Synthesis and Characterization of Iron Nanoparticles from a Bioflocculant Produced by Pichia kudriavzevii Isolated from Kombucha Tea SCOBY
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolate Activation for Fermentation
2.2. Extraction and Purification of the Bioflocculant
2.3. Biosynthesis of Iron Nanoparticles (FeNPs)
2.4. Characterization of Biosynthesized FeNPs
3. Results and Discussions
3.1. The FT-IR Analysis
3.2. UV–Visible Spectroscopy Analysis
3.3. X-Ray Diffraction Analysis
3.4. Thermogravimetric Analysis
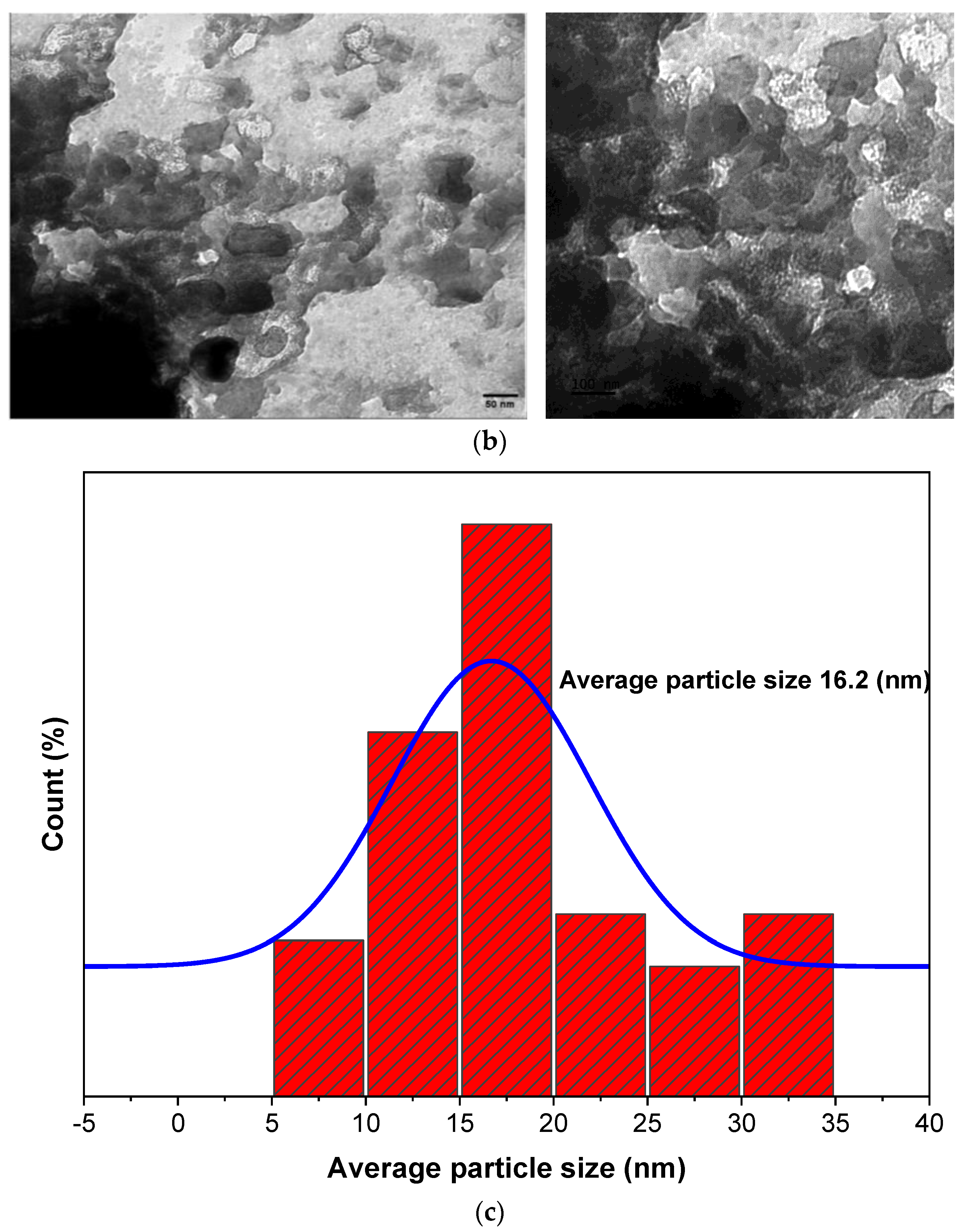
3.5. TEM Analysis
3.6. SEM Analysis
3.7. EDX Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwesh, O.M.; Ali, S.S.; Matter, I.A.; Elsamahy, T. Nanotextiles waste management: Controlling of release and remediation of wastes. In Nanosensors and Nanodevices for Smart Multifunctional Textiles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–286. [Google Scholar]
- Kumari, S.; Sarkar, L. A review on nanoparticles: Structure, classification, synthesis & applications. J. Sci. Res. 2021, 65, 42–46. [Google Scholar]
- Madkour, L.H.; Madkour, L.H. Processing of nanomaterials (NMs). In Nanoelectronic Materials; Springer: Berlin/Heidelberg, Germany, 2019; pp. 309–353. [Google Scholar]
- Subudhi, S.; Bisht, V.; Batta, N.; Pathak, M.; Devi, A.; Lal, B. Purification and characterization of exopolysaccharide bioflocculant produced by heavy metal resistant Achromobacter xylosoxidans. Carbohydr. Polym. 2016, 137, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, G.; Cheng, M.; Zhang, G.; Chen, S.; Liu, Y.; Li, Z.; Xue, W.; Lei, L.; Xiao, R. Optimal preparation of catalytic metal-organic framework derivatives and their efficient application in advanced oxidation processes. Chem. Eng. J. 2021, 421, 127817. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E. Plant-mediated synthesis of iron oxide nanoparticles and evaluation of the antimicrobial activity: A review. Mini-Rev. Org. Chem. 2021, 18, 725–734. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Acharya, B.S.; Kharel, G. Acid mine drainage from coal mining in the United States–An overview. J. Hydrol. 2020, 588, 125061. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchem. J. 2020, 152, 104296. [Google Scholar] [CrossRef]
- Tasharrofi, S.; Rouzitalab, Z.; Maklavany, D.M.; Esmaeili, A.; Rabieezadeh, M.; Askarieh, M.; Rashidi, A.; Taghdisian, H. Adsorption of cadmium using modified zeolite-supported nanoscale zero-valent iron composites as a reactive material for PRBs. Sci. Total Environ. 2020, 736, 139570. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. A Comparative study between Bimetallic Iron@ copper nanoparticles with iron and copper nanoparticles synthesized using a bioflocculant: Their applications and biosafety. Processes 2020, 8, 1125. [Google Scholar] [CrossRef]
- Shende, A.P.; MITRA, N. Green synthesis of iron nanoparticles using bioflocculant extracted from okra (Abelmoschus esculentus (L) Moench) and its application towards elimination of toxic metals from wastewater: A statistical approach. J. Water Environ. Nanotechnol. 2021, 6, 338–355. [Google Scholar]
- Karnena, M.K.; Saritha, V. Natural coagulants for the treatment of water and wastewater: A futuristic option for sustainable water clarification. Recent Innov. Chem. Eng. (Former. Recent Pat. Chem. Eng.) 2021, 14, 120–147. [Google Scholar]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 2. [Google Scholar]
- Das, N.; Shende, A.P.; Keerthana, G.; Mandal, S.K. Applications of microbial bioflocculants for environmental remediation: An overview. Res. J. Pharm. Technol. 2022, 15, 1883–1890. [Google Scholar]
- Tsilo, P.H.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V. Isolation and optimization of culture conditions of a bioflocculant-producing fungi from Kombucha tea SCOBY. Microbiol. Res. 2021, 12, 950–966. [Google Scholar] [CrossRef]
- Martínez Leal, J.; Valenzuela Suárez, L.; Jayabalan, R.; Huerta Oros, J.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA-J. Food 2018, 16, 390–399. [Google Scholar]
- Chen, C.; Liu, B. Changes in major components of tea fungus metabolites during prolonged fermentation. J. Appl. Microbiol. 2000, 89, 834–839. [Google Scholar]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Ngema, S.; Basson, A.; Maliehe, T. Synthesis, characterization and application of polyacrylamide grafted bioflocculant. Phys. Chem. Earth Parts A/B/C 2020, 115, 102821. [Google Scholar]
- Gottimukkala, K.; Harika, R.; Zamare, D. Green synthesis of iron nanoparticles using green tea leaves extract. J. Nanomed. Biother. Discov. 2017, 7, 151. [Google Scholar]
- Muthulakshmi, L.; Rajalu, A.V.; Kaliaraj, G.S.; Siengchin, S.; Parameswaranpillai, J.; Saraswathi, R. Preparation of cellulose/copper nanoparticles bionanocomposite films using a bioflocculant polymer as reducing agent for antibacterial and anticorrosion applications. Compos. Part B Eng. 2019, 175, 107177. [Google Scholar]
- More, T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Üstün, E.; Önbaş, S.C.; Çelik, S.K.; Ayvaz, M.Ç.; Şahin, N. Green synthesis of iron oxide nanoparticles by using Ficus carica leaf extract and its antioxidant activity. Biointerface Res. Appl. Chem. 2022, 12, 2108–2116. [Google Scholar]
- Katata-Seru, L.; Moremedi, T.; Aremu, O.S.; Bahadur, I. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq. 2018, 256, 296–304. [Google Scholar]
- Tyagi, P.K.; Gupta, S.; Tyagi, S.; Kumar, M.; Pandiselvam, R.; Daştan, S.D.; Sharifi-Rad, J.; Gola, D.; Arya, A. Green synthesis of iron nanoparticles from spinach leaf and banana peel aqueous extracts and evaluation of antibacterial potential. J. Nanomater. 2021, 2021, 1–11. [Google Scholar]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar]
- Mystrioti, C.; Sparis, D.; Papasiopi, N.; Xenidis, A.; Dermatas, D.; Chrysochoou, M. Assessment of polyphenol coated nano zero valent iron for hexavalent chromium removal from contaminated waters. Bull. Environ. Contam. Toxicol. 2015, 94, 302–307. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Chin, J.E. Biogenic synthesis of iron nanoparticles from apple peel extracts for decolorization of malachite green dye. Water Air Soil Pollut. 2020, 231, 278. [Google Scholar]
- Roy, A.; Singh, V.; Sharma, S.; Ali, D.; Azad, A.K.; Kumar, G.; Emran, T.B. Antibacterial and dye degradation activity of green synthesized iron nanoparticles. J. Nanomater. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of iron nanoparticles by various tea extracts: Comparative study of the reactivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 295–301. [Google Scholar] [CrossRef] [PubMed]
- El-Shahawi, M.; Hamza, A.; Bahaffi, S.; Al-Sibaai, A.; Abduljabbar, T. Analysis of some selected catechins and caffeine in green tea by high performance liquid chromatography. Food Chem. 2012, 134, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Markova, Z.; Novak, P.; Kaslik, J.; Plachtova, P.; Brazdova, M.; Jancula, D.; Siskova, K.M.; Machala, L.; Marsalek, B.; Zboril, R. Iron (II, III)—polyphenol complex nanoparticles derived from green tea with remarkable ecotoxicological impact. ACS Sustain. Chem. Eng. 2014, 2, 1674–1680. [Google Scholar] [CrossRef]
- Alqudami, A.; Annapoorni, S. Fluorescence from metallic silver and iron nanoparticles prepared by exploding wire technique. Plasmonics 2007, 2, 5–13. [Google Scholar] [CrossRef]
- Saini, J.; Kashyap, D.; Batra, B.; Kumar, S.; Kumar, R.; Malik, D.K. Green synthesis of silver nanoparticles by using Neem (Azadirachta indica) and Amla (Phyllanthus emblica) leaf Extract. Indian J. Appl. Res. 2013, 3, 209–210. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Zare-Hoseinabadi, A.; Berenjian, A.; Ghasemi, Y. Green synthesis and characterization of zero-valent iron nanoparticles using stinging nettle (Urtica dioica) leaf extract. Green Process. Synth. 2017, 6, 469–475. [Google Scholar] [CrossRef]
- Dlamini, N.G. Biosynthesis of Copper Nanoparticles Using a Bioflocculant from Alcaligenis faecalis, Characterization and Its Application. PhD Thesis, University of Zululand, Richards Bay, South Africa, 2017. [Google Scholar]
- Naz, S.; Islam, M.; Tabassum, S.; Fernandes, N.F.; de Blanco, E.J.C.; Zia, M. Green synthesis of hematite (α-Fe2O3) nanoparticles using Rhus punjabensis extract and their biomedical prospect in pathogenic diseases and cancer. J. Mol. Struct. 2019, 1185, 1–7. [Google Scholar] [CrossRef]
- Yu, W.-J.; Hou, P.-X.; Zhang, L.-L.; Li, F.; Liu, C.; Cheng, H.-M. Preparation and electrochemical property of Fe2O3 nanoparticles-filled carbon nanotubes. Chem. Commun. 2010, 46, 8576–8578. [Google Scholar] [CrossRef]
- Balamurugan, M.; Saravanan, S.; Soga, T. Synthesis of iron oxide nanoparticles by using Eucalyptus globulus plant extract. E-J. Surf. Sci. Nanotechnol. 2014, 12, 363–367. [Google Scholar] [CrossRef]
- Anchan, S.; Pai, S.; Sridevi, H.; Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Biogenic synthesis of ferric oxide nanoparticles using the leaf extract of Peltophorum pterocarpum and their catalytic dye degradation potential. Biocatal. Agric. Biotechnol. 2019, 20, 101251. [Google Scholar] [CrossRef]
- Groiss, S.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Structural characterization, antibacterial and catalytic effect of iron oxide nanoparticles synthesised using the leaf extract of Cynometra ramiflora. J. Mol. Struct. 2017, 1128, 572–578. [Google Scholar] [CrossRef]
- Jagathesan, G.; Rajiv, P. Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity. Biocatal. Agric. Biotechnol. 2018, 13, 90–94. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Maaza, M. Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev. 2017, 10, 186–201. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. Biosynthesis of bioflocculant passivated copper nanoparticles, characterization and application. Phys. Chem. Earth Parts A/B/C 2020, 118, 102898. [Google Scholar] [CrossRef]
- Hema, M.; Arasi, A.Y.; Tamilselvi, P.; Anbarasan, R. Titania nanoparticles synthesized by sol-gel technique. Chem. Sci. Trans. 2013, 2, 239–245. [Google Scholar] [CrossRef]
- Yadav, J.; Kumar, S.; Budhwar, L.; Yadav, A.; Yadav, M. Characterization and antibacterial activity of synthesized silver and iron nanoparticles using Aloe vera. J. Nanomed. Nanotechnol. 2016, 7, 2. [Google Scholar]
- Xu, S.; Lin, Y.; Huang, J.; Li, Z.; Xu, X.; Zhang, L. Construction of high strength hollow fibers by self-assembly of a stiff polysaccharide with short branches in water. J. Mater. Chem. A 2013, 1, 4198–4206. [Google Scholar] [CrossRef]
- Nguyen, T.-D. From formation mechanisms to synthetic methods toward shape-controlled oxide nanoparticles. Nanoscale 2013, 5, 9455–9482. [Google Scholar] [CrossRef]
- Naseem, T.; Farrukh, M.A. Antibacterial activity of green synthesis of iron nanoparticles using Lawsonia inermis and Gardenia jasminoides leaves extract. J. Chem. 2015, 2015, 912342. [Google Scholar] [CrossRef]
- Nahari, M.H.; Al Ali, A.; Asiri, A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J. Green Synthesis and Characterization of Iron Nanoparticles Synthesized from Aqueous Leaf Extract of Vitex leucoxylon and Its Biomedical Applications. Nanomaterials 2022, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, J.; Wang, Y. A novel technique by the citrate pyrolysis for preparation of iron oxide nanoparticles. Mater. Sci. Eng. B 2000, 77, 207–209. [Google Scholar] [CrossRef]
- Badni, N.; Benheraoua, F.; Tadjer, B.; Boudjemaa, A.; El Hameur, H.; Bachari, K. Green synthesis of α-Fe2O3 nanoparticles using Roman nettle. In Proceedings of the Third International Conference on Energy, Materials, Applied Energetics and Pollution ICEMAEP2016, Constantine, Algeria, 30–31 October 2016. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsilo, P.H.; Basson, A.K.; Ntombela, Z.G.; Dlamini, N.G.; Pullabhotla, V.S.R.R. Synthesis and Characterization of Iron Nanoparticles from a Bioflocculant Produced by Pichia kudriavzevii Isolated from Kombucha Tea SCOBY. Bioengineering 2024, 11, 1091. https://doi.org/10.3390/bioengineering11111091
Tsilo PH, Basson AK, Ntombela ZG, Dlamini NG, Pullabhotla VSRR. Synthesis and Characterization of Iron Nanoparticles from a Bioflocculant Produced by Pichia kudriavzevii Isolated from Kombucha Tea SCOBY. Bioengineering. 2024; 11(11):1091. https://doi.org/10.3390/bioengineering11111091
Chicago/Turabian StyleTsilo, Phakamani H., Albertus K. Basson, Zuzingcebo G. Ntombela, Nkosinathi G. Dlamini, and V. S. R. Rajasekhar Pullabhotla. 2024. "Synthesis and Characterization of Iron Nanoparticles from a Bioflocculant Produced by Pichia kudriavzevii Isolated from Kombucha Tea SCOBY" Bioengineering 11, no. 11: 1091. https://doi.org/10.3390/bioengineering11111091
APA StyleTsilo, P. H., Basson, A. K., Ntombela, Z. G., Dlamini, N. G., & Pullabhotla, V. S. R. R. (2024). Synthesis and Characterization of Iron Nanoparticles from a Bioflocculant Produced by Pichia kudriavzevii Isolated from Kombucha Tea SCOBY. Bioengineering, 11(11), 1091. https://doi.org/10.3390/bioengineering11111091







