A Co-Culture System for Studying Cellular Interactions in Vascular Disease
Abstract
1. Introduction
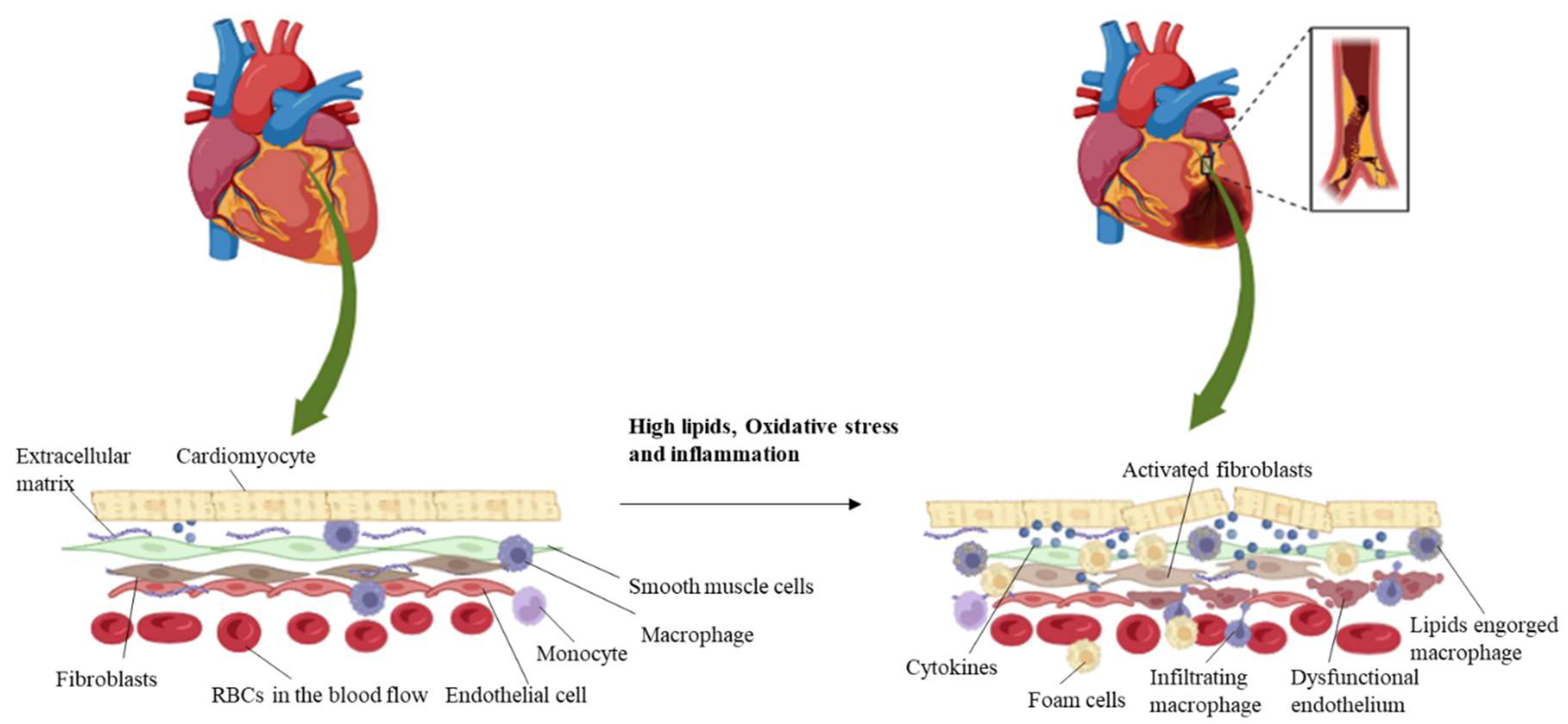
1.1. Role of Cellular Interactions in Vascular Disease
1.2. Endothelial Cells (ECs)
1.3. Smooth Muscle Cells (SMCs)
1.4. Immune Cells
1.5. Fibroblasts
1.6. Platelets
1.7. Cardiomyocytes (CMs)
1.8. Extracellular Matrix (ECM)
1.9. Pericytes
2. Interaction Dynamics in Vascular Disease
2.1. Endothelial–Smooth Muscle Cell Interaction
2.2. Endothelial–Immune Cell Interaction
2.3. Endothelial–Fibroblast Interactions
2.4. Smooth Muscle Cell–Immune Cell Interaction
2.5. Immune Cell–Fibroblast Interaction
2.6. Endothelial Cell and Cardiomyocyte Interactions
2.7. Endothelial Cell–Platelet Interactions
2.8. Endothelial Cell–Pericyte Interaction
3. Importance of In Vitro Co-Culture Systems
3.1. Mimicking Physiological Conditions
3.2. Studying Cell–Cell Communication
3.3. Modeling Disease Pathogenesis
3.4. Testing Drug Efficacy
3.5. Uncovering Novel Therapeutic Targets
3.6. Personalized Medicine and Tissue Engineering
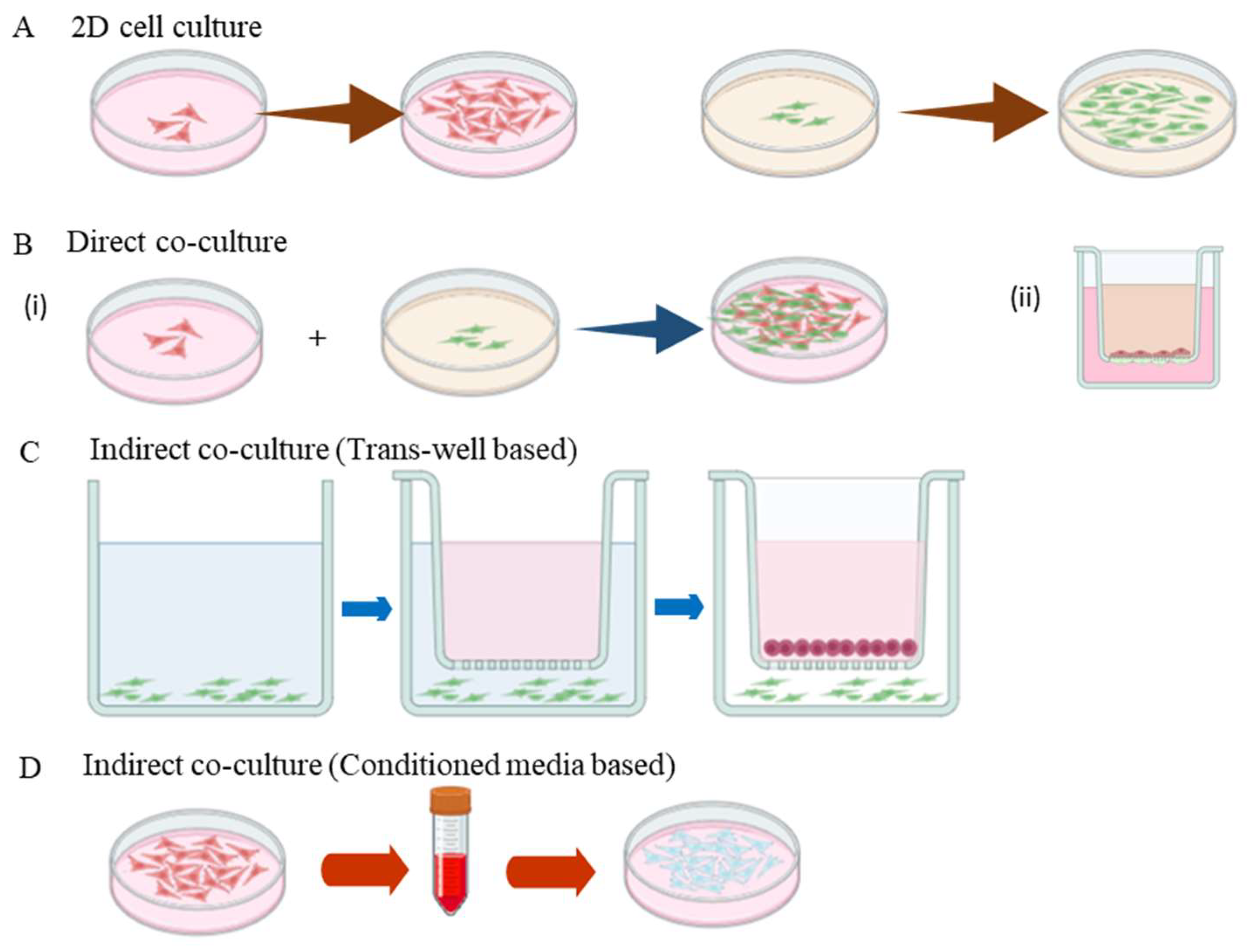
4. Types of Co-Culture Systems
4.1. Direct Co-Culture
4.2. Indirect (Trans-Well) Co-Culture Systems
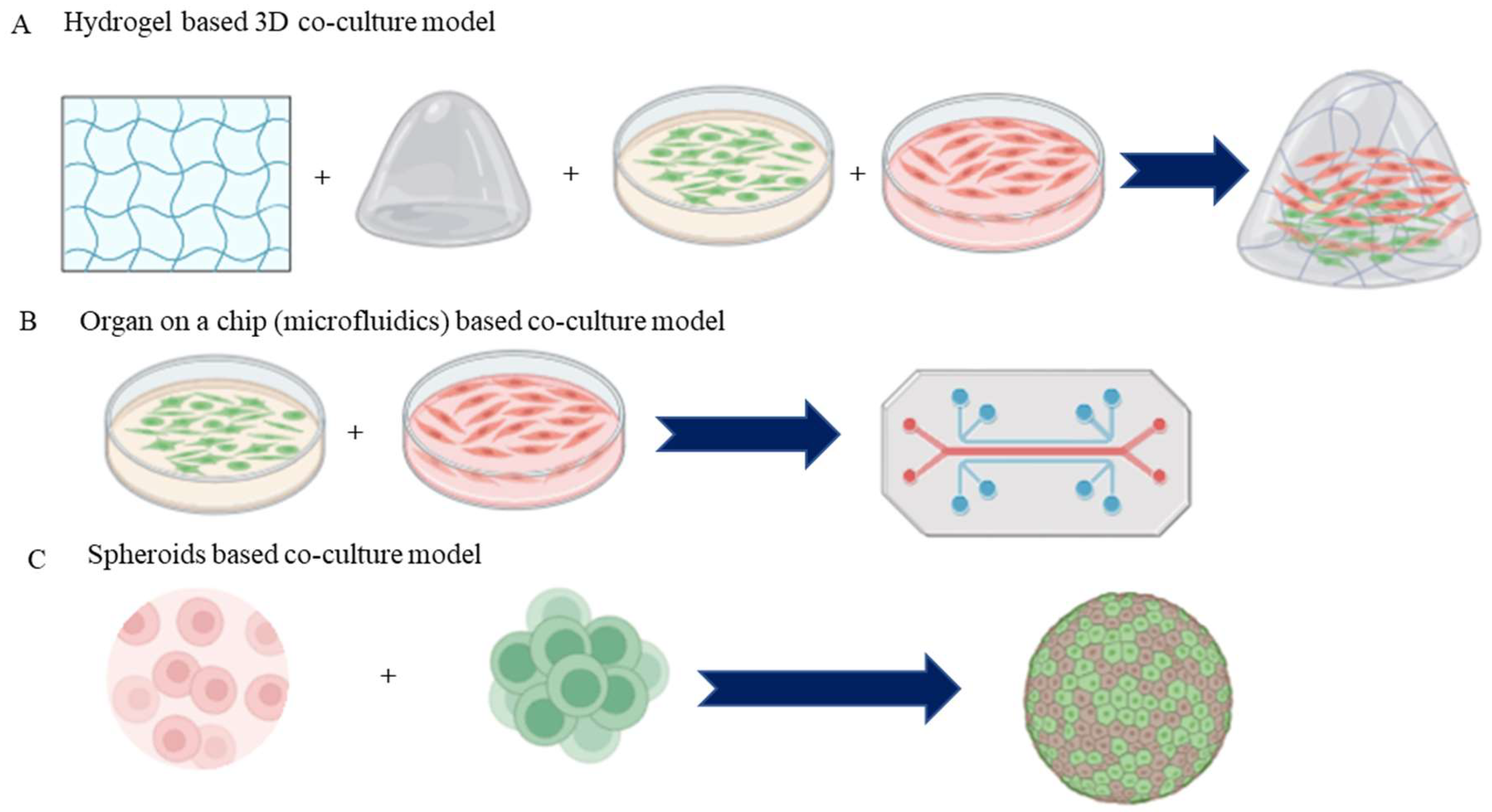
4.3. 3D Co-Culture Systems
4.4. Organ-on-a-Chip Co-Culture Systems
4.5. Spheroid and Organoid Co-Culture Systems
4.5.1. Spheroid Co-Culture Systems
4.5.2. Organoid Co-Culture Systems
4.6. Microcarrier-Based Co-Culture Systems
5. Applications of Co-Culture Systems in Drug Testing and Therapeutic Development: Screening Potential Therapeutics
5.1. Developing Targeted Therapies
5.2. Modeling the Effects of Existing Drugs
5.3. Investigating Cell Type-Specific Gene Expression In Vivo
6. Limitations and Challenges of Co-Culture Systems
7. Future Directions and Emerging Trends
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; As, A.K.; EngİN, M.; Koca, N.; Cander, S. Cardiovascular diseases and diabetes mellitus. Eur. Res. J. 2022, 8, 541–549. [Google Scholar] [CrossRef]
- Wolf, D.; Zirlik, A.; Ley, K. Beyond vascular inflammation—recent advances in understanding atherosclerosis. Cell. Mol. Life Sci. 2015, 72, 3853–3869. [Google Scholar] [CrossRef] [PubMed]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes. Nat. Rev. Cardiol. 2019, 17, 52–63. [Google Scholar] [CrossRef]
- Abdelbaky, A.; Corsini, E.; Figueroa, A.L.; Subramanian, S.; Fontanez, S.; Emami, H.; Hoffmann, U.; Narula, J.; Tawakol, A. Early aortic valve inflammation precedes calcification: A longitudinal FDG-PET/CT study. Atherosclerosis 2015, 238, 165–172. [Google Scholar] [CrossRef]
- Sullivan, G.W.; Sarembock, I.J.; Linden, J. The role of inflammation in vascular diseases. J. Leukoc. Biol. 2000, 67, 591–602. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arteroscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Kirkpatrick, C.J.; Fuchs, S.; Unger, R.E. Co-culture systems for vascularization—Learning from nature. Adv. Drug Deliv. Rev. 2011, 63, 291–299. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Lee, S.; Chen, T.T.; Barber, C.L.; Jordan, M.C.; Murdock, J.; Desai, S.; Ferrara, N.; Nagy, A.; Roos, K.P.; Iruela-Arispe, M.L. Autocrine VEGF Signaling Is Required for Vascular Homeostasis. Cell 2007, 130, 691–703. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.; Hong, L. Endothelial Reprogramming in Atherosclerosis. Bioengineering 2024, 11, 325. [Google Scholar] [CrossRef]
- Ashraf, J.V.; Zen, A.A.H. Role of Vascular Smooth Muscle Cell Phenotype Switching in Arteriogenesis. Int. J. Mol. Sci. 2021, 22, 10585. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, X.; Xia, Y.; Mao, L. An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis. Cell. Mol. Life Sci. 2021, 79, 1–19. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef]
- Markella, P.; Barbara, D. Extracellular matrix synthesis in vascular disease: Hypertension, and atherosclerosis. J. Biomed. Res. 2014, 28, 25–39. [Google Scholar] [CrossRef]
- Čejková, S.; Lesná, I.K.; Poledne, R. Monocyte adhesion to the endothelium is an initial stage of atherosclerosis development. Cor et Vasa 2015, 58, e419–e425. [Google Scholar] [CrossRef]
- Hulsmans, M.; Holvoet, P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J. Cell. Mol. Med. 2010, 14, 70–78. [Google Scholar] [CrossRef]
- Lintermans, L.L.; Stegeman, C.A.; Heeringa, P.; Abdulahad, W.H. T Cells in Vascular Inflammatory Diseases. Front. Immunol. 2014, 5, 504. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K. Role of Platelets in Atherothrombosis. Am. J. Cardiol. 2009, 103, 4A–10A. [Google Scholar] [CrossRef] [PubMed]
- Hundelshausen, P.V.; Lievens, D. Platelets in atherosclerosis. Thromb. Haemost. 2017, 106, 827–838. [Google Scholar] [CrossRef]
- Lebas, H.; Yahiaoui, K.; Martos, R.; Boulaftali, Y. Platelets Are at the Nexus of Vascular Diseases. Front. Cardiovasc. Med. 2019, 6, 132. [Google Scholar] [CrossRef]
- Severs, N.J. The cardiac muscle cell. BioEssays 2000, 22, 188–199. [Google Scholar] [CrossRef]
- Ingber, D.E. Mechanical Signaling and the Cellular Response to Extracellular Matrix in Angiogenesis and Cardiovascular Physiology. Circ. Res. 2002, 91, 877–887. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix Metalloproteinases in Vascular Remodeling and Atherogenesis. Circulation Research 2002, 90, 251–262. [Google Scholar] [CrossRef]
- Simões, G.; Pereira, T.; Caseiro, A. Matrix metaloproteinases in vascular pathology. Microvasc. Res. 2022, 143, 104398. [Google Scholar] [CrossRef]
- Caporarello, N.; D’angeli, F.; Cambria, M.T.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; Longo, A.; Giurdanella, G.; Anfuso, C.D.; et al. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019, 20, 6351. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Persidsky, Y.; Hill, J.; Zhang, M.; Dykstra, H.; Winfield, M.; Reichenbach, N.L.; Potula, R.; Mukherjee, A.; Ramirez, S.H.; Rom, S. Dysfunction of brain pericytes in chronic neuroinflammation. J. Cereb. Blood Flow Metab. 2015, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhou, T.; Pilli, V.S.S.; Phan, N.M.; Wang, Q.; Gupta, K.; Liu, Z.; Sheibani, N.; Liu, B. Novel Paracrine Functions of Smooth Muscle Cells in Supporting Endothelial Regeneration Following Arterial Injury. Circ. Res. 2019, 124, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef]
- Casscells, W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation 1992, 86, 723–729. [Google Scholar] [CrossRef]
- Simionescu, M. Implications of Early Structural-Functional Changes in the Endothelium for Vascular Disease. Arter. Thromb. Vasc. Biol. 2007, 27, 266–274. [Google Scholar] [CrossRef]
- Bkaily, G.; Abdallah, N.A.; Simon, Y.; Jazzar, A.; Jacques, D. Vascular smooth muscle remodeling in health and disease. Can. J. Physiol. Pharmacol. 2021, 99, 171–178. [Google Scholar] [CrossRef]
- Bkaily, G.; Jacques, D. Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1998. [Google Scholar] [CrossRef]
- Pirvulescu, M.M.; Gan, A.M.; Stan, D.; Simion, V.; Calin, M.; Butoi, E.; Manduteanu, I. Subendothelial resistin enhances monocyte transmigration in a co-culture of human endothelial and smooth muscle cells by mechanisms involving fractalkine, MCP-1 and activation of TLR4 and Gi/o proteins signaling. Int. J. Biochem. Cell Biol. 2014, 50, 29–37. [Google Scholar] [CrossRef]
- Mause, S.F.; Ritzel, E.; Deck, A.; Vogt, F.; Liehn, E.A. Endothelial Progenitor Cells Modulate the Phenotype of Smooth Muscle Cells and Increase Their Neointimal Accumulation Following Vascular Injury. Thromb. Haemost. 2021, 122, 456–469. [Google Scholar] [CrossRef]
- Qiu, J.; Zheng, Y.; Hu, J.; Liao, D.; Gregersen, H.; Deng, X.; Fan, Y.; Wang, G. Biomechanical regulation of vascular smooth muscle cell functions: From in vitro to in vivo understanding. J. R. Soc. Interface 2014, 11, 20130852. [Google Scholar] [CrossRef] [PubMed]
- Lavender, M.D.; Pang, Z.; Wallace, C.S.; Niklason, L.E.; Truskey, G.A. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials 2005, 26, 4642–4653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Chen, Y.; Wang, Y.; You, Y.; Yang, Q.; Weng, X.; Li, Q.; Zhu, X.; Zhou, B.; et al. Establishment of an interleukin-1β-induced inflammation-activated endothelial cell-smooth muscle cell-mononuclear cell co-culture model and evaluation of the anti-inflammatory effects of tanshinone IIA on atherosclerosis. Mol. Med. Rep. 2015, 12, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Deng, J.; Hao, S.; Wang, B. A Potential In Vitro 3D Cell Model to Study Vascular Diseases by Simulating the Vascular Wall Microenvironment and Its Application. Life 2022, 12, 427. [Google Scholar] [CrossRef]
- Fernandes, A.; Miéville, A.; Grob, F.; Yamashita, T.; Mehl, J.; Hosseini, V.; Emmert, M.Y.; Falk, V.; Vogel, V. Endothelial-Smooth Muscle Cell Interactions in a Shear-Exposed Intimal Hyperplasia on-a-Dish Model to Evaluate Therapeutic Strategies. Adv. Sci. 2022, 9, 2202317. [Google Scholar] [CrossRef]
- Lilly, B. We Have Contact: Endothelial Cell-Smooth Muscle Cell Interactions. Physiology 2014, 29, 234–241. [Google Scholar] [CrossRef]
- Torzewski, M.; Dahm, M.; Ochsenhirt, V.; Lehr, H.-A.; Lackner, K.J.; Vahl, C.-F.; Dorweiler, B. A novel in vitro model for the study of plaque development in atherosclerosis. Thromb. Haemost. 2006, 95, 182–189. [Google Scholar] [CrossRef]
- Wiejak, J.; Murphy, F.A.; Maffia, P.; Yarwood, S.J. Vascular smooth muscle cells enhance immune/vascular interplay in a 3-cell model of vascular inflammation. Sci. Rep. 2023, 13, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Li, B.; Li, Y.; Ye, K.; Qi, J.; Wang, Y. Macrophages Aggravate Hypoxia-Induced Cardiac Microvascular Endothelial Cell Injury via Peroxynitrite: Protection by Tongxinluo. Cell Commun. Adhes. 2015, 22, 39–47. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.-J.; Wang, X.; Xu, L.; Yang, X.-C.; Zhao, W.-S. PKC-Mediated Endothelin-1 Expression in Endothelial Cell Promotes Macrophage Activation in Atherogenesis. Am. J. Hypertens. 2019, 32, 880–889. [Google Scholar] [CrossRef]
- Tattersall, I.W.; Du, J.; Cong, Z.; Cho, B.S.; Klein, A.M.; Dieck, C.L.; Chaudhri, R.A.; Cuervo, H.; Herts, J.H.; Kitajewski, J. In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment. Angiogenesis 2016, 19, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.-H.; Lee, H.-S.; Seomun, Y.; Lee, Y.; Lee, K.-W. Monocyte-endothelium-smooth muscle cell interaction in co-culture: Proliferation and cytokine productions in response to advanced glycation end products. Biochim. Biophys. Acta (BBA) Gen. Subj. 2011, 1810, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, W.; Liu, G.; Cai, W.; Millard, R.W.; Wang, Y.; Chang, J.; Peng, T.; Fan, G.-C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014, 74, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.-K.; Fan, M.; Wang, Q. Curcumin Reduces Hypoxia/Reperfusion Injury of Cardiomyocytes by Stimulating Vascular Endothelial Cells to Secrete FGF2. Comb. Chem. High Throughput Screen. 2024, 27, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Kongpol, K.; Nernpermpisooth, N.; Prompunt, E.; Kumphune, S. Endothelial-Cell-Derived Human Secretory Leukocyte Protease Inhibitor (SLPI) Protects Cardiomyocytes against Ischemia/Reperfusion Injury. Biomolecules 2019, 9, 678. [Google Scholar] [CrossRef]
- Leucker, T.M.; Ge, Z.-D.; Procknow, J.; Liu, Y.; Shi, Y.; Bienengraeber, M.; Warltier, D.C.; Kersten, J.R. Impairment of Endothelial-Myocardial Interaction Increases the Susceptibility of Cardiomyocytes to Ischemia/Reperfusion Injury. PLoS ONE 2013, 8, e70088. [Google Scholar] [CrossRef]
- Legein, B.; Temmerman, L.; Biessen, E.A.L.; Lutgens, E. Inflammation and immune system interactions in atherosclerosis. Cell. Mol. Life Sci. 2013, 70, 3847–3869. [Google Scholar] [CrossRef]
- Nguyen, J.; Lin, Y.-Y.; Gerecht, S. The next generation of endothelial differentiation: Tissue-specific ECs. Cell Stem Cell 2021, 28, 1188–1204. [Google Scholar] [CrossRef]
- Kume, N.; Cybulsky, M.I.; Gimbrone, M.A., Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Investig. 1992, 90, 1138–1144. [Google Scholar] [CrossRef]
- Nakashima, Y.; Raines, E.W.; Plump, A.S.; Breslow, J.L.; Ross, R. Upregulation of VCAM-1 and ICAM-1 at Atherosclerosis-Prone Sites on the Endothelium in the ApoE-Deficient Mouse. Arter. Thromb. Vasc. Biol. 1998, 18, 842–851. [Google Scholar] [CrossRef]
- Imaizumi, T.; Itaya, H.; Fujita, K.; Kudoh, D.; Kudoh, S.; Mori, K.; Fujimoto, K.; Matsumiya, T.; Yoshida, H.; Satoh, K. Expression of Tumor Necrosis Factor-α in Cultured Human Endothelial Cells Stimulated with Lipopolysaccharide or Interleukin-1α. Arter. Thromb. Vasc. Biol. 2000, 20, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Certo, M.; Elkafrawy, H.; Pucino, V.; Cucchi, D.; Cheung, K.C.; Mauro, C. Endothelial cell and T-cell crosstalk: Targeting metabolism as a therapeutic approach in chronic inflammation. Br. J. Pharmacol. 2020, 178, 2041–2059. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Schriewer, J.; Tang, M.; Marlin, J.; Taylor, F.; Shohet, R.V.; Konorev, E.A. The TGF-β pathway mediates doxorubicin effects on cardiac endothelial cells. J. Mol. Cell. Cardiol. 2015, 90, 129–138. [Google Scholar] [CrossRef]
- Luu, R.J.; Hoefler, B.C.; Gard, A.L.; Ritenour, C.R.; Rogers, M.T.; Kim, E.S.; Coppeta, J.R.; Cain, B.P.; Isenberg, B.C.; Azizgolshani, H.; et al. Fibroblast activation in response to TGFβ1 is modulated by co-culture with endothelial cells in a vascular organ-on-chip platform. Front. Mol. Biosci. 2023, 10, 1160851. [Google Scholar] [CrossRef]
- Enzerink, A.; Vaheri, A. Fibroblast activation in vascular inflammation. J. Thromb. Haemost. 2011, 9, 619–626. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Gomez-Lazaro, M.; Ramalho, C.; Granja, P.L.; Soares, R.; Guerreiro, S.G. Fibroblast-Endothelial Partners for Vascularization Strategies in Tissue Engineering. Tissue Eng. Part A 2015, 21, 1055–1065. [Google Scholar] [CrossRef]
- McGettrick, H.M.; Smith, E.; Filer, A.; Kissane, S.; Salmon, M.; Buckley, C.D.; Rainger, G.E.; Nash, G.B. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur. J. Immunol. 2009, 39, 113–125. [Google Scholar] [CrossRef]
- Isali, I.; McClellan, P.; Wong, T.R.; Hijaz, S.; Fletcher, D.R.; Liu, G.; Bonfield, T.L.; Anderson, J.M.; Hijaz, A.; Akkus, O. Differential effects of macrophage subtype-specific cytokines on fibroblast proliferation and endothelial cell function in co-culture system. J. Biomed. Mater. Res. Part A 2024. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013, 9, 6981–6991. [Google Scholar] [CrossRef]
- Sukmana, I.; Vermette, P. The effects of co-culture with fibroblasts and angiogenic growth factors on microvascular maturation and multi-cellular lumen formation in HUVEC-oriented polymer fibre constructs. Biomaterials 2010, 31, 5091–5099. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, R.L.; Black, L.D. Cardiac Fibroblasts Support Endothelial Cell Proliferation and Sprout Formation but not the Development of Multicellular Sprouts in a Fibrin Gel Co-Culture Model. Ann. Biomed. Eng. 2014, 42, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Eckermann, C.W.; Lehle, K.; Schmid, S.A.; Wheatley, D.N.; Kunz-Schughart, L.A. Characterization and modulation of fibroblast/endothelial cell co-cultures for the in vitro preformation of three-dimensional tubular networks. Cell Biol. Int. 2011, 35, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Dikici, S.; Claeyssens, F.; MacNeil, S. Pre-Seeding of Simple Electrospun Scaffolds with a Combination of Endothelial Cells and Fibroblasts Strongly Promotes Angiogenesis. Tissue Eng. Regen. Med. 2020, 17, 445–458. [Google Scholar] [CrossRef]
- Rong, J.X.; Shapiro, M.; Trogan, E.; Fisher, E.A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl. Acad. Sci. USA 2003, 100, 13531–13536. [Google Scholar] [CrossRef]
- Feil, S.; Fehrenbacher, B.; Lukowski, R.; Essmann, F.; Schulze-Osthoff, K.; Schaller, M.; Feil, R. Transdifferentiation of Vascular Smooth Muscle Cells to Macrophage-Like Cells During Atherogenesis. Circ. Res. 2014, 115, 662–667. [Google Scholar] [CrossRef]
- He, X.; Lian, Z.; Yang, Y.; Wang, Z.; Fu, X.; Liu, Y.; Li, M.; Tian, J.; Yu, T.; Xin, H. Long Non-coding RNA PEBP1P2 Suppresses Proliferative VSMCs Phenotypic Switching and Proliferation in Atherosclerosis. Mol. Ther. Nucleic Acids 2020, 22, 84–98. [Google Scholar] [CrossRef]
- Peppel, K.; Zhang, L.; Orman, E.S.; Hagen, P.-O.; Amalfitano, A.; Brian, L.; Freedman, N.J. Activation of vascular smooth muscle cells by TNF and PDGF: Overlapping and complementary signal transduction mechanisms. Cardiovasc. Res. 2005, 65, 674–682. [Google Scholar] [CrossRef]
- Zhang, M.; Xin, W.; Yu, Y.; Yang, X.; Ma, C.; Zhang, H.; Liu, Y.; Zhao, X.; Guan, X.; Wang, X.; et al. Programmed death-ligand 1 triggers PASMCs pyroptosis and pulmonary vascular fibrosis in pulmonary hypertension. J. Mol. Cell. Cardiol. 2019, 138, 23–33. [Google Scholar] [CrossRef]
- van Amerongen, M.J.; Harmsen, M.C.; van Rooijen, N.; Petersen, A.H.; van Luyn, M.J.A. Macrophage Depletion Impairs Wound Healing and Increases Left Ventricular Remodeling after Myocardial Injury in Mice. Am. J. Pathol. 2007, 170, 818–829. [Google Scholar]
- Waltenberger, J.; Lundin, L.; Oberg, K.; Wilander, E.; Miyazono, K.; Heldin, C.H.; Funa, K. Involvement of transforming growth factor-beta in the formation of fibrotic lesions in carcinoid heart disease. Am. J. Pathol. 1993, 142, 71–78. [Google Scholar] [PubMed]
- Jiang, S.; Li, T.; Yang, Z.; Yi, W.; Di, S.; Sun, Y.; Wang, D.; Yang, Y. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res. Rev. 2017, 38, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, R.; Zhai, R.; Yang, S.; Peng, T.; Zheng, F.; Shen, Y.; Li, M.; Li, L. Matrix stiffness regulates macrophage polarization in atherosclerosis. Pharmacol. Res. 2022, 179, 106236. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Hsieh, P.C.; Davis, M.E.; Lisowski, L.K.; Lee, R.T. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu. Rev. Physiol. 2006, 68, 51–66. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Kaplan, Z.S.; Jackson, S.P. The Role of Platelets in Atherothrombosis. Hematol. 2011, 2011, 51–61. [Google Scholar] [CrossRef]
- Cerletti, C.; De Gaetano, G.; Lorenzet, R. Platelet-Leukocyte Interactions: Multiple Links between Inflammation, Blood Coagulation and Vascular Risk. Mediterr. J. Hematol. Infect. Dis. 2010, 2, e2010023. [Google Scholar] [CrossRef]
- Huilcaman, R.; Veliz-Olivos, N.; Venturini, W.; Olate-Briones, A.; Treuer, A.V.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Endothelial transmigration of platelets depends on soluble factors released by activated endothelial cells and monocytes. Platelets 2021, 32, 1113–1119. [Google Scholar] [CrossRef]
- Martin, A.; Bailie, J.; Robson, T.; McKeown, S.; Al-Assar, O.; McFarland, A.; Hirst, D. Retinal Pericytes Control Expression of Nitric Oxide Synthase and Endothelin-1 in Microvascular Endothelial Cells. Microvasc. Res. 2000, 59, 131–139. [Google Scholar] [CrossRef]
- McIlroy, M.; Orourke, M.; McKeown, S.R.; Hirst, D.G.; Robson, T. Pericytes influence endothelial cell growth characteristics: Role of plasminogen activator inhibitor type 1 (PAI-1). Cardiovasc. Res. 2006, 69, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Koutsakis, C.; Piperigkou, Z.; Karamanos, N.K. Recreating the extracellular matrix: Novel 3D cell culture platforms in cancer research. FEBS J. 2023, 290, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef] [PubMed]
- Peiró, C.; Redondo, J.; Rodríguez-Martínez, M.A.; Angulo, J.; Marín, J.; Sánchez-Ferrer, C.F. Influence of Endothelium on Cultured Vascular Smooth Muscle Cell Proliferation. Hypertension 1995, 25, 748–751. [Google Scholar] [CrossRef]
- Ramel, D.; Gayral, S.; Sarthou, M.-K.; Augé, N.; Nègre-Salvayre, A.; Laffargue, M. Immune and Smooth Muscle Cells Interactions in Atherosclerosis: How to Target a Breaking Bad Dialogue? Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Dubland, J.A.; Allahverdian, S.; Asonye, E.; Sahin, B.; Jaw, J.E.; Sin, D.D.; Seidman, M.A.; Leeper, N.J.; Francis, G.A. Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 876–887. [Google Scholar] [CrossRef]
- Cai, Q.; Lanting, L.; Natarajan, R.; Meng, L.; Park, J.; Reddy, M.A.; Parmentier, J.-H.; Zhang, C.; Estes, A.; Schaefer, S.; et al. Growth factors induce monocyte binding to vascular smooth muscle cells: Implications for monocyte retention in atherosclerosis. Am. J. Physiol. Physiol. 2004, 287, C707–C714. [Google Scholar] [CrossRef]
- Cai, Q.; Lanting, L.; Natarajan, R. Interaction of Monocytes with Vascular Smooth Muscle Cells Regulates Monocyte Survival and Differentiation Through Distinct Pathways. Arter. Thromb. Vasc. Biol. 2004, 24, 2263–2270. [Google Scholar] [CrossRef]
- Majesky, M.W.; Horita, H.; Ostriker, A.; Lu, S.; Regan, J.N.; Bagchi, A.; Dong, X.R.; Poczobutt, J.; Nemenoff, R.A.; Weiser-Evans, M.C. Differentiated Smooth Muscle Cells Generate a Subpopulation of Resident Vascular Progenitor Cells in the Adventitia Regulated by Klf4. Circ. Res. 2016, 120, 296–311. [Google Scholar] [CrossRef]
- Kenny, H.A.; Lal-Nag, M.; White, E.A.; Shen, M.; Chiang, C.-Y.; Mitra, A.K.; Zhang, Y.; Curtis, M.; Schryver, E.M.; Bettis, S.; et al. Quantitative high throughput screening using a primary human three-dimensional organotypic culture predicts in vivo efficacy. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Farlik, M.; Sheffield, N.C. Multi-Omics of Single Cells: Strategies and Applications. Trends Biotechnol. 2016, 34, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; A Ganio, E.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Nakahama, K.-I. Cellular communications in bone homeostasis and repair. Cell. Mol. Life Sci. 2010, 67, 4001–4009. [Google Scholar] [CrossRef]
- Anderson, J.M.; McNally, A.K. Biocompatibility of implants: Lymphocyte/macrophage interactions. Semin. Immunopathol. 2011, 33, 221–233. [Google Scholar] [CrossRef]
- Wu, M.-H.; Huang, S.-B.; Lee, G.-B. Microfluidic cell culture systems for drug research. Lab Chip 2010, 10, 939–956. [Google Scholar] [CrossRef]
- Gupta, R.K. Adipocytes. Curr. Biol. 2014, 24, R988–R993. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Joshi, A.; Singh, N. Generation of Patterned Cocultures in 2D and 3D: State of the Art. ACS Omega 2023, 8, 34249–34261. [Google Scholar] [CrossRef]
- Monsuur, H.N.; Boink, M.A.; Weijers, E.M.; Roffel, S.; Breetveld, M.; Gefen, A.; Broek, L.J.v.D.; Gibbs, S. Methods to study differences in cell mobility during skin wound healing in vitro. J. Biomech. 2016, 49, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Cheng, H.; Qian, J.; Dai, X.; Huang, Y.; Fan, Y. In vitro fluidic systems: Applying shear stress on endothelial cells. Med. Nov. Technol. Devices 2022, 15. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Cao, T.; Lee, E.H. Directing Stem Cell Differentiation into the Chondrogenic Lineage In Vitro. STEM CELLS 2004, 22, 1152–1167. [Google Scholar] [CrossRef]
- Zhang, Q.; Bu, S.; Sun, J.; Xu, M.; Yao, X.; He, K.; Lai, D. Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef]
- Beilmann, M. Human primary co-culture angiogenesis assay reveals additive stimulation and different angiogenic properties of VEGF and HGF. Cytokine 2004, 26, 178–185. [Google Scholar] [CrossRef]
- Ou, D.-B.; Zeng, D.; Jin, Y.; Liu, X.-T.; Teng, J.-W.; Guo, W.-G.; Wang, H.-T.; Su, F.-F.; He, Y.; Zheng, Q.-S. The Long-Term Differentiation of Embryonic Stem Cells into Cardiomyocytes: An Indirect Co-Culture Model. PLoS ONE 2013, 8, e55233. [Google Scholar] [CrossRef]
- Grellier, M.; Bordenave, L.; Amédée, J. Cell-to-cell communication between osteogenic and endothelial lineages: Implications for tissue engineering. Trends Biotechnol. 2009, 27, 562–571. [Google Scholar] [CrossRef]
- Martinoli, M.-G.; Renaud, J. Development of an Insert Co-culture System of Two Cellular Types in the Absence of Cell-Cell Contact. J. Vis. Exp. 2016, 113, e54356. [Google Scholar]
- Lee, D.; Chambers, M. A co-culture model of the bovine alveolus. F1000Research 2019, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Babaei, S.; Stewart, D.J. Overexpression of endothelial NO synthase induces angiogenesis in a co-culture model. Cardiovasc. Res. 2002, 55, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Frister, A.; Wang, S.; Ludwig, A.; Behr, H.; Pippig, S.; Li, B.; Simm, A.; Hofmann, B.; Pilowski, C.; et al. Interaction of vascular smooth muscle cells and monocytes by soluble factors synergistically enhances IL-6 and MCP-1 production. Am. J. Physiol. Circ. Physiol. 2009, 296, H987–H996. [Google Scholar] [CrossRef]
- Lim, Y.-H.; Kim, Y.-K. Roles of non-coding RNAs in intercellular crosstalk in cardiovascular diseases. Korean J. Physiol. Pharmacol. 2023, 27, 289–298. [Google Scholar] [CrossRef]
- Chiu, J.-J.; Chen, L.-J.; Lee, P.-L.; Lee, C.-I.; Lo, L.-W.; Usami, S.; Chien, S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 2003, 101, 2667–2674. [Google Scholar] [CrossRef]
- Li, X.; Speer, M.Y.; Yang, H.; Bergen, J.; Giachelli, C.M. Vitamin D Receptor Activators Induce an Anticalcific Paracrine Program in Macrophages. Arter. Thromb. Vasc. Biol. 2010, 30, 321–326. [Google Scholar] [CrossRef]
- Weinert, S.; Poitz, D.M.; Auffermann-Gretzinger, S.; Eger, L.; Herold, J.; Medunjanin, S.; Schmeisser, A.; Strasser, R.H.; Braun-Dullaeus, R.C. The lysosomal transfer of LDL/cholesterol from macrophages into vascular smooth muscle cells induces their phenotypic alteration. Cardiovasc. Res. 2012, 97, 544–552. [Google Scholar] [CrossRef]
- Zhu, Y.; Hojo, Y.; Ikeda, U.; Takahashi, M.; Shimada, K. Interaction Between Monocytes and Vascular Smooth Muscle Cells Enhances Matrix Metalloproteinase-1 Production. J. Cardiovasc. Pharmacol. 2000, 36, 152–161. [Google Scholar] [CrossRef]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of Bone-Type Alkaline Phosphatase in Human Vascular Smooth Muscle Cells. Circ. Res. 2002, 91, 9–16. [Google Scholar] [CrossRef]
- Vuorenpää, H.; Valtonen, J.; Penttinen, K.; Koskimäki, S.; Hovinen, E.; Ahola, A.; Gering, C.; Parraga, J.; Kelloniemi, M.; Hyttinen, J.; et al. Gellan gum-gelatin based cardiac models support formation of cellular networks and functional cardiomyocytes. Cytotechnology 2024, 76, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Mostert, D.; Jorba, I.; Groenen, B.G.; Passier, R.; Goumans, M.-J.T.; van Boxtel, H.A.; Kurniawan, N.A.; Bouten, C.V.; Klouda, L. Methacrylated human recombinant collagen peptide as a hydrogel for manipulating and monitoring stiffness-related cardiac cell behavior. iScience 2023, 26, 106423. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, H.; Xu, F.; Wang, X.; Sun, W. Microfluidics in cardiovascular disease research: State of the art and future outlook. Microsystems Nanoeng. 2021, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Kim, Y.-J.; Son, M.-Y.; Oh, M.-S.; Kim, J.; Ryu, B.; Kang, K.-R.; Baek, J.; Chung, G.; Woo, D.H.; et al. Generation of human iPSCs derived heart organoids structurally and functionally similar to heart. Biomaterials 2022, 290, 121860. [Google Scholar] [CrossRef]
- Sahara, M. Recent Advances in Generation of In Vitro Cardiac Organoids. Int. J. Mol. Sci. 2023, 24, 6244. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Maffini, M.V.; Soto, A.; Sonnenschein, C.; Kaplan, D.L. A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function. Biomaterials 2010, 31, 3920–3929. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Moncada-Saucedo, N.K.; Carriquiry-Chequer, P.A.; Valencia-Gómez, L.E.; Matthews, E.R.; Gómez-Flores, V.; Simental-Mendía, M.; Delgado-Gonzalez, P.; Delgado-Gallegos, J.L.; et al. Artificial Scaffolds in Cardiac Tissue Engineering. Life 2022, 12, 1117. [Google Scholar] [CrossRef]
- Patino-Guerrero, A.; Veldhuizen, J.; Zhu, W.; Migrino, R.Q.; Nikkhah, M. Three-dimensional scaffold-free microtissues engineered for cardiac repair. J. Mater. Chem. B 2020, 8, 7571–7590. [Google Scholar] [CrossRef]
- Mathur, A.; Ma, Z.; Loskill, P.; Jeeawoody, S.; Healy, K.E. In vitro cardiac tissue models: Current status and future prospects. Adv. Drug Deliv. Rev. 2016, 96, 203–213. [Google Scholar] [CrossRef]
- Babaliari, E.; Ranella, A.; Stratakis, E. Microfluidic Systems for Neural Cell Studies. Bioengineering 2023, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Jackman, C.P.; Ganapathi, A.M.; Asfour, H.; Qian, Y.; Allen, B.W.; Li, Y.; Bursac, N. Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 2018, 159, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Sayer, S.; Zandrini, T.; Markovic, M.; Van Hoorick, J.; Van Vlierberghe, S.; Baudis, S.; Holnthoner, W.; Ovsianikov, A. Guiding cell migration in 3D with high-resolution photografting. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Sebastián-Jaraba, I.S.; Fernández-Gómez, M.J.; Blázquez-Serra, R.; Sanz-Andrea, S.; Blanco-Colio, L.M.; Méndez-Barbero, N. Modelo de cocultivo 3D in vitro de células endoteliales y vasculares de músculo liso humanas para el estudio del remodelado vascular patológico. Clin. E Investig. En Arter. 2024. [Google Scholar] [CrossRef]
- Beauchamp, P.; Jackson, C.B.; Ozhathil, L.C.; Agarkova, I.; Galindo, C.L.; Sawyer, D.B.; Suter, T.M.; Zuppinger, C. 3D Co-culture of hiPSC-Derived Cardiomyocytes with Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- De Spirito, M.; Palmieri, V.; Perini, G.; Papi, M. Bridging the Gap: Integrating 3D Bioprinting and Microfluidics for Advanced Multi-Organ Models in Biomedical Research. Bioengineering 2024, 11, 664. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef]
- Zhang, B.; Radisic, M. Organ-on-a-chip devices advance to market. Lab a Chip 2017, 17, 2395–2420. [Google Scholar] [CrossRef]
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl. Vitr. Toxicol. 2016, 2, 82–96. [Google Scholar] [CrossRef]
- Ramadan, Q.; Zourob, M. Organ-on-a-chip engineering: Toward bridging the gap between lab and industry. Biomicrofluidics 2020, 14, 041501. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Jiang, B.; Wang, D.; Zhang, W.; Wang, Z.; Jiang, X. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab a Chip 2012, 12, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, F.; Balachandran, W.; Rasekh, M. Organ-on-a-Chip: Design and Simulation of Various Microfluidic Channel Geometries for the Influence of Fluid Dynamic Parameters. Appl. Sci. 2022, 12, 3829. [Google Scholar] [CrossRef]
- Franzen, N.; van Harten, W.H.; Retèl, V.P.; Loskill, P.; van den Eijnden-van Raaij, J.; Ijzerman, M. Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov. Today 2019, 24, 1720–1724. [Google Scholar] [PubMed]
- Lemarié, L.; Dargar, T.; Grosjean, I.; Gache, V.; Courtial, E.J.; Sohier, J. Human Induced Pluripotent Spheroids’ Growth Is Driven by Viscoelastic Properties and Macrostructure of 3D Hydrogel Environment. Bioengineering 2023, 10, 1418. [Google Scholar] [CrossRef]
- Mihara, H.; Kugawa, M.; Sayo, K.; Tao, F.; Shinohara, M.; Nishikawa, M.; Sakai, Y.; Akama, T.; Kojima, N. Improved Oxygen Supply to Multicellular Spheroids Using A Gas-permeable Plate and Embedded Hydrogel Beads. Cells 2019, 8, 525. [Google Scholar] [CrossRef]
- Courau, T.; Bonnereau, J.; Chicoteau, J.; Bottois, H.; Remark, R.; Miranda, L.A.; Toubert, A.; Blery, M.; Aparicio, T.; Allez, M.; et al. Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment. J. Immunother. Cancer 2019, 7, 74. [Google Scholar] [CrossRef]
- Huch, M.; Koo, B.-K. Modeling mouse and human development using organoid cultures. Development 2015, 142, 3113–3125. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef]
- Kasendra, M.; Tovaglieri, A.; Sontheimer-Phelps, A.; Jalili-Firoozinezhad, S.; Bein, A.; Chalkiadaki, A.; Scholl, W.; Zhang, C.; Rickner, H.; Richmond, C.A.; et al. Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Gordon, A.; Yoon, S.-J.; Tran, S.S.; Makinson, C.D.; Park, J.Y.; Andersen, J.; Valencia, A.M.; Horvath, S.; Xiao, X.; Huguenard, J.R.; et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 2021, 24, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Recaldin, T.; Steinacher, L.; Gjeta, B.; Harter, M.F.; Adam, L.; Kromer, K.; Mendes, M.P.; Bellavista, M.; Nikolaev, M.; Lazzaroni, G.; et al. Human organoids with an autologous tissue-resident immune compartment. Nature 2024, 633, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, S.; Tsumoto, K.; Sano, E. Establishment of a microcarrier culture system with serial sub-cultivation for functionally active human endothelial cells. J. Biotechnol. 2012, 160, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Stoecklein, D.; Kommajosula, A.; Lin, J.; Owsley, K.; Ganapathysubramanian, B.; Di Carlo, D. Shaped 3D microcarriers for adherent cell culture and analysis. Microsystems Nanoeng. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Rogers, R.E.; Haskell, A.; White, B.P.; Dalal, S.; Lopez, M.; Tahan, D.; Pan, S.; Kaur, G.; Kim, H.; Barreda, H.; et al. A Scalable System for Generation of Mesenchymal Stem Cells Derived from Induced Pluripotent Cells Employing Bioreactors and Degradable Microcarriers. STEM CELLS Transl. Med. 2021, 10, 1650–1665. [Google Scholar] [CrossRef]
- Truskey, G.A. Endothelial vascular smooth muscle cell coculture assay for high throughput screening assays to identify antiangiogenic and other therapeutic molecules. Int. J. High Throughput Screen. 2010, 1, 171–181. [Google Scholar] [CrossRef]
- Rogers, M.T.; Gard, A.L.; Gaibler, R.; Mulhern, T.J.; Strelnikov, R.; Azizgolshani, H.; Cain, B.P.; Isenberg, B.C.; Haroutunian, N.J.; Raustad, N.E.; et al. A high-throughput microfluidic bilayer co-culture platform to study endothelial-pericyte interactions. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Liu, M.; Samant, S.; Vasa, C.H.; Pedrigi, R.M.; Oguz, U.M.; Ryu, S.; Wei, T.; Anderson, D.R.; Agrawal, D.K.; Chatzizisis, Y.S. Co-culture models of endothelial cells, macrophages, and vascular smooth muscle cells for the study of the natural history of atherosclerosis. PLoS ONE 2023, 18, e0280385. [Google Scholar] [CrossRef]
- Lee, H.I.; Heo, Y.; Baek, S.-W.; Kim, D.-S.; Song, D.H.; Han, D.K. Multifunctional Biodegradable Vascular PLLA Scaffold with Improved X-ray Opacity, Anti-Inflammation, and Re-Endothelization. Polymers 2021, 13, 1979. [Google Scholar] [CrossRef]
- Margaritis, M.; Channon, K.M.; Antoniades, C. Statins as Regulators of Redox State in the Vascular Endothelium: Beyond Lipid Lowering. Antioxidants Redox Signal. 2014, 20, 1198–1215. [Google Scholar] [CrossRef]
- Huang, D.; Ma, N.; Li, X.; Gou, Y.; Duan, Y.; Liu, B.; Xia, J.; Zhao, X.; Wang, X.; Li, Q.; et al. Advances in single-cell RNA sequencing and its applications in cancer research. J. Hematol. Oncol. 2023, 16, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, X.; Chai, Y.; Zhu, Z.; Yi, P.; Feng, G.; Li, W.; Ou, G. Conditional Knockouts Generated by Engineered CRISPR-Cas9 Endonuclease Reveal the Roles of Coronin in C. elegans Neural Development. Dev. Cell 2014, 30, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jedrychowski, M.P.; Vinayagam, A.; Wu, N.; Shyh-Chang, N.; Hu, Y.; Min-Wen, C.; Moore, J.K.; Asara, J.M.; Lyssiotis, C.A.; et al. Proteomic and Metabolomic Characterization of a Mammalian Cellular Transition from Quiescence to Proliferation. Cell Rep. 2017, 20, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Meng, X.; Yu, X.; Wang, G.; Dong, Z.; Zhou, Z.; Qi, M.; Yu, X.; Ji, T.; Wang, F. From 2D to 3D Co-Culture Systems: A Review of Co-Culture Models to Study the Neural Cells Interaction. Int. J. Mol. Sci. 2022, 23, 13116. [Google Scholar] [CrossRef]
- Mazan, A.; Marusiak, A.A. Protocols for Co-Culture Phenotypic Assays with Breast Cancer Cells and THP-1-Derived Macrophages. J. Mammary Gland. Biol. Neoplasia 2024, 29, 1–10. [Google Scholar] [CrossRef]
- Bogdanowicz, D.R.; Lu, H.H. Studying cell-cell communication in co-culture. Biotechnol. J. 2013, 8, 395–396. [Google Scholar] [CrossRef]
- Wu, C.A.; Zhu, Y.; Woo, Y.J. Advances in 3D Bioprinting: Techniques, Applications, and Future Directions for Cardiac Tissue Engineering. Bioengineering 2023, 10, 842. [Google Scholar] [CrossRef]
| Cellular Interactions | Condition | Signaling Pathways Involved | References |
|---|---|---|---|
| Macrophages and human cardiac microvascular endothelial cells | Hypoxia mediated endothelial dysfunction | Peroxynitrite increased the expressions of hypoxia-inducible factors, (HIF)-1α, HIF-2α, endothelin-converting enzyme (ECE)-1, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). Reduction in prostacyclin synthase (PGIS) | [49] |
| Macrophages and endothelial cells | Oxidized low-density, lipoprotein-stimulated atherogenesis | Oxidized LDL-stimulated release of ET-1 from endothelial cells could affect macrophages. Increased expressions of iNOS, COX-2, IL-6, and TNF-α, and decreased expression of Arg-1, mannose receptor C type 1, and IL-10 were found. | [50] |
| Endothelial cells, macrophages, and pericytes | Vascular angiogenesis | Notch and Jagged signaling | [51] |
| Monocytes, aortic vascular smooth muscle cells, and endothelial cells | Hyperglycemia-induced arteriosclerosis | Changes in cytokines, interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) expression | [52] |
| Mouse cardiac endothelial cells and cardiomyocytes from GK rats | Diabetic vascular disease | Exosomes from the myocytes increased the levels of miR-320. Reduced levels of miR-126 | [53] |
| Endothelial cells and cardiomyocytes | Hypoxia-reoxygenation injury | Curcumin treatment inhibited apoptosis and autophagy of cardiomyocytes. Increased the FGF2 levels | [54] |
| Endothelial cells overexpressing rhSLPI and cardiomyocytes | Hypoxia-reoxygenation injury | Reduced reactive oxygen species production and Bax/Bcl-2, caspase-3, and caspase-8. Activation of p38MAPK and Akt signaling | [55] |
| Cardiomyocytes and endothelial cells | Ischemia reperfusion injury | Increased NO production Reduction of LDH activity | [56] |
| Endothelial cells and smooth muscle cells exposed to laminar pulsatile and disturbed flow | Intimal hyperplasia | Defective endothelial monolayer Incorporation of fibronectin in smooth muscle cells | [45] |
| Endothelial cells and smooth muscle cells in a fibrin gel scaffold, with addition of monocytes later | Atherosclerosis | Infiltration of lipids into macrophages Development of foam cell was studied. | [47] |
| Endothelial cells, smooth muscle cells, and macrophages | Atherosclerosis | Increase in the expression of IL6, IL8, CXCL1/GROα, and CCL2/MCP1 Elevation of inflammatory pathways such as JAK/STAT, NFκB, and Jun signaling | [48] |
| Type of Co-Culture | Description | Advantages | Limitations | References |
|---|---|---|---|---|
| Direct co-culture | Various cell types are seeded in the same culture dish, which allows cell-to-cell communication via gap junction, adherens, and paracrine signaling. | Able to analyze contact-based and non-contact-based cellular interactions. Simple, easy, and cost-effective way to culture. | Difficult to achieve equal amounts of cellular densities of both the cells studied. One cell type could grow fast/slow and might not mimic the exact vascular environment. Culture media used should be adaptable for both the cell types used. | [123,124] |
| Direct co-culture with trans-well | Different cell types are seeded on the upper and lower sides of the porous trans-well inserts. | Direct cell–cell contact allows for study of the physical contact interactions between the cell types. Can demonstrate cell adhesion, permeability, and migration towards the other cell type. Different culture media can be used for the different cell populations across the trans-well. | Trans-well membranes are expensive, and they cannot be reused. There is no difference whether the pathological condition developed is based on contact-dependent or contact-independent signals. | [125,126] |
| Indirect co-culture, trans-well based | Two cell types are cultured in different chambers of the trans-well membrane, and the distance between them allows communication only through soluble factors in the culture media. | Can be used to study cell–cell interactions, drug permeability, and drug transport. | Cellular communication restricts to soluble secretions (growth factors, cytokines, and extracellular vesicles) alone and lacks signaling through physical contact to mimic in vivo environment. Expensive. | [98,127,128] |
| Conditioned media-based indirect co-culture | Cell secretions of one cell type (conditioned media) when transferred to another cell type, which can modulate the cell behavior. | Easy to establish and provides secretory factors to modulate the other cell type. Conditioned media can be frozen and can be used on other cell type later. | Unidirectional. It is used to study only secretory factor-based signaling and lacks contact signaling. | [129,130] |
| 3D co-culture, scaffold based | Encapsulating different cell types in a 3D scaffold, which can provide topography and mechanical stimulus needed reflecting physiological microenvironment. | Mimics more of an in vivo condition and allows for the study of cell morphology, behavior, function, cell–cell contact signaling, and paracrine signaling. Recapitulates the vascular microenvironment realistically. Multiple cellular interactions (both physical and secretory) are feasible. | Expensive. Needs more time to optimize the culture. Proteolytic separation of a single layer of cells is difficult. Repeatability of the experiments is difficult. | [131,132] |
| Microfluidics-based co-culture | Dynamic fluid manipulation system designed for micrometer sized channels. It mimics physiological microenvironment, which can culture multiple cell types. | Regulation of signal gradients and perform simulation of physiological microenvironment such as shear stress. Reliable platform for drug screening and vascular modeling. | Needs external devices like pumps, connectors, and valves to function. Difficult to optimize and repeat the experiments. Expensive. | [133] |
| Organoids-based co-culture | Self-organizing 3D cellular structures that can recapitulate organs (cardiac organoid–endothelial cells, cardiomyocytes, fibroblasts, etc.). | Modeling cardiogenesis, drug screening, and testing and also in tissue engineering. | Difficult to optimize. Hard to reproduce. Less or insufficient vascularization limits the applicability of cardiac organoid. | [134,135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padmanaban, A.M.; Ganesan, K.; Ramkumar, K.M. A Co-Culture System for Studying Cellular Interactions in Vascular Disease. Bioengineering 2024, 11, 1090. https://doi.org/10.3390/bioengineering11111090
Padmanaban AM, Ganesan K, Ramkumar KM. A Co-Culture System for Studying Cellular Interactions in Vascular Disease. Bioengineering. 2024; 11(11):1090. https://doi.org/10.3390/bioengineering11111090
Chicago/Turabian StylePadmanaban, Abirami M., Kumar Ganesan, and Kunka Mohanram Ramkumar. 2024. "A Co-Culture System for Studying Cellular Interactions in Vascular Disease" Bioengineering 11, no. 11: 1090. https://doi.org/10.3390/bioengineering11111090
APA StylePadmanaban, A. M., Ganesan, K., & Ramkumar, K. M. (2024). A Co-Culture System for Studying Cellular Interactions in Vascular Disease. Bioengineering, 11(11), 1090. https://doi.org/10.3390/bioengineering11111090






