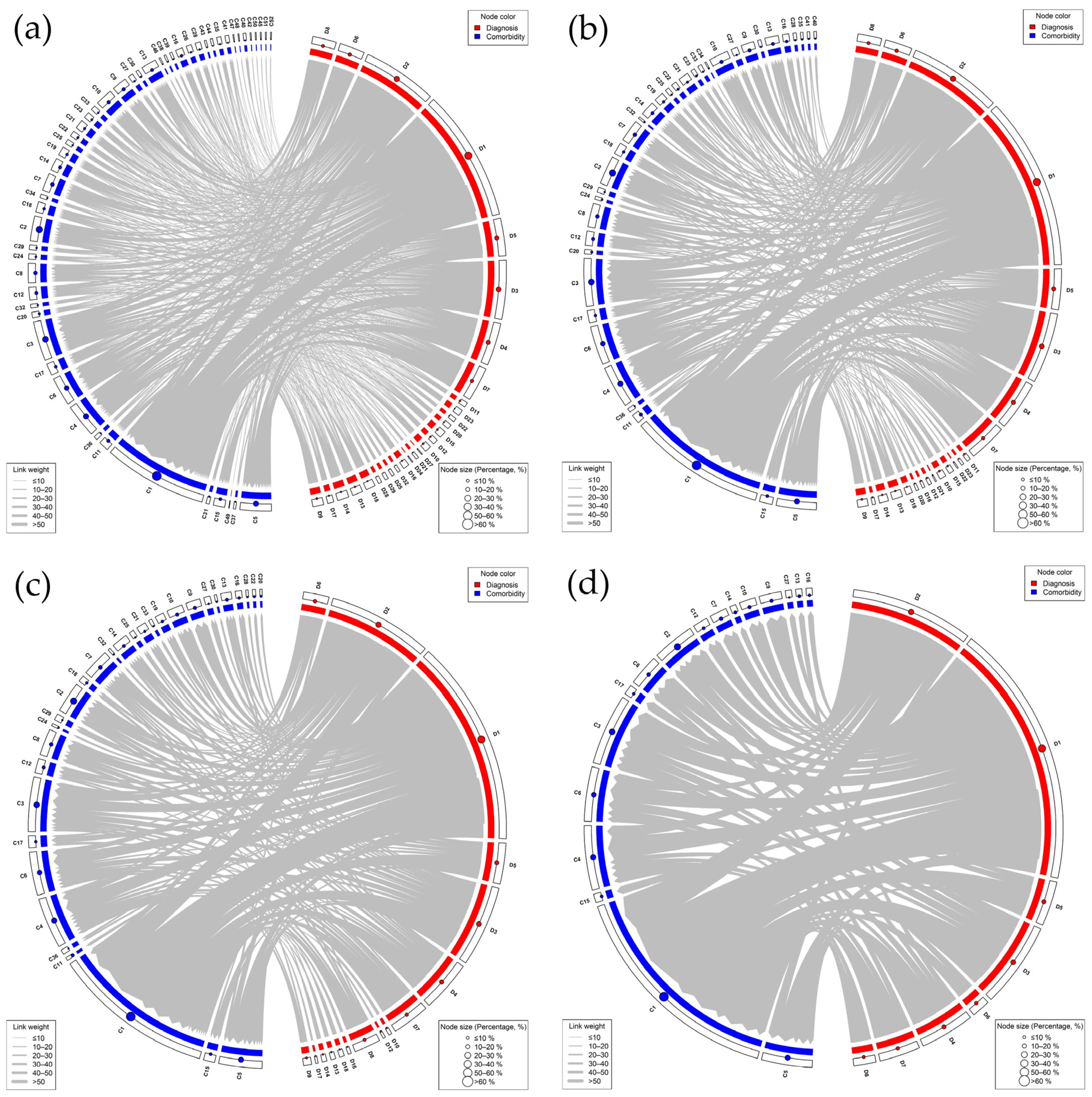
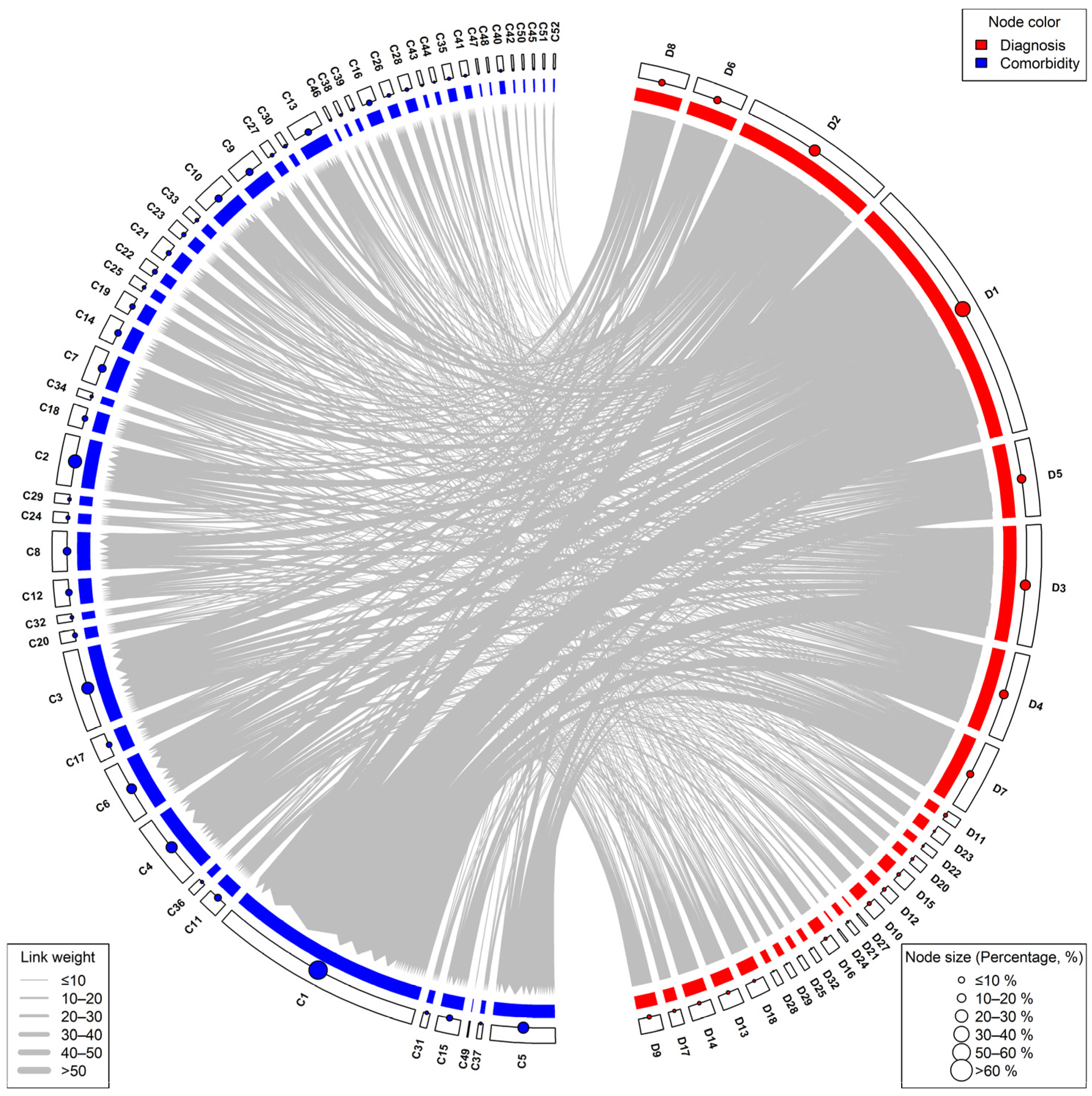
3.4. Co-Occurrence Frequency of Diagnoses and Comorbidities
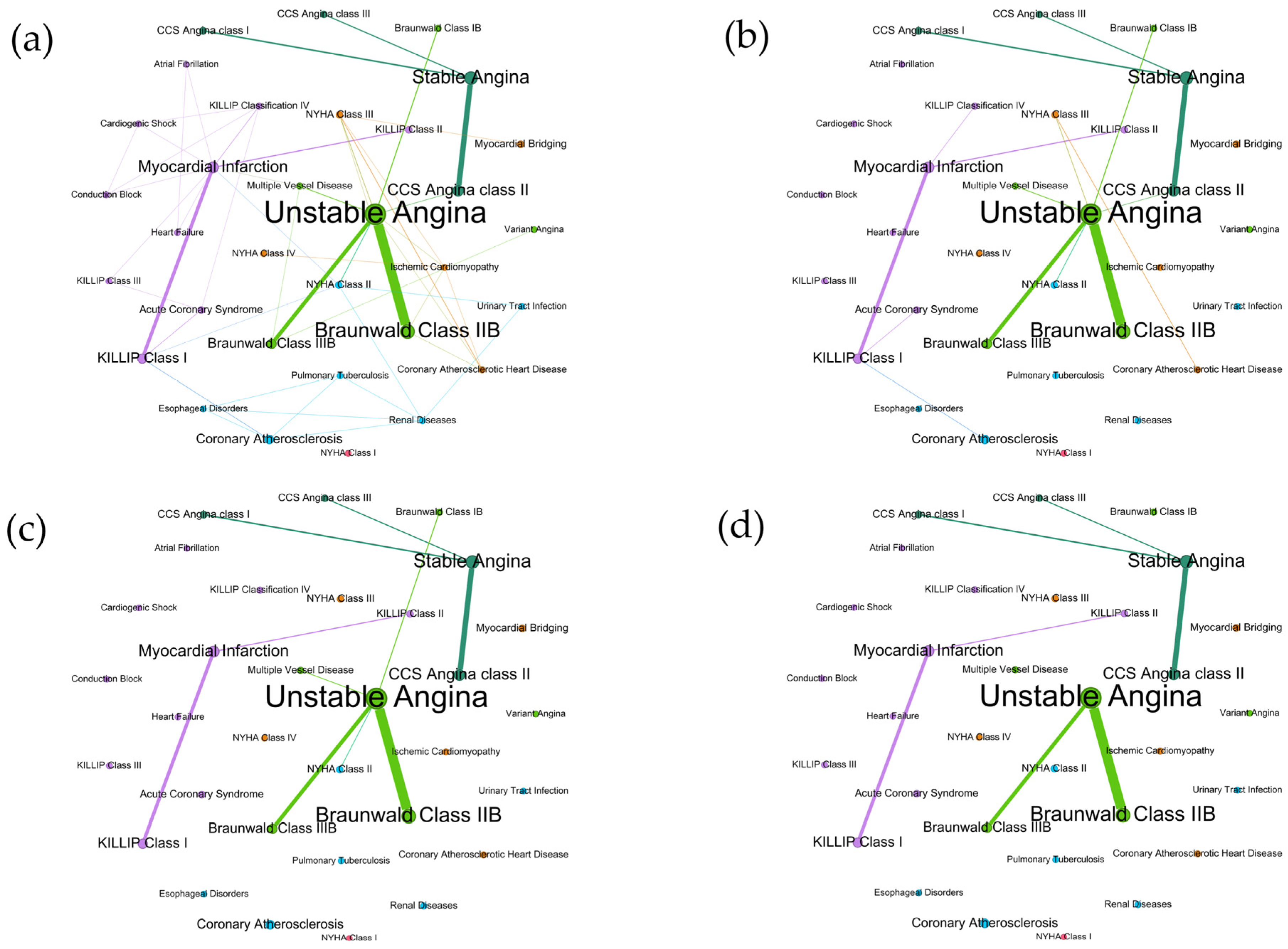
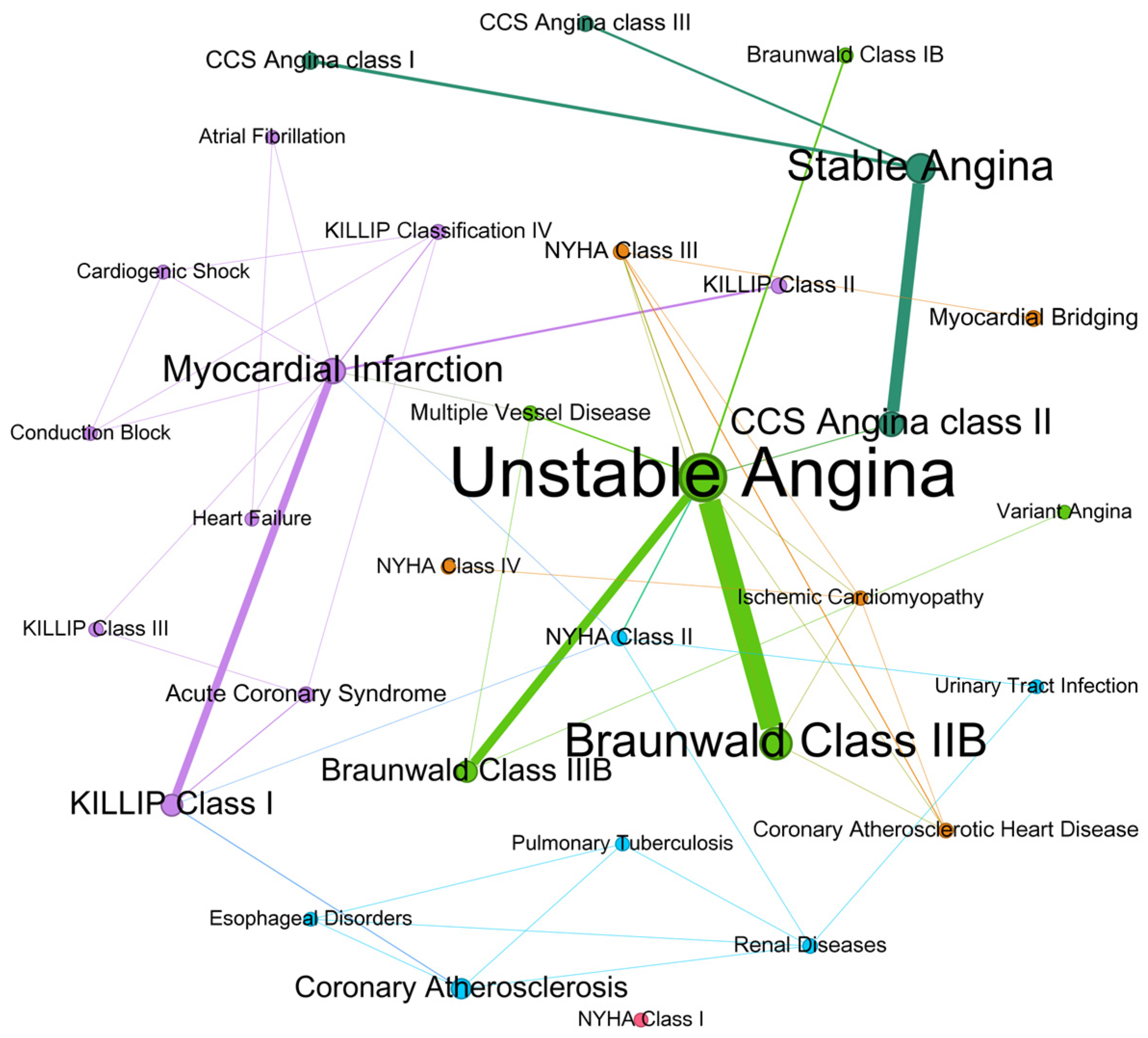
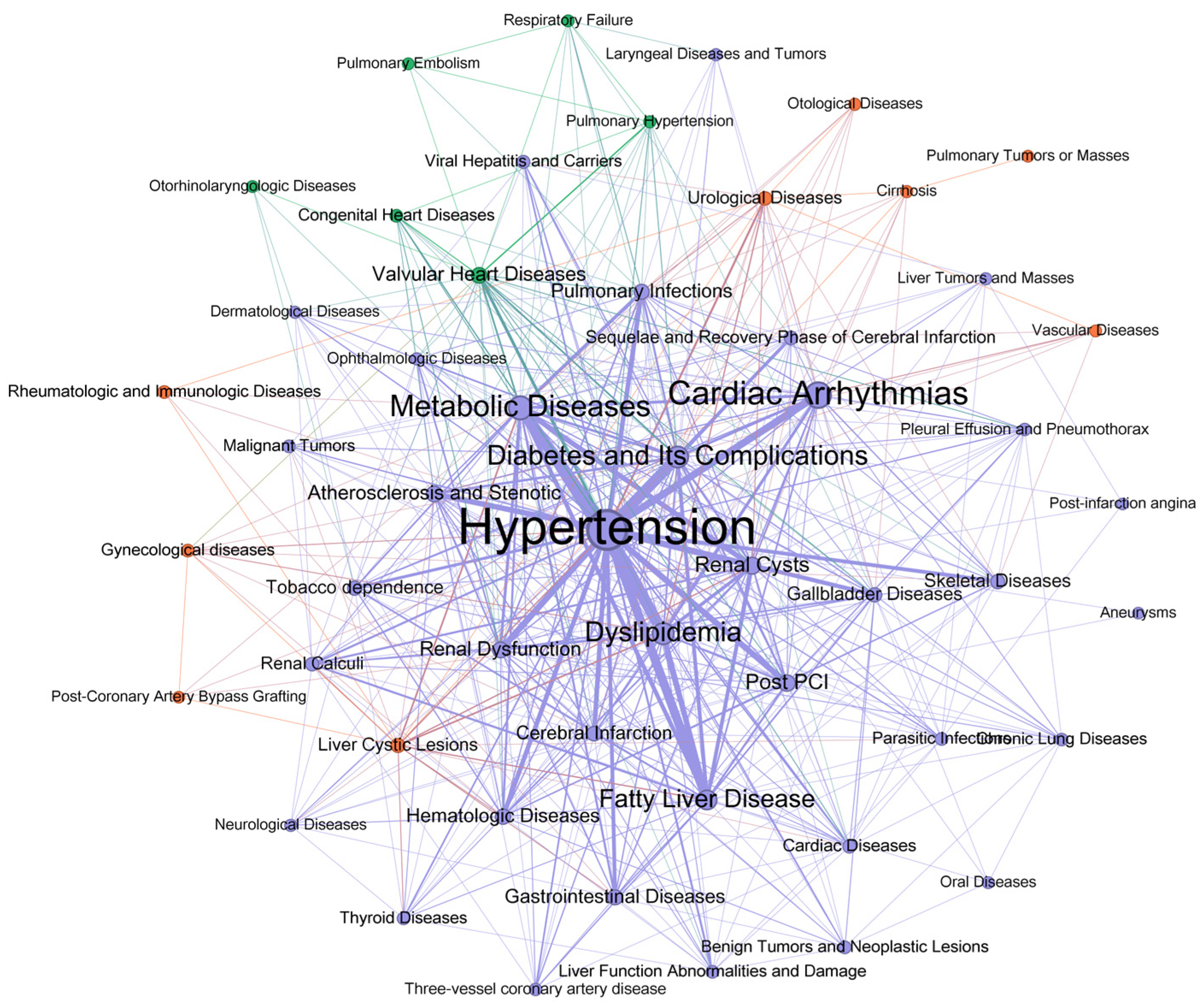
3.5. Network Analysis of Diagnoses and Comorbidities
3.6. Sensitivity Analysis of Co-Occurrence Networks
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Code of Node | Name of Diagnosis | Diagnosis Abbreviation | Frequency (Count) |
|---|---|---|---|
| D1 | Unstable Angina | UA | 82 |
| D2 | Braunwald Class II B | 45 | |
| D3 | Stable Angina | SA | 38 |
| D4 | Myocardial Infarction | MI | 28 |
| D5 | CCS Angina Class II | 27 | |
| D6 | KILLIP Class I | 20 | |
| D7 | Braunwald Class III B | 19 | |
| D8 | Coronary Atherosclerosis | CA | 16 |
| D9 | CCS Angina class I | 7 | |
| D10 | Myocardial Bridging | 6 | |
| D11 | Acute Coronary Syndrome | ACS | 6 |
| D12 | KILLIP Class II | 5 | |
| D13 | NYHA Class III | 5 | |
| D14 | CCS Angina class III | 5 | |
| D15 | NYHA Class II | 5 | |
| D16 | Multiple Vessel Disease | 4 | |
| D17 | Braunwald Class I B | 4 | |
| D18 | Coronary Atherosclerotic Heart Disease | 3 | |
| D19 | Renal Diseases | 3 | |
| D20 | KILLIP Classification IV | 3 | |
| D21 | KILLIP Class III | 2 | |
| D22 | NYHA Class IV | 2 | |
| D23 | Ischemic Cardiomyopathy | 2 | |
| D24 | Variant Angina | 1 | |
| D25 | Conduction Block | 1 | |
| D26 | Urinary Tract Infection | 1 | |
| D27 | NYHA Class I | 1 | |
| D28 | Heart Failure | 1 | |
| D29 | Atrial Fibrillation | 1 | |
| D30 | Esophageal Disorders | 1 | |
| D31 | Pulmonary Tuberculosis | 1 | |
| D32 | Cardiogenic Shock | 1 |
| Code of Node | Name of Comorbidity | Comorbidity Abbreviation | Frequency (Count) |
|---|---|---|---|
| C1 | Hypertension | 122 | |
| C2 | Cardiac Arrhythmias | Arrhythmias | 62 |
| C3 | Metabolic Diseases | 52 | |
| C4 | Dyslipidemia | 44 | |
| C5 | Diabetes and Its Complications | Diabetes | 43 |
| C6 | Fatty Liver Disease | 35 | |
| C7 | Renal Cysts | 23 | |
| C8 | Post-Percutaneous Coronary Intervention | Post PCI | 22 |
| C9 | Atherosclerosis and Stenotic | Atherosclerosis | 19 |
| C10 | Renal Dysfunction | 19 | |
| C11 | Valvular Heart Diseases | 18 | |
| C12 | Hematologic Diseases | 16 | |
| C13 | Pulmonary Infections | 16 | |
| C14 | Gastrointestinal Diseases | 16 | |
| C15 | Cerebral Infarction | CI | 14 |
| C16 | Skeletal Diseases | 12 | |
| C17 | Gallbladder Diseases | 12 | |
| C18 | Tobacco dependence | 12 | |
| C19 | Liver Cystic Lesions | 11 | |
| C20 | Renal Calculi | 9 | |
| C21 | Sequelae and Recovery Phase of Cerebral Infarction | 9 | |
| C22 | Urological Diseases | 9 | |
| C23 | Cardiac Diseases | 7 | |
| C24 | Viral Hepatitis and Carriers | 6 | |
| C25 | Parasitic Infections | 5 | |
| C26 | Congenital Heart Diseases | 5 | |
| C27 | Thyroid Diseases | 5 | |
| C28 | Liver Function Abnormalities and Damage | 5 | |
| C29 | Malignant Tumors | 5 | |
| C30 | Rheumatologic and Immunologic Diseases | 5 | |
| C31 | Gynecological diseases | 5 | |
| C32 | Benign Tumors and Neoplastic Lesions | 5 | |
| C33 | Chronic Lung Diseases | 5 | |
| C34 | Pleural Effusion and Pneumothorax | 4 | |
| C35 | Ophthalmologic Diseases | 4 | |
| C36 | Three-vessel coronary artery disease | 4 | |
| C37 | Cirrhosis | 3 | |
| C38 | Neurological Diseases | 3 | |
| C39 | Dermatological Diseases | 3 | |
| C40 | Liver Tumors and Masses | 3 | |
| C41 | Pulmonary Hypertension | 3 | |
| C42 | Oral Diseases | 2 | |
| C43 | Laryngeal Diseases and Tumors | 2 | |
| C44 | Respiratory Failure | 2 | |
| C45 | Otological Diseases | 2 | |
| C46 | Vascular Diseases | 2 | |
| C47 | Otorhinolaryngologic Diseases | 1 | |
| C48 | Post-Coronary Artery Bypass Grafting | 1 | |
| C49 | Pulmonary Tumors or Masses | 1 | |
| C50 | Post-infarction angina | 1 | |
| C51 | Aneurysms | 1 | |
| C52 | Pulmonary Embolism | 1 |
| Sensitivity Analysis | Diagnosis Co-Occurrence Network | Comorbidity Co-Occurrence Network | Diagnosis–Comorbidity Bipartite Network | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Network Topology Properties | a * | b * | c * | d * | a | b | c | d | a | b | c | d |
| Node | 32 | 32 | 32 | 32 | 52 | 52 | 52 | 52 | 84 | 84 | 84 | 84 |
| Edge | 50 | 16 | 10 | 7 | 467 | 200 | 110 | 45 | 423 | 191 | 118 | 52 |
| Average Degree | 3.13 | 1 | 0.63 | 0.44 | 17.96 | 7.69 | 4.23 | 1.73 | 5.04 | 2.27 | 1.41 | 0.62 |
| Average Weighted Degree | 11.06 | 8.94 | 8.19 | 7.56 | 42.77 | 32.5 | 25.58 | 17.12 | 13.51 | 10.75 | 9.01 | 6.27 |
| Density | 0.10 | 0.03 | 0.02 | 0.01 | 0.35 | 0.15 | 0.08 | 0.03 | 0.06 | 0.03 | 0.02 | 0.01 |
| Diameter | 6 | 5 | 2 | 2 | 4 | 3 | 3 | 2 | 1 | 1 | 1 | 1 |
| Average Path length | 3.20 | 2.42 | 1.58 | 1.41 | 1.70 | 1.81 | 1.86 | 1.8 | 1 | 1 | 1 | 1 |
| Modularity | 0.61 | 0.59 | 0.58 | 0.61 | 0.12 | 0.14 | 0.19 | 0.22 | 0.21 | 0.25 | 0.26 | 0.27 |
| Description Length | 183.25 | 142.33 | 129.62 | 121.48 | 999.69 | 775.10 | 575.55 | 296.71 | 1497.60 | 1113.54 | 629.56 | 500.24 |
| Average Clustering Coefficient | 0.54 | 0 | 0 | 0 | 0.74 | 0.75 | 0.76 | 0.76 | 0 | 0 | 0 | 0 |

References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E. Heart disease and stroke statistics—2023 update: A report from the american heart association. Circulation 2023, 147, e93–e621. [Google Scholar] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, Y.; Liu, L.; Liu, Y.; Liu, J. Trend, regional variation and socioeconomic inequality in cardiovascular disease among the elderly population in China: Evidence from a nationwide longitudinal study during 2011–2018. BMJ Glob. Health 2023, 8, e013311. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from ischemic heart disease: Analysis of data from the world health organization and coronary artery disease risk factors From NCD Risk Factor Collaboration. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef]
- Gouda, H.N.; Charlson, F.; Sorsdahl, K.; Ahmadzada, S.; Ferrari, A.J.; Erskine, H.; Leung, J.; Santamauro, D.; Lund, C.; Aminde, L.N. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: Results from the Global Burden of Disease Study 2017. Lancet Glob. Health 2019, 7, e1375–e1387. [Google Scholar] [CrossRef]
- Sharma, A.K.; DAR, M.I.; Iqbal, M.; Tramboo, N.A. Gender-based differences in coronary artery disease: A prospective observational study from a North Indian state. Heart India 2020, 8, 85–92. [Google Scholar]
- Elia, E.; Bruno, F.; Crimi, G.; Wańha, W.; Leonardi, S.; Mauro, M.; Roubin, S.R.; Fabris, E.; Giannino, G.; Mancone, M. Gender differences in the development of heart failure after acute coronary syndrome: Insight from the CORALYS registry. Int. J. Cardiol. 2024, 397, 131622. [Google Scholar] [CrossRef]
- Zilio, F.; Musella, F.; Ceriello, L.; Ciliberti, G.; Pavan, D.; Manes, M.T.; Selimi, A.; Scicchitano, P.; Iannopollo, G.; Albani, S.; et al. Sex differences in patients presenting with acute coronary syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102486. [Google Scholar] [CrossRef] [PubMed]
- Balagopalan, J.P.; Abdullakutty, J.; Sastry, S.L.; Nanjappa, V.; Vaidyanathan, P.R.; Chopra, V.K.; Sengupta, S.; Arimpur, G.Z.; Routray, S.; Ravindranath, K. Gender-based differences in the presentation and outcomes of acute decompensated heart failure patients in developing countries. J. Am. Coll. Cardiol. 2024, 83, 622. [Google Scholar] [CrossRef]
- Lakshmanan, S.; Frumento, P.; Dalmiani, S.; Giorgetti, A.; Gimelli, A. Gender differences in referral trends and clinical characteristics in the evaluation of stable coronary artery disease by myocardial perfusion scintigraphy: A 20-year analysis from a tertiary referral centre. Eur. Heart J.-Imaging Methods Pract. 2024, 2, qyae013. [Google Scholar] [CrossRef] [PubMed]
- Peerwani, G.; Hanif, B.; Rahim, K.A.; Kashif, M.; Virani, S.S.; Sheikh, S. Presentation, management, and early outcomes of young acute coronary syndrome patients-analysis of 23,560 South Asian patients from 2012 to 2021. BMC Cardiovasc. Disord. 2024, 24, 378. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Chen, A.; Wu, Y.-l.; Lee, M.-S. Gender and age differences in the evaluation and clinical outcomes of patients with palpitations. J. Gen. Intern. Med. 2024, 39, 3035–3041. [Google Scholar] [CrossRef]
- Bao, Y.; Lu, P.; Wang, M.; Zhang, X.; Song, A.; Gu, X.; Ma, T.; Su, S.; Wang, L.; Shang, X. Exploring multimorbidity profiles in middle-aged inpatients: A network-based comparative study of China and the United Kingdom. BMC Med. 2023, 21, 495. [Google Scholar] [CrossRef]
- Uddin, S.; Wang, S.; Lu, H.; Khan, A.; Hajati, F.; Khushi, M. Comorbidity and multimorbidity prediction of major chronic diseases using machine learning and network analytics. Expert Syst. Appl. 2022, 205, 117761. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, H.; Luo, L.; Zhou, L. Age-and sex-specific differences in multimorbidity patterns and temporal trends on assessing hospital discharge records in Southwest China: Network-based study. J. Med. Internet Res. 2022, 24, e27146. [Google Scholar] [CrossRef]
- Maas, A.H.; Appelman, Y.E. Gender differences in coronary heart disease. Neth. Heart J. 2010, 18, 598–603. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Murray, C.J.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdollahi, M.; Abedi, P.; Abedi, A.; Abolhassani, H. Five insights from the global burden of disease study 2019. Lancet 2020, 396, 1135–1159. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.E.; Uddin, S.; Khan, A. Network analytics and machine learning for predictive risk modelling of cardiovascular disease in patients with type 2 diabetes. Expert Syst. Appl. 2021, 164, 113918. [Google Scholar] [CrossRef]
- Cruz-Ávila, H.A.; Vallejo, M.; Martínez-García, M.; Hernández-Lemus, E. Comorbidity networks in cardiovascular diseases. Front. Physiol. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Barabási, A.-L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Lusis, A.J.; Weiss, J.N. Cardiovascular networks: Systems-based approaches to cardiovascular disease. Circulation 2010, 121, 157–170. [Google Scholar] [CrossRef]
- Monchka, B.A.; Leung, C.K.; Nickel, N.C.; Lix, L.M. The effect of disease co-occurrence measurement on multimorbidity networks: A population-based study. BMC Med. Res. Methodol. 2022, 22, 165. [Google Scholar] [CrossRef]
- Prados-Torres, A.; Poblador-Plou, B.; Calderón-Larrañaga, A.; Gimeno-Feliu, L.A.; González-Rubio, F.; Poncel-Falcó, A.; Sicras-Mainar, A.; Alcalá-Nalvaiz, J.T. Multimorbidity patterns in primary care: Interactions among chronic diseases using factor analysis. PLoS ONE 2012, 7, e32190. [Google Scholar] [CrossRef]
- Halu, A.; De Domenico, M.; Arenas, A.; Sharma, A. The multiplex network of human diseases. NPJ Syst. Biol. Appl. 2019, 5, 15. [Google Scholar] [CrossRef]
- Infante, T.; Del Viscovo, L.; De Rimini, M.L.; Padula, S.; Caso, P.; Napoli, C. Network medicine: A clinical approach for precision medicine and personalized therapy in coronary heart disease. J. Atheroscler. Thromb. 2020, 27, 279–302. [Google Scholar] [CrossRef]
- Deng, P.; Fu, Y.; Chen, M.; Wang, D.; Si, L. Temporal trends in inequalities of the burden of cardiovascular disease across 186 countries and territories. Int. J. Equity Health 2023, 22, 164. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Liu, Y.; Ye, P.; Xu, C.; Yin, P.; Liu, J.; Qi, J.; You, J.; Lin, L. Spatiotemporal trends and ecological determinants of cardiovascular mortality among 2844 counties in mainland China, 2006–2020: A Bayesian modeling study of national mortality registries. BMC Med. 2022, 20, 467. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, G.-y.; Jing, W.-z.; Liu, J.; Liu, M. Global trends and regional differences in incidence and mortality of cardiovascular disease, 1990−2019: Findings from 2019 global burden of disease study. Eur. J. Prev. Cardiol. 2023, 30, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Ye, P.; Liu, J.; Yin, P.; Qi, J.; You, J.; Lin, L.; Wang, F.; Wang, L. Trends and associated factors in place of death among individuals with cardiovascular disease in China, 2008–2020: A population-based study. Lancet Reg. Health–West. Pac. 2022, 21, 100383. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american college of physicians, american association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J. Am. Coll. Cardiol. 2012, 60, e44–e164. [Google Scholar] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 61, e78–e140. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Wang, J.; Zeng, D.D.; Song, H.; Wang, L.; Cao, Z. Comorbidity analysis according to sex and age in hypertension patients in China. Int. J. Med. Sci. 2016, 13, 99. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, P.; Wang, L. DCNeT: A disease comorbidity network-based temporal deep learning framework to predict cardiovascular risk in patients with mental disorders. Expert Syst. Appl. 2024, 254, 124312. [Google Scholar] [CrossRef]
- Cramer, A.O.; Waldorp, L.J.; Van Der Maas, H.L.; Borsboom, D. Comorbidity: A network perspective. Behav. Brain Sci. 2010, 33, 137–150. [Google Scholar] [CrossRef]
- Neal, Z.P.; Cadieux, A.; Garlaschelli, D.; Gotelli, N.J.; Saracco, F.; Squartini, T.; Shutters, S.T.; Ulrich, W.; Wang, G.; Strona, G. Pattern detection in bipartite networks: A review of terminology, applications, and methods. PLoS Complex Syst. 2024, 1, e0000010. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Mehra, A.; Brass, D.J.; Labianca, G. Network analysis in the social sciences. Science 2009, 323, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Brandes, U. A faster algorithm for betweenness centrality. J. Math. Sociol. 2001, 25, 163–177. [Google Scholar] [CrossRef]
- Latapy, M. Main-memory triangle computations for very large (sparse (power-law)) graphs. Theor. Comput. Sci. 2008, 407, 458–473. [Google Scholar] [CrossRef]
- Blondel, V.D.; Guillaume, J.-L.; Lambiotte, R.; Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, 2008, P10008. [Google Scholar] [CrossRef]
- Zhang, L.; Peixoto, T.P. Statistical inference of assortative community structures. Phys. Rev. Res. 2020, 2, 043271. [Google Scholar] [CrossRef]
- Peixoto, T.P. Bayesian stochastic blockmodeling. In Advances in Network Clustering and Blockmodeling; Doreian, P., Batagelj, V., Ferligoj, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 289–332. [Google Scholar] [CrossRef]
- Opsahl, T.; Agneessens, F.; Skvoretz, J. Node centrality in weighted networks: Generalizing degree and shortest paths. Soc. Netw. 2010, 32, 245–251. [Google Scholar] [CrossRef]
- Fruchterman, T.M.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Web and Social Media, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Gebhard, C. Gender medicine: Effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef]
- Pilote, L.; Dasgupta, K.; Guru, V.; Humphries, K.H.; McGrath, J.; Norris, C.; Rabi, D.; Tremblay, J.; Alamian, A.; Barnett, T. A comprehensive view of sex-specific issues related to cardiovascular disease. CMAJ 2007, 176, S1–S44. [Google Scholar] [CrossRef] [PubMed]
- Appelman, Y.; van Rijn, B.B.; Ten Haaf, M.E.; Boersma, E.; Peters, S.A. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015, 241, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, L.; Figtree, G.A.; Schutte, A.E.; Patel, S.; Woodward, M.; Arnott, C. Cardiovascular disease in women: From pathophysiology to novel and emerging risk factors. Heart Lung Circ. 2021, 30, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Lu, H.; Hatfield, L.A.; Al-Azazi, S.; Bakx, P.; Banerjee, A.; Burrack, N.; Chen, Y.-C.; Fu, C.; Gordon, M.; Heine, R. Sex-Based Disparities in Acute Myocardial Infarction Treatment Patterns and Outcomes in Older Adults Hospitalized Across 6 High-Income Countries: An Analysis From the International Health Systems Research Collaborative. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e010144. [Google Scholar] [CrossRef]
- Kuehnemund, L.; Koeppe, J.; Feld, J.; Wiederhold, A.; Illner, J.; Makowski, L.; Gerß, J.; Reinecke, H.; Freisinger, E. Gender differences in acute myocardial infarction—A nationwide German real-life analysis from 2014 to 2017. Clin. Cardiol. 2021, 44, 890–898. [Google Scholar] [CrossRef]
- Bucholz, E.M.; Butala, N.M.; Rathore, S.S.; Dreyer, R.P.; Lansky, A.J.; Krumholz, H.M. Sex differences in long-term mortality after myocardial infarction: A systematic review. Circulation 2014, 130, 757–767. [Google Scholar] [CrossRef]
- Chen, W.; Woods, S.L.; Puntillo, K.A. Gender differences in symptoms associated with acute myocardial infarction: A review of the research. Heart Lung 2005, 34, 240–247. [Google Scholar] [CrossRef]
- Hung, M.-J. Diabetes, Hypertension and Cardiovascular Disease: Clinical Insights, Mechanisms and Pharmacotherapies. Medicina 2024, 60, 566. [Google Scholar] [CrossRef]
- Kraja, A.T.; Hunt, S.C.; Rao, D.; Dávila-Román, V.G.; Arnett, D.K.; Province, M.A. Genetics of hypertension and cardiovascular disease and their interconnected pathways: Lessons from large studies. Curr. Hypertens. Rep. 2011, 13, 46–54. [Google Scholar] [CrossRef]
- Hosseinpour-Niazi, S.; Afaghi, S.; Hadaegh, P.; Mahdavi, M.; Farhadnejad, H.; Tohidi, M.; Mirmiran, P.; Azizi, F.; Hadaegh, F. The association between metabolic syndrome and insulin resistance with risk of cardiovascular events in different states of cardiovascular health status. J. Diabetes Investig. 2024, 15, 208–218. [Google Scholar] [CrossRef]

| Diagnosis | Count (%) | Male (%) | Female (%) | OR (95% CI) |
|---|---|---|---|---|
| Unstable Angina | 82 (42.05) | 55 (39.86) | 27 (47.37) | 0.74 (0.40, 1.37) |
| Braunwald Class II B | 45 (23.08) | 32 (23.19) | 13 (22.81) | 1.01 (0.49, 2.08) |
| Stable Angina | 38 (19.49) | 30 (21.74) | 8 (14.04) | 1.64 (0.71, 3.76) |
| Myocardial Infarction | 28 (14.36) | 23 (16.67) | 5 (8.77) | 1.94 (0.73, 5.20) |
| CCS Angina class II | 27 (13.85) | 20 (14.49) | 7 (12.28) | 1.16 (0.47, 2.86) |
| KILLIP Class I | 20 (10.26) | 20 (14.49) | 0 (0.00) | INF * |
| Braunwald Class III B | 19 (9.74) | 13 (9.42) | 6 (10.53) | 0.85 (0.32, 2.29) |
| Coronary Atherosclerosis | 16 (8.21) | 9 (6.52) | 7 (12.28) | 0.49 (0.18, 1.36) |
| CCS Angina class I | 7 (3.59) | 7 (5.07) | 0 (0.00) | INF * |
| Post-Percutaneous Coronary Intervention | 6 (3.08) | 3 (2.17) | 3 (5.26) | 0.40 (0.09, 1.83) |
| Acute Coronary Syndrome | 6 (3.08) | 5 (3.62) | 1 (1.75) | 1.55 (0.25, 9.69) |
| Myocardial Bridging | 6 (3.08) | 3 (2.17) | 3 (5.26) | 0.40 (0.09, 1.83) |
| NYHA Class III | 5 (2.56) | 3 (2.17) | 2 (3.51) | 0.57 (0.11, 2.99) |
| NYHA Class II | 5 (2.56) | 3 (2.17) | 2 (3.51) | 0.57 (0.11, 2.99) |
| KILLIP Class II | 5 (2.56) | 4 (2.90) | 1 (1.75) | 1.26 (0.19, 8.21) |
| CCS Angina class III | 5 (2.56) | 4 (2.90) | 1 (1.75) | 1.26 (0.19, 8.21) |
| Multiple Vessel Disease | 4 (2.05) | 2 (1.45) | 2 (3.51) | 0.41 (0.07, 2.41) |
| Braunwald Class I B | 4 (2.05) | 3 (2.17) | 1 (1.75) | 0.97 (0.14, 6.76) |
| KILLIP Classification IV | 3 (1.54) | 1 (0.72) | 2 (3.51) | 0.24 (0.03, 1.88) |
| Coronary Atherosclerotic Heart Disease | 3 (1.54) | 3 (2.17) | 0 (0.00) | INF * |
| Comorbidities | Count (%) | Male (%) | Female (%) | OR (95% CI) |
|---|---|---|---|---|
| Hypertension | 120 (61.54) | 81 (58.70) | 39 (68.42) | 0.66 (0.35, 1.27) |
| Metabolic Diseases | 43 (22.05) | 35 (25.36) | 8 (14.04) | 2.00 (0.88, 4.54) |
| Dyslipidemia | 41 (21.03) | 23 (16.67) | 18 (31.58) | 0.43 (0.21, 0.88) |
| Diabetes and Its Complications | 38 (19.49) | 28 (20.29) | 10 (17.54) | 1.17 (0.53, 2.56) |
| Fatty Liver Disease | 35 (17.95) | 29 (21.01) | 6 (10.53) | 2.13 (0.86, 5.31) |
| Cardiac Arrhythmias | 28 (14.36) | 23 (16.67) | 5 (8.77) | 1.94 (0.73, 5.20) |
| Renal Cysts | 23 (11.79) | 21 (15.22) | 2 (3.51) | 4.06 (1.06, 15.64) |
| Post PCI | 22 (11.28) | 18 (13.04) | 4 (7.02) | 1.83 (0.62, 5.37) |
| Renal Dysfunction | 18 (9.23) | 13 (9.42) | 5 (8.77) | 1.03 (0.36, 2.91) |
| Pulmonary Infections | 16 (8.21) | 11 (7.97) | 5 (8.77) | 0.86 (0.30, 2.50) |
| Hematologic Diseases | 15 (7.69) | 8 (5.80) | 7 (12.28) | 0.44 (0.16, 1.23) |
| Gastrointestinal Diseases | 15 (7.69) | 9 (6.52) | 6 (10.53) | 0.58 (0.20, 1.66) |
| Atherosclerosis and Stenotic | 15 (7.69) | 11 (7.97) | 4 (7.02) | 1.07 (0.34, 3.34) |
| Cerebral Infarction | 14 (7.18) | 9 (6.52) | 5 (8.77) | 0.70 (0.23, 2.10) |
| Gallbladder Diseases | 12 (6.15) | 8 (5.80) | 4 (7.02) | 0.77 (0.24, 2.54) |
| Tobacco dependence | 12 (6.15) | 12 (8.70) | 0 (0.00) | INF * |
| Myocardial Infarction | 11 (5.64) | 11 (7.97) | 0 (0.00) | INF * |
| Liver Cystic Lesions | 11 (5.64) | 10 (7.25) | 1 (1.75) | 3.08 (0.54, 17.52) |
| Skeletal Diseases | 10 (5.13) | 5 (3.62) | 5 (8.77) | 0.39 (0.12, 1.34) |
| Valvular Heart Diseases | 9 (4.62) | 7 (5.07) | 2 (3.51) | 1.27 (0.29, 5.48) |
| Group * | Top1 | Top2 | Top3 | Top4 | Top5 | |
|---|---|---|---|---|---|---|
| Age | 30–39 | UA | Braunwald Class II B | Braunwald Class II B | / | / |
| 40–49 | MI | KILLIP Class I | SA | Braunwald Class III B | CCS Angina Class II | |
| 50–59 | UA | Braunwald Class II B | MI | KILLIP Class I | CA | |
| 60–69 | UA | Braunwald Class II B | SA | CCS Angina Class II | Braunwald Class III B | |
| 70–79 | UA | SA | Braunwald Class II B | CCS Angina Class II | CA | |
| 80–89 | UA | SA | CCS Angina Class II | Braunwald Class II B | Braunwald Class III B | |
| Sex | Male | UA | Braunwald Class II B | SA | MI | CCS Angina Class II |
| Female | UA | Braunwald Class II B | SA | CCS Angina Class II | CA | |
| Year | 2013 | UA | Braunwald Class II B | MI | SA | CA |
| 2014 | SA | CCS Angina Class II | MI | UA | KILLIP Class I | |
| 2015 | UA | Braunwald Class II B | CCS Angina Class II | CA | Braunwald Class I B | |
| 2016 | UA | Braunwald Class II B | CCS Angina Class II | SA | ACS | |
| 2017 | SA | CCS Angina Class II | UA | MI | Braunwald Class II B | |
| 2018 | UA | Braunwald Class II B | SA | Braunwald Class III B | CA | |
| 2019 | UA | Braunwald Class II B | Braunwald Class III B | CA | SA | |
| 2020 | UA | Braunwald Class III B | MI | Braunwald Class II B | KILLIP Class I | |
| Group * | Top1 | Top2 | Top3 | Top4 | Top5 | |
|---|---|---|---|---|---|---|
| Age | 30–39 | CI | Dyslipidemia | Fatty Liver Disease | Atherosclerosis | Post PCI |
| 40–49 | Hypertension | Fatty Liver Disease | Dyslipidemia | Metabolic Diseases | Post PCI | |
| 50–59 | Hypertension | Arrhythmias | Fatty Liver Disease | Dyslipidemia | Metabolic Diseases | |
| 60–69 | Hypertension | Arrhythmias | Dyslipidemia | Metabolic Diseases | Diabetes | |
| 70–79 | Hypertension | Arrhythmias | Metabolic Diseases | Diabetes | Dyslipidemia | |
| 80–89 | Hypertension | Arrhythmias | Diabetes | Atherosclerosis | Metabolic Diseases | |
| Sex | Male | Hypertension | Arrhythmias | Metabolic Diseases | Diabetes | Fatty Liver Disease |
| Female | Hypertension | Dyslipidemia | Arrhythmias | Diabetes | Metabolic Diseases | |
| Year | 2013 | Hypertension | Diabetes | Atherosclerosis | Dyslipidemia | Post PCI |
| 2014 | Hypertension | Arrhythmias | Diabetes | Fatty Liver Disease | Metabolic Diseases | |
| 2015 | Hypertension | Diabetes | Atherosclerosis | Post PCI | Pulmonary Infections | |
| 2016 | Arrhythmias | Hypertension | Diabetes | Dyslipidemia | Metabolic Diseases | |
| 2017 | Hypertension | Arrhythmias | Atherosclerosis | CI | Diabetes | |
| 2018 | Hypertension | Metabolic Diseases | Dyslipidemia | Fatty Liver Disease | Arrhythmias | |
| 2019 | Hypertension | Dyslipidemia | Arrhythmias | Diabetes | Metabolic Diseases | |
| 2020 | Hypertension | Metabolic Diseases | Arrhythmias | Dyslipidemia | Fatty Liver Disease | |
| Diagnosis | Comorbidity | Co-Occurrence Frequency |
|---|---|---|
| Unstable Angina | Hypertension | 59 |
| Braunwald Class II B | Hypertension | 31 |
| Stable Angina | Hypertension | 24 |
| Unstable Angina | Dyslipidemia | 20 |
| CCS Angina class II | Hypertension | 20 |
| Unstable Angina | Metabolic Diseases | 17 |
| Myocardial Infarction | Hypertension | 17 |
| Unstable Angina | Fatty Liver Disease | 16 |
| Unstable Angina | Diabetes and Its Complications | 14 |
| Braunwald Class III B | Hypertension | 14 |
| Braunwald Class II B | Metabolic Diseases | 12 |
| Unstable Angina | Post PCI | 12 |
| Unstable Angina | Cardiac Arrhythmias | 11 |
| Braunwald Class II B | Dyslipidemia | 10 |
| Unstable Angina | Atherosclerosis and Stenotic | 10 |
| Diagnosis | Number of Associated Comorbidities | Diagnosis | Number of Associated Comorbidities |
|---|---|---|---|
| Unstable Angina | 45 | CCS Angina class III | 16 |
| Braunwald Class II B | 40 | NYHA Class III | 15 |
| Stable Angina | 36 | Coronary Atherosclerotic Heart Disease | 14 |
| Myocardial Infarction | 32 | CCS Angina class I | 13 |
| CCS Angina class II | 31 | Ischemic Cardiomyopathy | 11 |
| Braunwald Class III B | 28 | NYHA Class II | 11 |
| KILLIP Class I | 23 | Braunwald Class I B | 10 |
| Coronary Atherosclerosis | 22 | Multiple Vessel Disease | 10 |
| Comorbidity | Number of Associated Diagnoses | Comorbidity | Number of Associated Diagnoses |
|---|---|---|---|
| Hypertension | 27 | Renal Cysts | 12 |
| Metabolic Diseases | 21 | Hematologic Diseases | 12 |
| Diabetes and Its Complications | 19 | Fatty Liver Disease | 11 |
| Pulmonary Infections | 19 | Cerebral Infarction | 11 |
| Cardiac Arrhythmias | 17 | Gallbladder Diseases | 11 |
| Renal Dysfunction | 16 | Tobacco dependence | 11 |
| Dyslipidemia | 15 | Congenital Heart Diseases | 11 |
| Atherosclerosis and Stenotic | 14 | Gastrointestinal Diseases | 10 |
| Valvular Heart Diseases | 13 | Cardiac Diseases | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qi, Z.; Liu, X.; Li, X.; Cao, Z.; Zeng, D.D.; Wang, H. Population and Co-Occurrence Characteristics of Diagnoses and Comorbidities in Coronary Artery Disease Patients: A Case Study from a Hospital in Guangxi, China. Bioengineering 2024, 11, 1284. https://doi.org/10.3390/bioengineering11121284
Wang J, Qi Z, Liu X, Li X, Cao Z, Zeng DD, Wang H. Population and Co-Occurrence Characteristics of Diagnoses and Comorbidities in Coronary Artery Disease Patients: A Case Study from a Hospital in Guangxi, China. Bioengineering. 2024; 11(12):1284. https://doi.org/10.3390/bioengineering11121284
Chicago/Turabian StyleWang, Jiaojiao, Zhixuan Qi, Xiliang Liu, Xin Li, Zhidong Cao, Daniel Dajun Zeng, and Hong Wang. 2024. "Population and Co-Occurrence Characteristics of Diagnoses and Comorbidities in Coronary Artery Disease Patients: A Case Study from a Hospital in Guangxi, China" Bioengineering 11, no. 12: 1284. https://doi.org/10.3390/bioengineering11121284
APA StyleWang, J., Qi, Z., Liu, X., Li, X., Cao, Z., Zeng, D. D., & Wang, H. (2024). Population and Co-Occurrence Characteristics of Diagnoses and Comorbidities in Coronary Artery Disease Patients: A Case Study from a Hospital in Guangxi, China. Bioengineering, 11(12), 1284. https://doi.org/10.3390/bioengineering11121284









