Comparison of Chondrocyte Behaviors Between Silk Microfibers and Polycaprolactone Microfibers in Tissue Engineering and Regenerative Medicine Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Microfiber Preparation
2.2. Culture and Seeding of Cells
2.3. Cell Growth Assay
2.4. Western Blotting
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Dimethyl Methylene Blue (DMMB) Assay
2.7. Statistical Analysis
3. Results
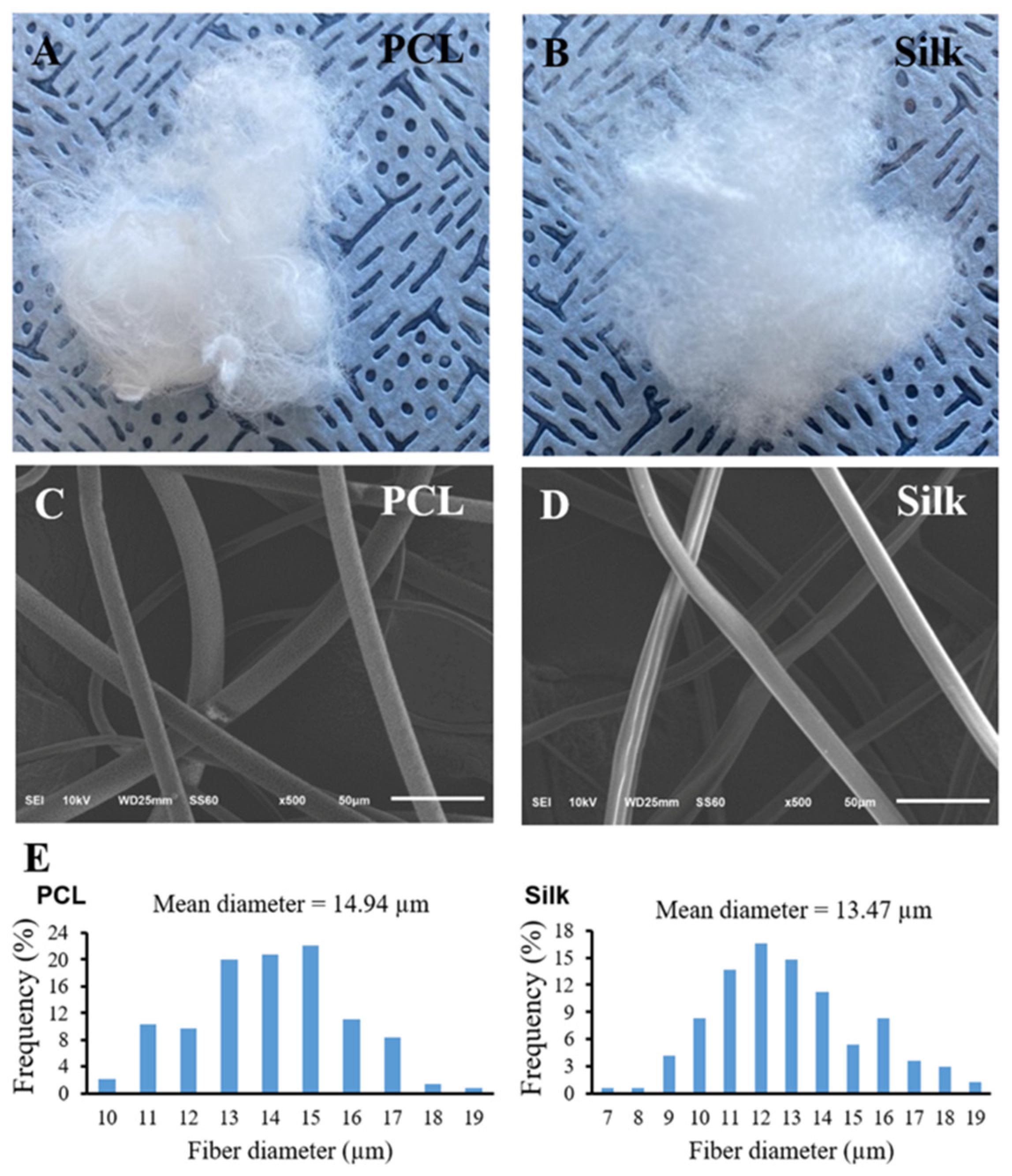
3.1. Morphology and Characteristic of Both Microfibers
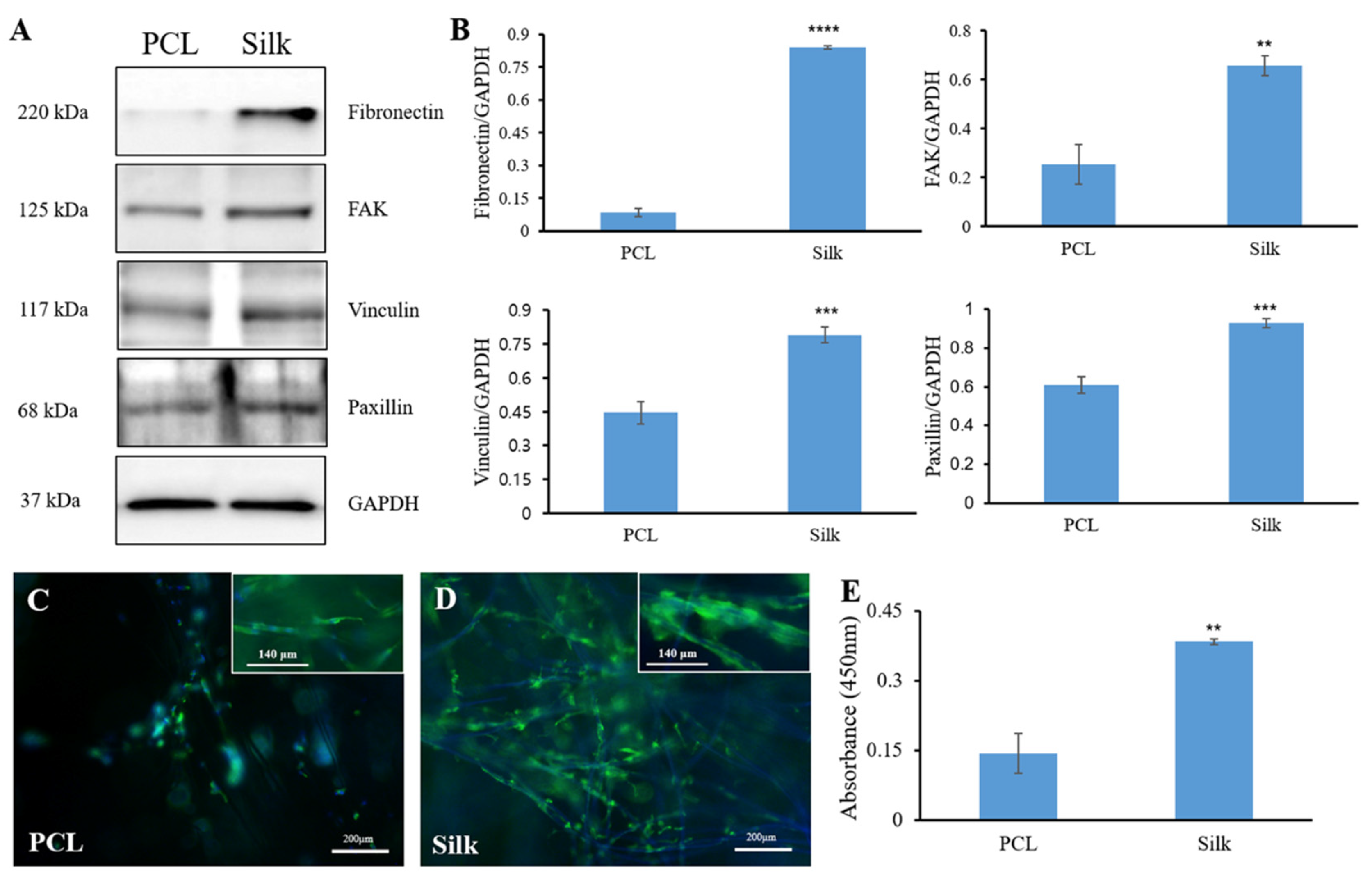
3.2. Cell Adhesion-Related Protein Expression
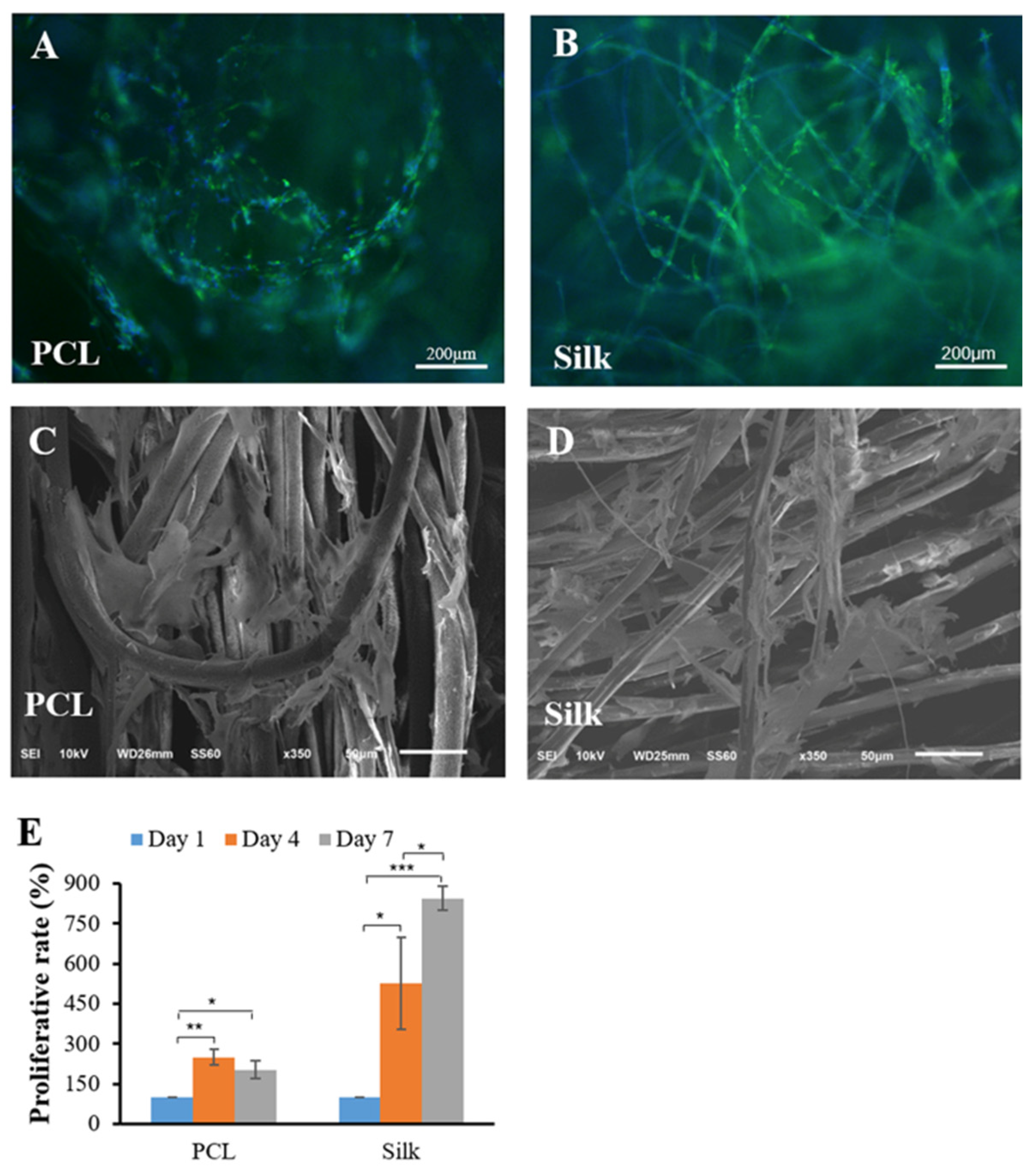
3.3. Cell Growth and Proliferation
3.4. Chondrogenic Phenotype Expression
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Setiawati, A.; Jang, D.; Cho, D.; Cho, S.; Jeong, H.; Park, S.; Gwak, J.; Ryu, S.R.; Jung, W.H.; Ju, B.-G.; et al. An Accelerated Wound-Healing Surgical Suture Engineered with an Extracellular Matrix. Adv. Healthc. Mater. 2021, 10, 2001686. [Google Scholar] [CrossRef] [PubMed]
- Miguel, G.A.; Álvarez-López, C. Extraction and Antioxidant Activity of Sericin, a Protein from Silk. Braz. J. Food Technol. 2020, 23, e2019058. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Pramanik, K. Enhanced Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Silk Fibroin/Chitosan/Glycosaminoglycan Scaffolds under Dynamic Culture Condition. Differentiation 2019, 110, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Yodmuang, S.; McNamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.-A.N.; Chao, P.G.; Hung, C.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk Microfiber-Reinforced Silk Hydrogel Composites for Functional Cartilage Tissue Repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (ε-Caprolactone) Fiber: An Overview. J. Eng. Fibers Fabr. 2014, 9, 74–90. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.M.; Ju, Y.M.; Jung, J.W.; Kang, H.-W.; Lee, S.J.; Yoo, J.J.; Kim, S.W.; Kim, S.H.; Cho, D.-W. A Novel Tissue-Engineered Trachea with a Mechanical Behavior Similar to Native Trachea. Biomaterials 2015, 62, 106–115. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Cheng, J.; Sun, M.; Yang, P.; Zhao, F.; Zhang, J.; Duan, X.; Fu, X.; Zhang, J.; et al. Biomechanically, Structurally and Functionally Meticulously Tailored Polycaprolactone/Silk Fibroin Scaffold for Meniscus Regeneration. Theranostics 2020, 10, 5090–5106. [Google Scholar] [CrossRef]
- Chen, X.; Hou, D.; Wang, L.; Zhang, Q.; Zou, J.; Sun, G. Antibacterial Surgical Silk Sutures Using a High-Performance Slow-Release Carrier Coating System. ACS Appl. Mater. Interfaces 2015, 7, 22394–22403. [Google Scholar] [CrossRef]
- Bigalke, C.; Luderer, F.; Wulf, K.; Storm, T.; Löbler, M.; Arbeiter, D.; Rau, B.M.; Nizze, H.; Vollmar, B.; Schmitz, K.-P.; et al. VEGF-Releasing Suture Material for Enhancement of Vascularization: Development, in Vitro and in Vivo Study. Acta Biomater. 2014, 10, 5081–5089. [Google Scholar] [CrossRef]
- Hamada, Y.; Katoh, S.; Hibino, N.; Kosaka, H.; Hamada, D.; Yasui, N. Effects of Monofilament Nylon Coated with Basic Fibroblast Growth Factor on Endogenous Intrasynovial Flexor Tendon Healing. J. Hand Surg. Am. 2006, 31, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Reckhenrich, A.K.; Kirsch, B.M.; Wahl, E.A.; Schenck, T.L.; Rezaeian, F.; Harder, Y.; Foehr, P.; Machens, H.-G.; Egaña, J.T. Surgical Sutures Filled with Adipose-Derived Stem Cells Promote Wound Healing. PLoS ONE 2014, 9, e91169. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Woon, C.Y.-L.; Behn, A.; Korotkova, T.; Park, D.-Y.; Gajendran, V.; Smith, R.L. The Effect of Suture Coated with Mesenchymal Stem Cells and Bioactive Substrate on Tendon Repair Strength in a Rat Model. J. Hand Surg. Am. 2012, 37, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-Z.; Kim, H.-W. Efficacy of Collagen and Alginate Hydrogels for the Prevention of Rat Chondrocyte Dedifferentiation. J. Tissue Eng. 2018, 9, 2041731418802438. [Google Scholar] [CrossRef] [PubMed]
- Kashte, S.; Jaiswal, A.K.; Kadam, S. Artificial Bone via Bone Tissue Engineering: Current Scenario and Challenges. Tissue Eng. Regen. Med. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk Fibroin in Tissue Engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Nguyen, Q.T.; Chen, A.C.; Kaplan, D.L.; Sah, R.L.; Kundu, S.C. Potential of 3-D Tissue Constructs Engineered from Bovine Chondrocytes/Silk Fibroin-Chitosan for in Vitro Cartilage Tissue Engineering. Biomaterials 2011, 32, 5773–5781. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Zheng, L.; Zhang, X.; Liu, P.; Yang, T.; Han, B. Electrospun Fibrous Silk Fibroin/Poly(L-Lactic Acid) Scaffold for Cartilage Tissue Engineering. Tissue Eng. Regen. Med. 2016, 13, 516–526. [Google Scholar] [CrossRef]
- Wang, Y.; Blasioli, D.J.; Kim, H.-J.; Kim, H.S.; Kaplan, D.L. Cartilage Tissue Engineering with Silk Scaffolds and Human Articular Chondrocytes. Biomaterials 2006, 27, 4434–4442. [Google Scholar] [CrossRef]
- Voga, M.; Drnovsek, N.; Novak, S.; Majdic, G. Silk Fibroin Induces Chondrogenic Differentiation of Canine Adipose-Derived Multipotent Mesenchymal Stromal Cells/Mesenchymal Stem Cells. J. Tissue Eng. 2019, 10, 2041731419835056. [Google Scholar] [CrossRef]
- Talukdar, S.; Nguyen, Q.T.; Chen, A.C.; Sah, R.L.; Kundu, S.C. Effect of Initial Cell Seeding Density on 3D-Engineered Silk Fibroin Scaffolds for Articular Cartilage Tissue Engineering. Biomaterials 2011, 32, 8927–8937. [Google Scholar] [CrossRef] [PubMed]
- Kachi, N.D.; Otaka, A.; Sim, S.; Kuwana, Y.; Tamada, Y.; Sunaga, J.; Adachi, T.; Tomita, N. Observation of Chondrocyte Aggregate Formation and Internal Structure on Micropatterned Fibroin-Coated Surface. Biomed. Mater. Eng. 2010, 20, 55–63. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Yi, H.; Yang, G.; Wang, J. Biosynthesis of a Potentially Functional Polypeptide Derived from Silk Fibroin. Biomed. Mater. Eng. 2014, 24, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Igarashi, Y.; Takasu, Y.; Saito, H.; Tsubouchi, K. Identification of Fibroin-Derived Peptides Enhancing the Proliferation of Cultured Human Skin Fibroblasts. Biomaterials 2004, 25, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Quintana, L.; zur Nieden, N.I.; Semino, C.E. Morphogenetic and Regulatory Mechanisms during Developmental Chondrogenesis: New Paradigms for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-Z.; Kim, H.-W. Chondrogenic Potential of Dedifferentiated Rat Chondrocytes Reevaluated in Two- and Three-Dimensional Culture Conditions. Tissue Eng. Regen. Med. 2018, 15, 163–172. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Chawla, S.; Chameettachal, S.; Murab, S.; Bhavesh, N.S.; Ghosh, S. Role of Chondroitin Sulphate Tethered Silk Scaffold in Cartilaginous Disc Tissue Regeneration. Biomed. Mater. 2016, 11, 025014. [Google Scholar] [CrossRef]
- Ortega, Z.; Alemán, M.E.; Donate, R. Nanofibers and Microfibers for Osteochondral Tissue Engineering. In Osteochondral Tissue Engineering: Nanotechnology, Scaffolding-Related Developments and Translation; Oliveira, J.M., Pina, S., Reis, R.L., San Roman, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 97–123. ISBN 978-3-319-76711-6. [Google Scholar]
- Yan, J.; Zhou, G.; Knight, D.P.; Shao, Z.; Chen, X. Wet-Spinning of Regenerated Silk Fiber from Aqueous Silk Fibroin Solution: Discussion of Spinning Parameters. Biomacromolecules 2010, 11, 1–5. [Google Scholar] [CrossRef]
- Lister, J. On a New Method of Treating Compound Fracture, Abscess, etc.: With Observations on the Conditions of Suppuration. Lancet 1867, 89, 326–329. [Google Scholar] [CrossRef]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk Matrix for Tissue Engineered Anterior Cruciate Ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Liang, R.; Zhao, J.; Li, B.; Cai, P.; Loh, X.J.; Xu, C.; Chen, P.; Kai, D.; Zheng, L. Implantable and Degradable Antioxidant Poly(ε-Caprolactone)-Lignin Nanofiber Membrane for Effective Osteoarthritis Treatment. Biomaterials 2020, 230, 119601. [Google Scholar] [CrossRef]

| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| Sox9 | 5′-CTGAAGGGCTACGACTGGAC-3′ | 5′-TACTGGTCTGCCAGCTTCCT-3′ |
| Type II collagen | 5′-GAGTGGAAGAGCGGAGACTACTG-3′ | 5′-CTCCATGTTGCAGAAGACTTTCA-3′ |
| Aggrecan | 5′-CTAGCTGCTTAGCAGGGATAACG-3′ | 5′-TGACCCGCAGAGTCACAAAG-3′ |
| Type I collagen | 5′-CGTGACCAAAAACCAAAAGT-3′ | 5′-GGGGTGGAGAAAGGAACAGA-3′ |
| Type X collagen | 5′-GATCATGGAGCTCACGGAAAA-3′ | 5′-CCGTTCGATTCCGCATTG-3′ |
| GAPDH | 5′-TGAACGGGAAGCTCACTGG-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, G.-Z. Comparison of Chondrocyte Behaviors Between Silk Microfibers and Polycaprolactone Microfibers in Tissue Engineering and Regenerative Medicine Applications. Bioengineering 2024, 11, 1209. https://doi.org/10.3390/bioengineering11121209
Jin G-Z. Comparison of Chondrocyte Behaviors Between Silk Microfibers and Polycaprolactone Microfibers in Tissue Engineering and Regenerative Medicine Applications. Bioengineering. 2024; 11(12):1209. https://doi.org/10.3390/bioengineering11121209
Chicago/Turabian StyleJin, Guang-Zhen. 2024. "Comparison of Chondrocyte Behaviors Between Silk Microfibers and Polycaprolactone Microfibers in Tissue Engineering and Regenerative Medicine Applications" Bioengineering 11, no. 12: 1209. https://doi.org/10.3390/bioengineering11121209
APA StyleJin, G.-Z. (2024). Comparison of Chondrocyte Behaviors Between Silk Microfibers and Polycaprolactone Microfibers in Tissue Engineering and Regenerative Medicine Applications. Bioengineering, 11(12), 1209. https://doi.org/10.3390/bioengineering11121209






