Metabolomic Analysis and Biochemical Profiling of Cadmium-Induced Metabolic Impairment and Its Amelioration by Resveratrol
Abstract
1. Introduction
2. Materials and Methods
2.1. Cadmium Solution
2.2. Resveratrol Solution
2.3. Study Design
2.4. Measurement of Glycemic Index Parameters
2.5. Assessment of Liver Biomarkers
2.6. Estimation of Kidney Function Biomarkers
2.7. Estimation of Inflammatory Biomarkers
2.8. Estimation of the Lipid Profile
2.9. Metabolomic Analysis via LC-MS/MS
2.9.1. Sample Preparation for LC–MS/MS Analysis
2.9.2. Conditions of the Instrument
2.9.3. Analysis of Metabolomes by LC-MS/MS
2.10. Estimation of Cadmium in Serum, Urine, and Organs via ICP-OES
2.10.1. Reagents and Standard Solutions
2.10.2. Sample Preparation for ICP-OES Analysis
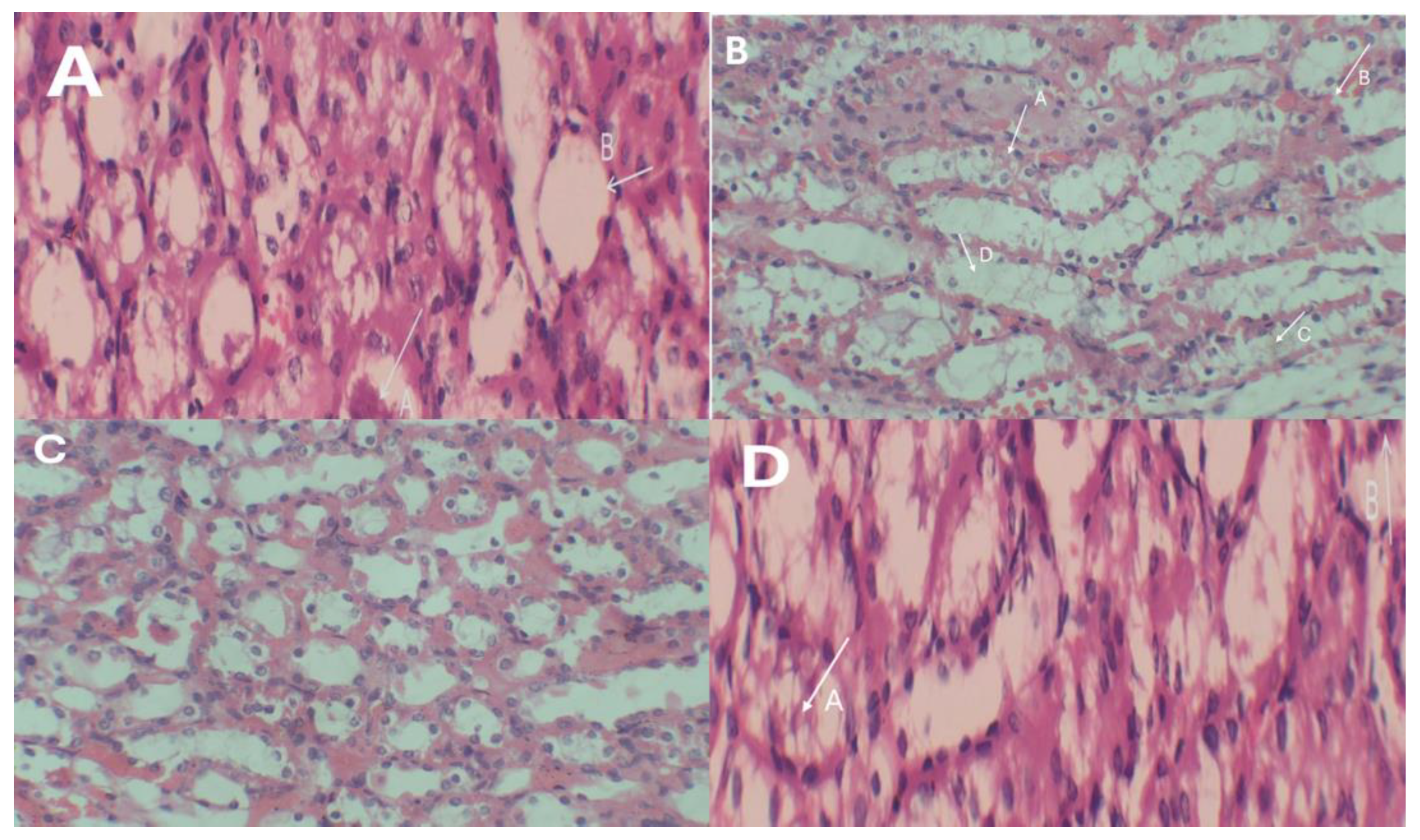
2.11. Histopathological Assessment
2.12. Statistical Analysis
3. Results
3.1. Evaluation of Metabolic Impairment Through Biochemical Analysis in Animal Models
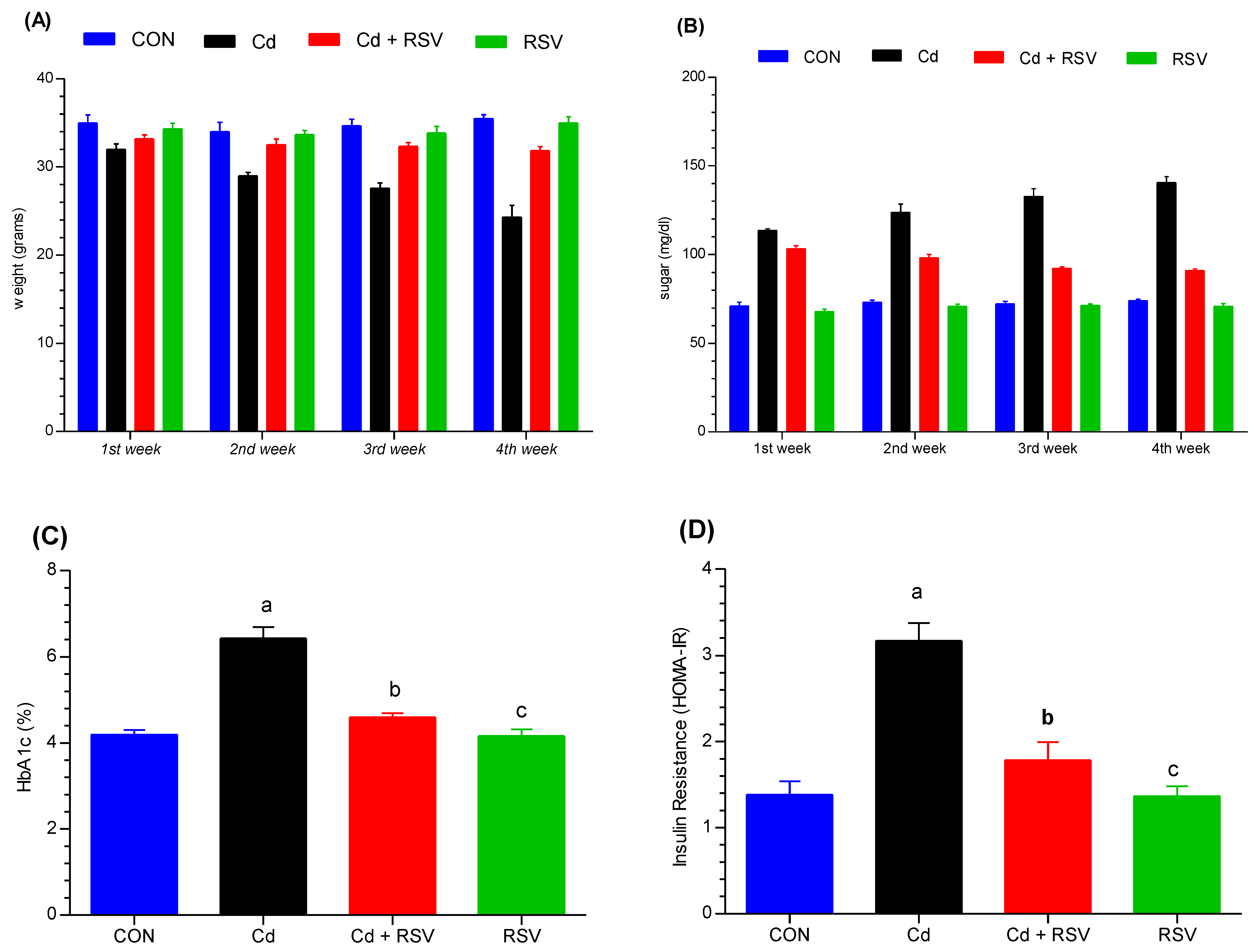
3.1.1. Body Weight Changes
3.1.2. Assessment of Glycemic Index Changes
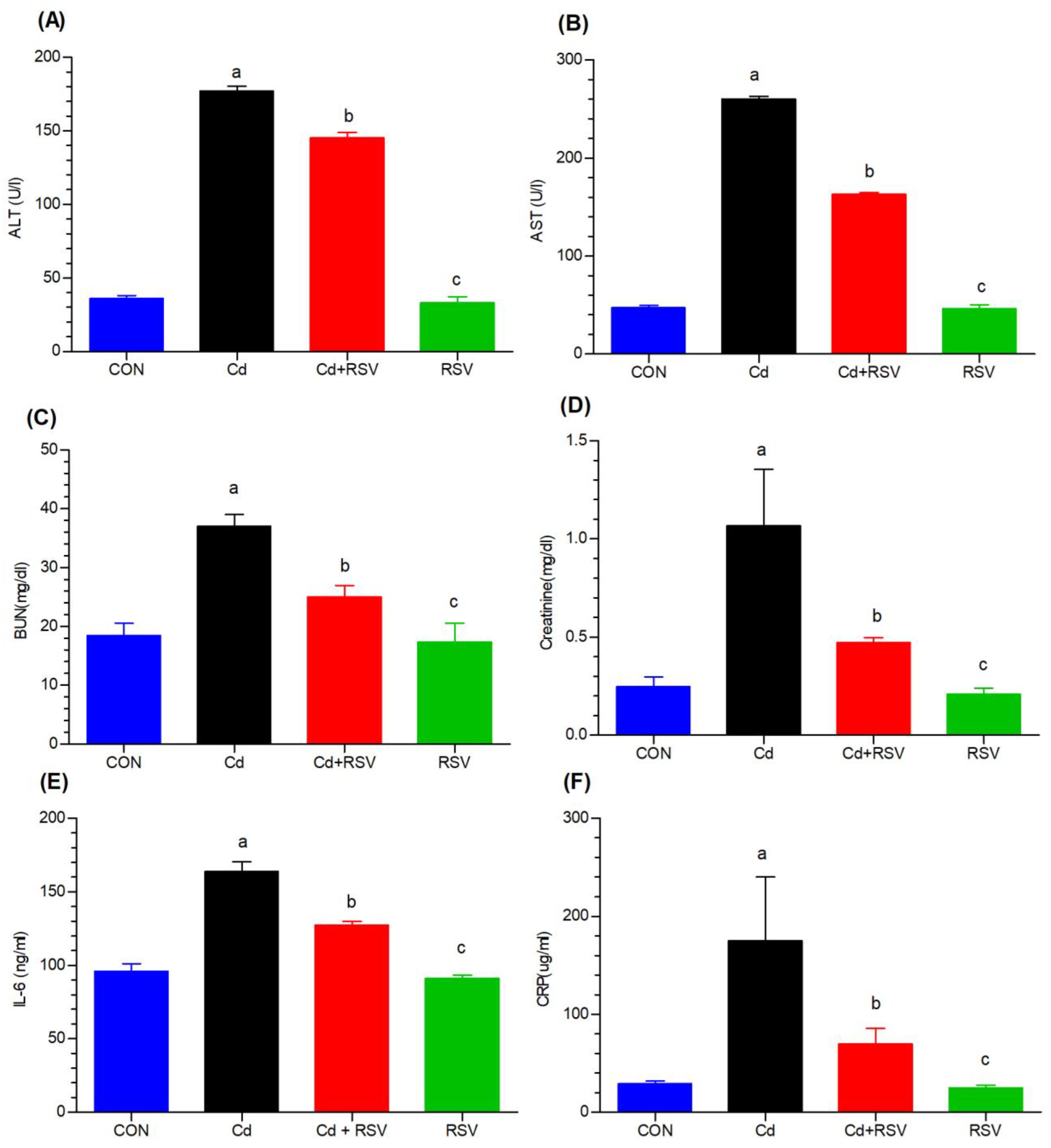
3.1.3. Effects on Liver, Kidney, and Inflammatory Biomarkers
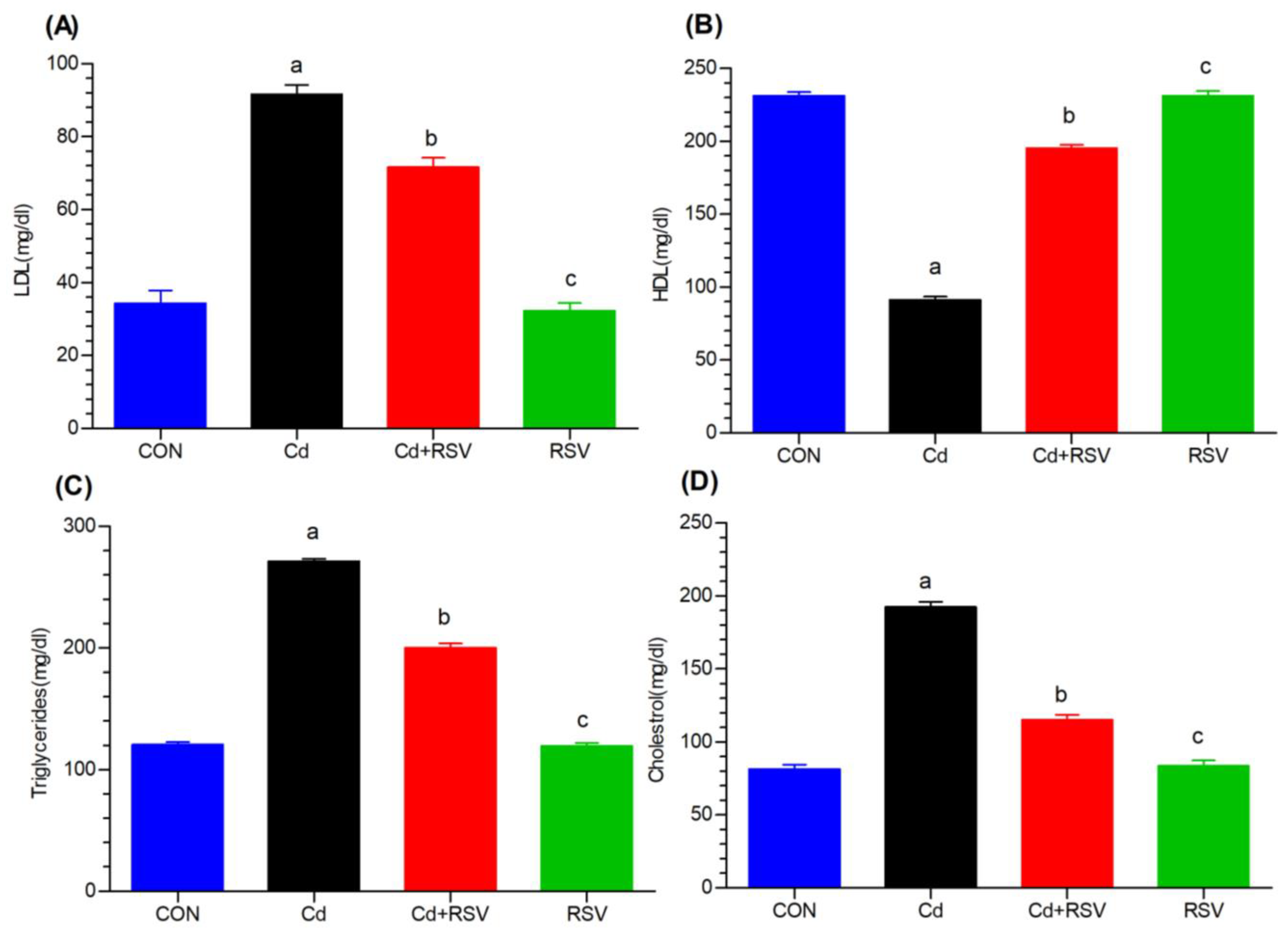
3.1.4. Effects on Lipid Biomarkers
3.2. Analysis of Metabolomes by LC-MS/MS
3.2.1. Comparative Analysis of Lipid Metabolites on the Basis of Peak Analysis
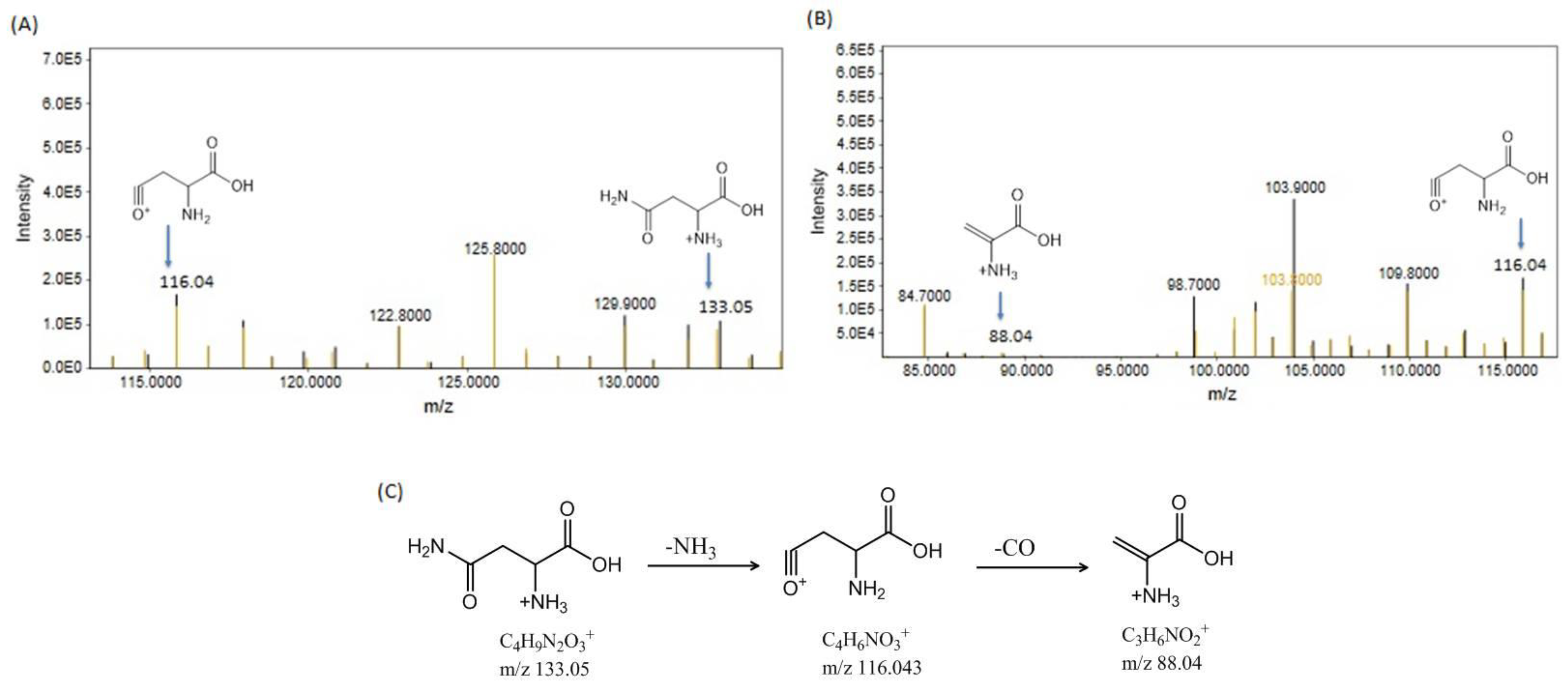
3.2.2. Metabolome Profiling via Fragmentation Analysis
Phenylalanine
Arginine
Asparagine
Histidine
Tryptophan
Sphinganine
Lysophosphatidylcholine (LPC 16:0)
Lysophosphatidylcholine (LGPC 11:0)
Carnitine
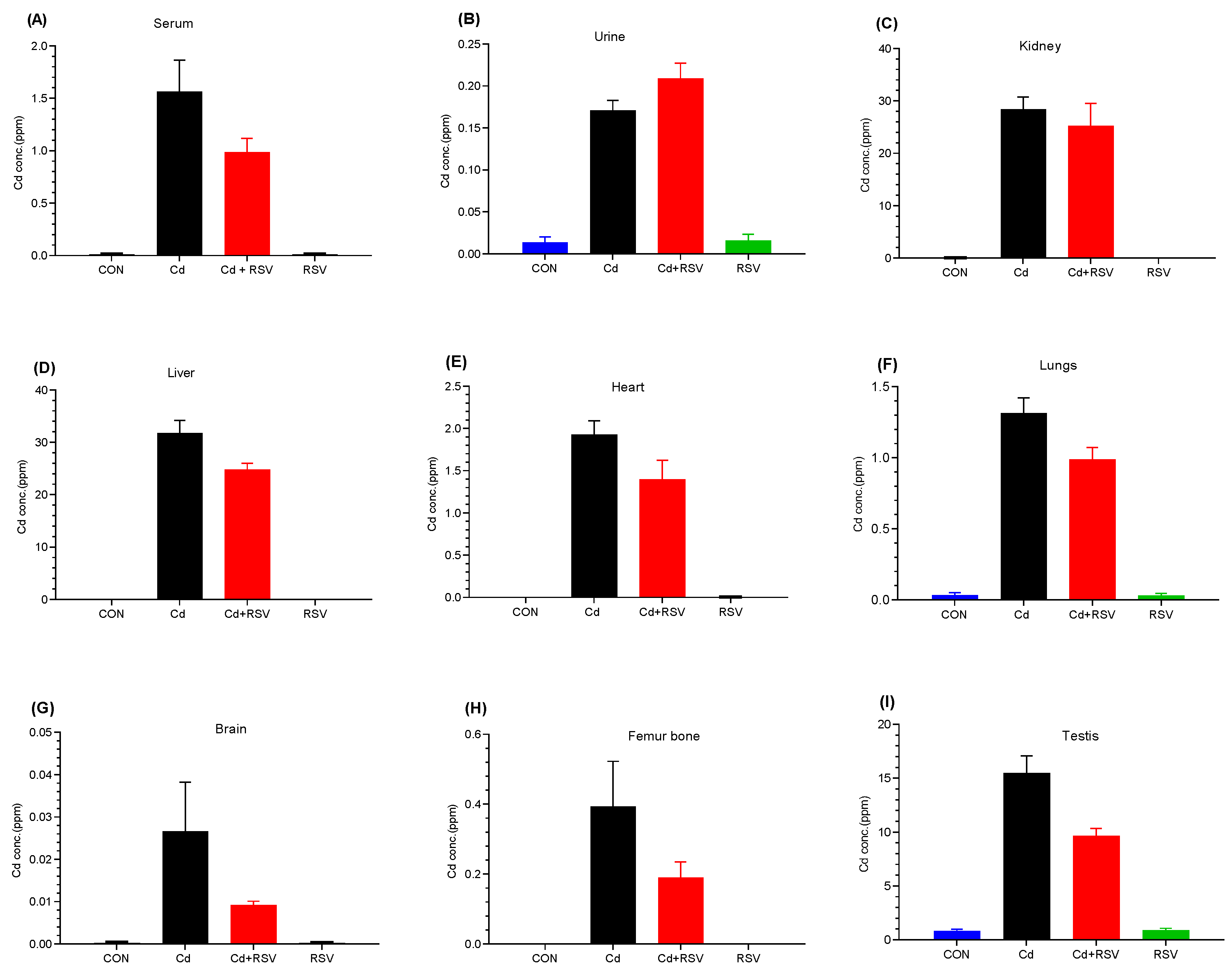
3.3. Detection of Cd by ICP-OES
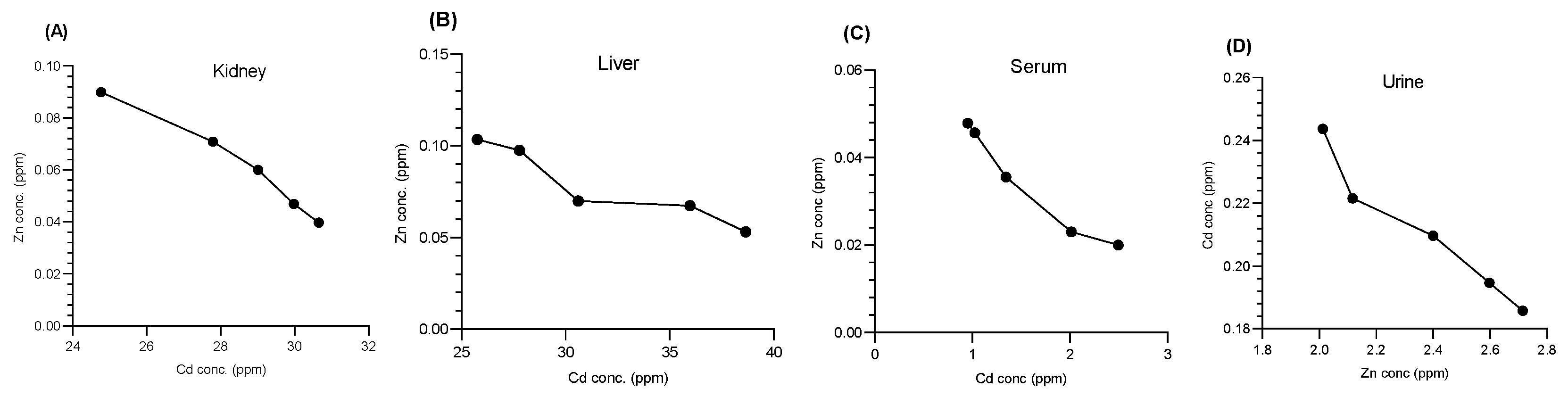
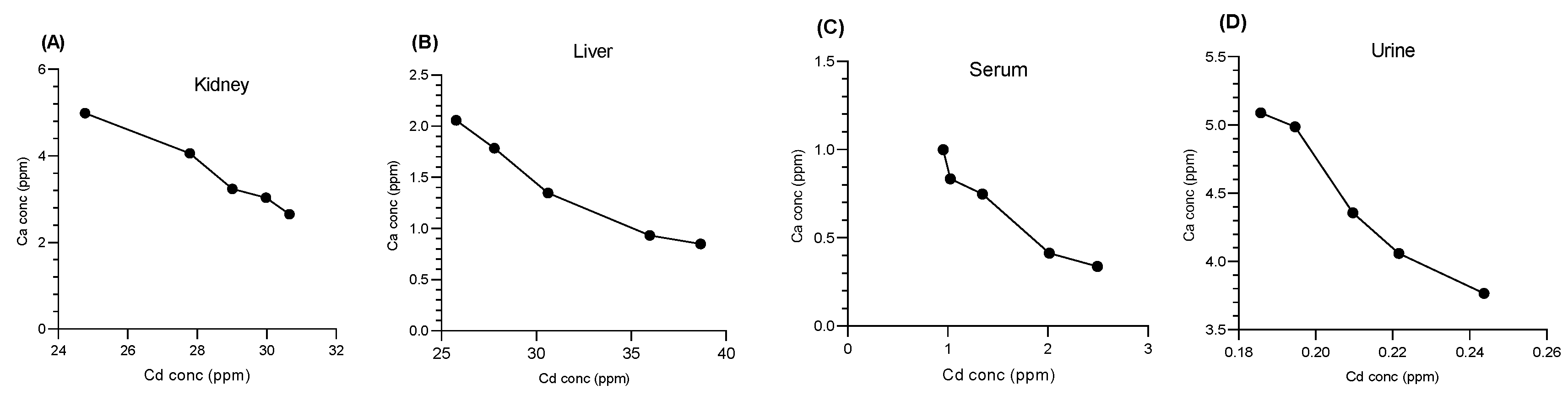
3.4. Impact of Cd on Essential Metals
3.4.1. Impact of Cd on Zn Cations
3.4.2. Impact of Cd on Ca Cations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef] [PubMed]
- Chunhabundit, R. Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Qu, F.; Zheng, W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Liu, T.; Castro, S.M.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009, 41, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, R.; Wang, X.; Shen, X.; Wang, P.; Sun, N.; Li, X.; Li, X.; Hai, C. Effects of sub-chronic, low-dose cadmium exposure on kidney damage and potential mechanisms. Ann. Transl. Med. 2019, 7, 177. [Google Scholar] [CrossRef]
- Alvi, M.; Rehman, K.; Akash, M.S.H.; Yaqoob, A.; Shoaib, S.M. Determination of Metabolomics Profiling in BPA-Induced Impaired Metabolism. Pharmaceutics 2022, 14, 2496. [Google Scholar] [CrossRef]
- Hadi, N.I.; Jamal, Q.; Iqbal, A.; Shaikh, F.; Somroo, S.; Musharraf, S.G. Serum Metabolomic Profiles for Breast Cancer Diagnosis, Grading and Staging by Gas Chromatography-Mass Spectrometry. Sci. Rep. 2017, 7, 1715. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Wahl, D.; Diéguez, C.; Bernier, M.; De Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef]
- Saba, S.; Akash, M.S.H.; Rehman, K.; Saleem, U.; Fiayyaz, F.; Ahmad, T. Assessment of heavy metals by ICP-OES and their impact on insulin stimulating hormone and carbohydrate metabolizing enzymes. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Dhania, G. Cadmium as an environmental pollutant: Ecotoxicological effects, health hazards, and bioremediation approaches for its detoxification from contaminated sites. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Springer: Singapore, 2020; pp. 357–387. [Google Scholar]
- Nguyen, J.; Patel, A.; Gensburg, A.; Bokhari, R.; Lamar, P.; Edwards, J. Diabetogenic and obesogenic effects of cadmium in Db/Db mice and rats at a clinically relevant level of exposure. Toxics 2022, 10, 107. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217. [Google Scholar] [CrossRef]
- Borné, Y.; Fagerberg, B.; Persson, M.; Sallsten, G.; Forsgard, N.; Hedblad, B.; Barregard, L.; Engström, G. Cadmium exposure and incidence of diabetes mellitus-results from the Malmö Diet and Cancer study. PLoS ONE 2014, 9, e112277. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Baig, J.A.; Sarfraz, R.A. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res. Clin. Pr. 2008, 80, 280–288. [Google Scholar] [CrossRef]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef]
- Xiao, L.; Li, W.; Zhu, C.; Yang, S.; Zhou, M.; Wang, B.; Wang, X.; Wang, D.; Ma, J.; Zhou, Y.; et al. Cadmium exposure, fasting blood glucose changes, and type 2 diabetes mellitus: A longitudinal prospective study in China. Environ. Res. 2020, 192, 110259. [Google Scholar] [CrossRef]
- Satarug, S. Is Chronic Kidney Disease Due to Cadmium Exposure Inevitable and Can It Be Reversed? Biomedicines 2024, 12, 718. [Google Scholar] [CrossRef]
- Zhang, K.; Long, M.; Dong, W.; Li, J.; Wang, X.; Liu, W.; Huang, Q.; Ping, Y.; Zou, H.; Song, R.; et al. Cadmium Induces Kidney Iron Deficiency and Chronic Kidney Injury by Interfering with the Iron Metabolism in Rats. Int. J. Mol. Sci. 2024, 25, 763. [Google Scholar] [CrossRef] [PubMed]
- Mognetti, B.; Franco, F.; Castrignano, C.; Bovolin, P.; Berta, G.N. Mechanisms of Phytoremediation by Resveratrol against Cadmium Toxicity. Antioxidants 2024, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Chung, M.; Cosgrove, D.; Herman, J.M.; Li, Z.; Firoozmand, A.; Gurakar, A.; Koteish, A.; Pawlik, T.M. Cadmium exposure and liver disease among US adults. J. Gastrointest. Surg. 2013, 17, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Al-Baqami, N.M.; Hamza, R.Z. Protective Effect of Resveratrol against Hepatotoxicity of Cadmium in Male Rats: Antioxidant and Histopathological Approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- Hong, D.; Min, J.-Y.; Min, K.-B. Association Between Cadmium Exposure and Liver Function in Adults in the United States: A Cross-sectional Study. J. Prev. Med. Public Health 2021, 54, 471–480. [Google Scholar] [CrossRef]
- Chae, M.S.; Moon, K.U.; Chung, H.S.; Park, C.S.; Lee, J.; Choi, J.H.; Hong, S.H. Serum interleukin-6 and tumor necrosis factor-α are associated with early graft regeneration after living donor liver transplantation. PLoS ONE 2018, 13, e0195262. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef]
- Hosseini, H.; Koushki, M.; Khodabandehloo, H.; Fathi, M.; Panahi, G.; Teimouri, M.; Majidi, Z.; Meshkani, R. The Effect of Resveratrol Supplementation on C-reactive Protein (CRP) in Type 2 Diabetic Patients: Results from a Systematic Review and Meta-analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 49, 102251. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Larregle, E.V.; Varas, S.M.; Oliveros, L.B.; Martinez, L.D.; Antón, R.; Marchevsky, E.; Giménez, M.S. Lipid metabolism in liver of rat exposed to cadmium. Food Chem. Toxicol. 2008, 46, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Guerrero-Romero, F. Effect of resveratrol supplementation on lipid profile in subjects with dyslipidemia: A randomized double-blind, placebo-controlled trial. Nutrition 2019, 58, 7–10. [Google Scholar] [CrossRef]
- Ahmed, H.A.M.; Alatawi, A.O. Comparison of the ICP OES viewing modes efficiency in the estimation of cadmium (Cd) and lead (Pb) in whole blood samples. Arab. J. Basic Appl. Sci. 2022, 29, 208–214. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Chou, S.-H.; Cheng, C.-Y.; Ho, C.-T.; Lin, H.-C.; Jung, S.-M.; Chu, P.-H.; Ko, F.-H. The preferential accumulation of cadmium ions among various tissues in mice. Toxicol. Rep. 2022, 9, 111–119. [Google Scholar] [CrossRef]
- Rahil-Khazen, R.; Bolann, B.J.; Myking, A.; Ulvik, R.J. Multielement analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES). J. Trace Elem. Med. Biol. 2002, 16, 15–25. [Google Scholar] [CrossRef]
- Nordberg, M.; Nordberg, G.F. Metallothionein and cadmium toxicology—Historical review and commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef]
- Mosna, K.; Jurczak, K.; Krężel, A. Differentiated Zn(II) binding affinities in animal, plant, and bacterial metallothioneins define their zinc buffering capacity at physiological pZn. Metallomics 2023, 15, mfad061. [Google Scholar] [CrossRef] [PubMed]
- Grzybowska, E.A. Calcium-binding proteins with disordered structure and their role in secretion, storage, and cellular signaling. Biomolecules 2018, 8, 42. [Google Scholar] [CrossRef]
- Yu, H.-T.; Zhen, J.; Leng, J.-Y.; Cai, L.; Ji, H.-L.; Keller, B.B. Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol. Sin. 2020, 42, 340–346. [Google Scholar] [CrossRef]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, W.; Shi, H.; Hou, Y.; Xu, Q. Calcium homeostasis disruption-a bridge connecting cadmium-induced apoptosis, autophagy and tumorigenesis. Oncol. Res. Treat. 2015, 38, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Sticco, K.L. Arginase Deficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Paľove-Balang, P.; Kisová, A.; Pavlovkin, J.; Mistrík, I. Effect of manganese on cadmium toxicity in maize seedlings. Plant Soil Environ. 2006, 52, 143–149. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Have, G.A.M.T.; Wolfe, R.R.; Deutz, N.E.P. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.-L.; Ritter, J.K. Role of nitric oxide in the cardiovascular and renal systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, W.-W.; Chen, J.-C.; Zhang, H.-L.; Liu, J.; Lin, Y.; Lin, P.-C.; Wu, B.-X.; An, Y.-P.; Huang, L.; et al. Phenylalanine impairs insulin signaling and inhibits glucose uptake through modification of IRβ. Nat. Commun. 2022, 13, 4291. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain2. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Sumaily, K.M.; Mujamammi, A.H. Phenylketonuria: A new look at an old topic, advances in laboratory diagnosis, and therapeutic strategies. Int. J. Health Sci. 2017, 11, 63–70. [Google Scholar]
- Lu, Y.; Wang, Y.; Liang, X.; Zou, L.; Ong, C.N.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Serum amino acids in association with prevalent and incident type 2 diabetes in a Chinese population. Metabolites 2019, 9, 14. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Alexander, C.S.W.; Dhanasekaran, M.; Moore, T. The influence of kynurenine metabolites on neurodegenerative pathologies. Int. J. Mol. Sci. 2024, 25, 853. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Side effects of amino acid supplements. Physiol. Res. 2022, 71, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yin, L.; He, J.; Zeng, Q.; Liang, Y.; Shen, Y.; Zu, X. Metabolism of asparagine in the physiological state and cancer. Cell Commun. Signal. 2024, 22, 163. [Google Scholar] [CrossRef]
- Aliu, E.; Kanungo, S.; Arnold, G.L. Amino acid disorders. Ann. Transl. Med. 2018, 6, 471. [Google Scholar] [CrossRef]
- Górska, M.; Dobrzyń, A.; Baranowski, M. Concentrations of sphingosine and sphinganine in plasma of patients with type 2 diabetes. Med. Sci. Monit. 2005, 11, CR35–CR38. [Google Scholar]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef]
- Knuplez, E.; Marsche, G. An Updated Review of Pro- and Anti-Inflammatory Properties of Plasma Lysophosphatidylcholines in the Vascular System. Int. J. Mol. Sci. 2020, 21, 4501. [Google Scholar] [CrossRef]
- Radulović, S.; Gottschalk, B.; Hörl, G.; Zardoya-Laguardia, P.; Schilcher, I.; Hallström, S.; Vujić, N.; Schmidt, K.; Trieb, M.; Graier, W.F.; et al. Endothelial lipase increases eNOS activating capacity of high-density lipoprotein. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158612. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef] [PubMed]




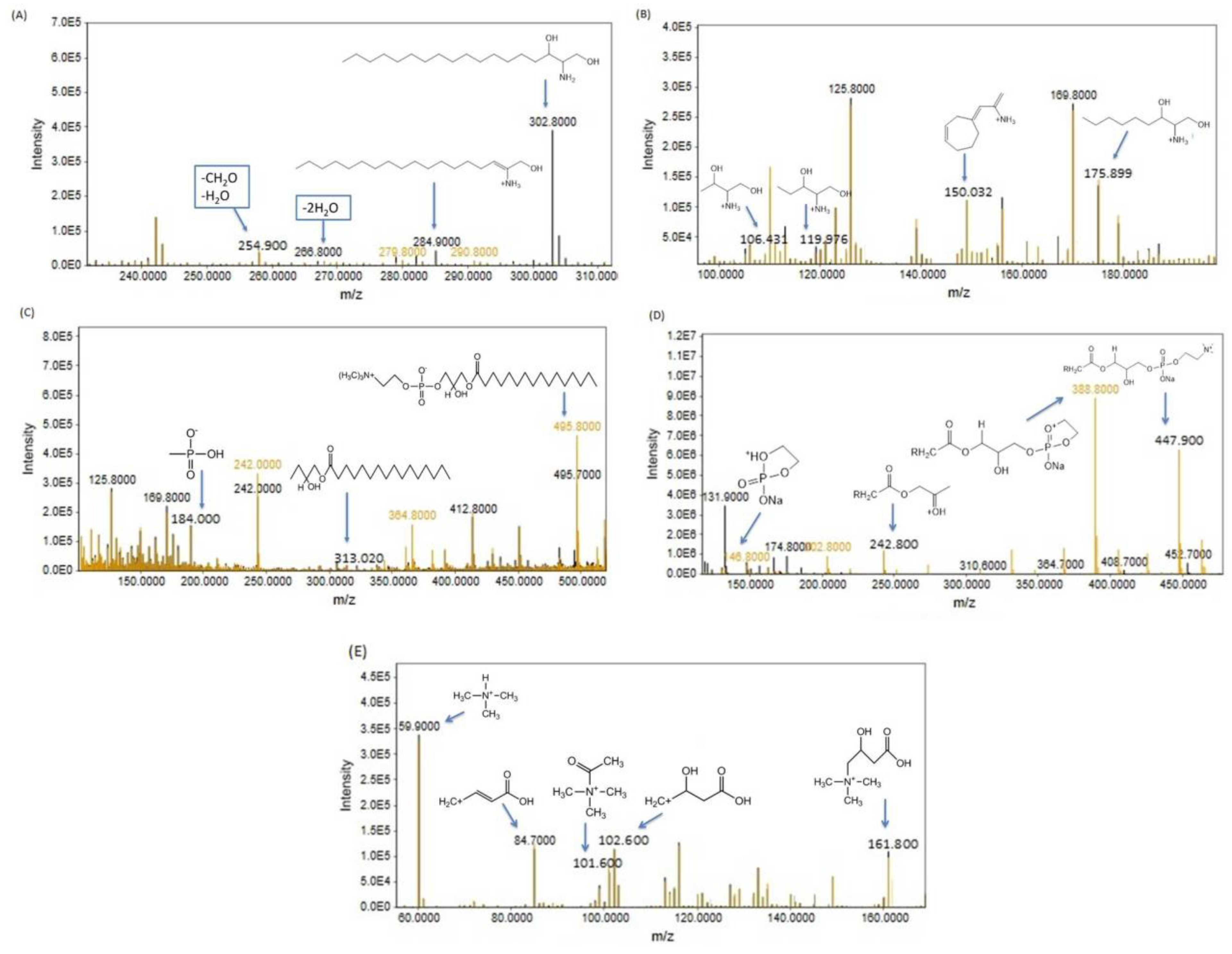

| Sr.# | R.T (min) | m/z | Metabolite | Regulation | Fold Change | p Value | Significance |
|---|---|---|---|---|---|---|---|
| 1 | 14.917 | 116.1 | Proline | Downregulated | −0.00519 | 0.621 | Non-significant |
| 2 | 4.199 | 118.1 | Valine | Upregulated | 0.78912 | <0.001 | Significant |
| 3 | 4.285 | 132.2 | Leucine | Upregulated | 6.071174 | <0.001 | Significant |
| 4 | 3.483 | 133.1 | L-Ornithine | Upregulated | 3.264767 | <0.001 | Significant |
| 5 | 14.012 | 147.2 | Glutamine | Upregulated | 0.08801 | 0.040 | Significant |
| 6 | 6.173 | 197.1 | L-Arginine | Downregulated | −0.15066 | 0.209 | Non-significant |
| 7 | 5.819 | 205.1 | Tryptophan | Upregulated | 1.84339 | 0.006 | Significant |
| 8 | 12.078 | 263.1 | L-Asparagine | Downregulated | −0.12964 | 0.549 | Non-significant |
| 9 | 4.969 | 156 | Histidine | Upregulated | 0.58638 | <0.001 | Significant |
| 10 | 20.861 | 166.1 | Phenylalanine | Downregulated | −0.08173 | 0.226 | Non-significant |
| 11 | 4.852 | 175.1 | Arginine | Upregulated | 0.379477 | <0.001 | Significant |
| Sr.# | R.T (min) | m/z | Metabolite | Regulation | Fold Change | p Value | Significance |
|---|---|---|---|---|---|---|---|
| 1 | 3.46 | 302.5 | Sphinganine | Upregulated | 0.082136 | 0.623 | Non-significant |
| 2 | 6.503 | 326 | Cer20:0;O2|Cer 12:0;O2/8:0 | Upregulated | 0.151338 | 0.011 | Significant |
| 3 | 6.503 | 326 | CAR 3:0 | Upregulated | 0.151338 | 0.011 | Significant |
| 4 | 4.749 | 338 | CAR 12:3 | Upregulated | 0.507756 | 0.0002 | Significant |
| 5 | 16.033 | 496 | PC (0:0/16:0) | Downregulated | −0.35501 | <0.001 | Significant |
| 6 | 6.802 | 522.4 | LPC 18:1 | Upregulated | 0.556376 | <0.001 | Significant |
| 7 | 4.372 | 524.3 | PE 20:0 | Downregulated | −0.1426 | 0.0006 | Significant |
| 8 | 9.365 | 560.5 | Cer34:1;O2|Cer 12:0;O2/22:1 | Upregulated | 0.1185 | 0.0048 | Significant |
| 9 | 8.744 | 583.3 | TG 31:4|TG 8:0_8:0_15:4 | Upregulated | 0.552885 | <0.001 | Significant |
| 10 | 7.659 | 616.4 | PC 24:3|PC 5:0_19:3 | Downregulated | −0.37606 | <0.001 | Significant |
| 11 | 9.216 | 627.4 | DG 36:10 | Downregulated | −0.36206 | <0.001 | Significant |
| 12 | 6.794 | 651.8 | DG 37:5 | Downregulated | −0.2914 | <0.001 | Significant |
| 14 | 4.191 | 652.4 | PC 27:6|PC 5:0_22:6 | Upregulated | 1.501611 | <0.001 | Significant |
| 15 | 5.772 | 657.4 | SM 31:3;O2|SM 12:1;O2/19:2 | Downregulated | −0.19907 | <0.001 | Significant |
| 16 | 7.533 | 646.5 | SM 20:1;O2/10:0 | Upregulated | 1.7152057 | 0.0278 | Significant |
| 17 | 3.106 | 764.6 | AHexCer39:2;O2|AHexCer (O-15:1)12:1;O2/12:0 | Downregulated | −0.078 | <0.001 | Significant |
| 18 | 4.427 | 794.7 | Cer52:6;O4|Cer 14:1;O3/38:5(2OH) | Upregulated | 0.720853 | <0.001 | Significant |
| 19 | 2.972 | 898.7 | SL 55:8;O2|SL 19:2;O/36:6;O | Upregulated | 0.88367 | <0.001 | Significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas, K.; Rehman, K.; Iqbal, H.; Hussain, A.; Akash, M.S.H.; Shahid, M.; Sadaf, B. Metabolomic Analysis and Biochemical Profiling of Cadmium-Induced Metabolic Impairment and Its Amelioration by Resveratrol. Bioengineering 2024, 11, 1141. https://doi.org/10.3390/bioengineering11111141
Ilyas K, Rehman K, Iqbal H, Hussain A, Akash MSH, Shahid M, Sadaf B. Metabolomic Analysis and Biochemical Profiling of Cadmium-Induced Metabolic Impairment and Its Amelioration by Resveratrol. Bioengineering. 2024; 11(11):1141. https://doi.org/10.3390/bioengineering11111141
Chicago/Turabian StyleIlyas, Kainat, Kanwal Rehman, Hajra Iqbal, Amjad Hussain, Muhammad Sajid Hamid Akash, Mudassar Shahid, and Bushra Sadaf. 2024. "Metabolomic Analysis and Biochemical Profiling of Cadmium-Induced Metabolic Impairment and Its Amelioration by Resveratrol" Bioengineering 11, no. 11: 1141. https://doi.org/10.3390/bioengineering11111141
APA StyleIlyas, K., Rehman, K., Iqbal, H., Hussain, A., Akash, M. S. H., Shahid, M., & Sadaf, B. (2024). Metabolomic Analysis and Biochemical Profiling of Cadmium-Induced Metabolic Impairment and Its Amelioration by Resveratrol. Bioengineering, 11(11), 1141. https://doi.org/10.3390/bioengineering11111141









