Investigating the Antibacterial Ability of Sodium Hypochlorite Solution Activated with PUI and XPF File Against Enterococcus faecalis Using CFU, RT-PCR, and SEM
Abstract
1. Introduction
2. Results
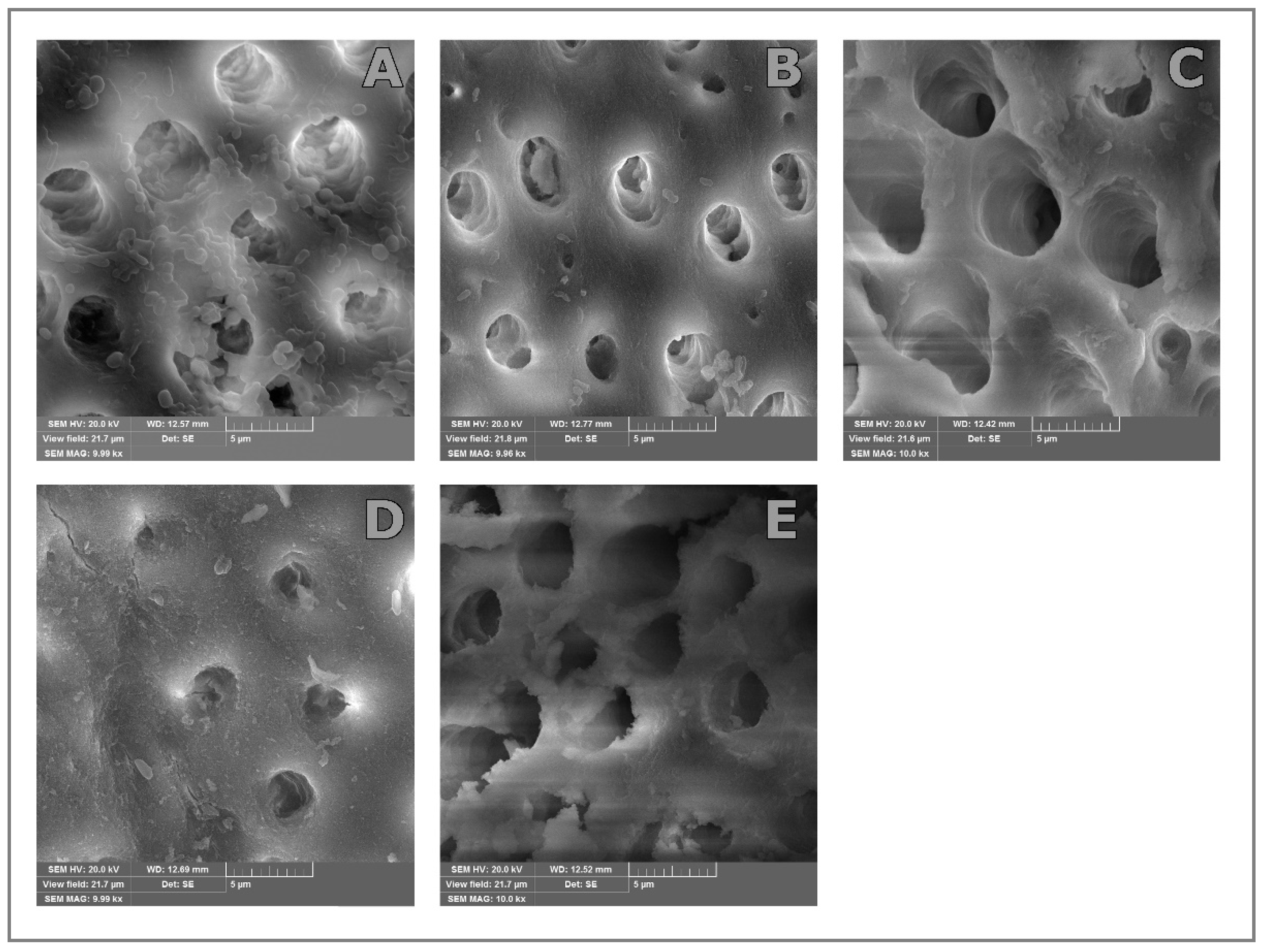
SEM
3. Discussion
4. Materials and Methods
Statistical Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plotino, G.; Colangeli, M.; Özyürek, T.; DeDeus, G.; Panzetta, C.; Castagnola, R.; Grande, N.M.; Marigo, L. Evaluation of smear layer and debris removal by stepwise intraoperative activation (SIA) of sodium hypochlorite. Clin. Oral. Investig. 2021, 25, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral Patol. Oral Cir. Bucal. 2019, 364–372, e364–e372. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2022, 55, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Bonsor, S. Survival rates of teeth treated with bacterial photo-dynamic therapy during disinfection of the root canal system. Br. Dent. J. 2019, 226, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Siqueira Junior, J.F., Jr.; Rôças, I.D.N.; Marceliano-Alves, M.F.; Pérez, A.R.; Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral Res. 2018, 32, 65–84. [Google Scholar] [CrossRef]
- Gomes, B.; Berber, V.B.; Kokaras, A.S.; Chen, T.; Paster, B.J. Microbiomes of endodontic periodontal lesions before and after chemomechanical preparation. J. Endod. 2015, 41, 1975–1984. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]
- Zou, X.; Zheng, X.; Liang, Y.; Zhang, C.; Fan, B.; Liang, J.; Ling, J.; Bian, Z.; Yu, Q.; Hou, B.; et al. Expert consensus on irrigation and intracanal medication in root canal therapy. Int. J. Oral. Sci. 2024, 16, 23. [Google Scholar] [CrossRef]
- Guivarc’h, M.; Ordioni, U.; Ahmed, H.M.; Cohen, S.; Catherine, J.H.; Bukiet, F. Sodium Hypochlorite Accident: A Systematic Review. J. Endod. 2017, 43, 16–24. [Google Scholar] [CrossRef]
- Xu, J.; He, J.; Shen, Y.; Zhou, X.; Huang, D.; Gao, Y.; Haapasalo, M. Influence of Endodontic Procedure on the Adherence of Enterococcus faecalis. J. Endod. 2019, 45, 943–949. [Google Scholar] [CrossRef]
- Gaeta, C.; Marruganti, C.; Ali, I.A.A.; Fabbro, A.; Pinzauti, D.; Santoro, F.; Neelakantan, P.; Pozzi, G.; Grandini, S. The presence of Enterococcus faecalis in saliva as a risk factor for endodontic infection. Front. Cell. Infect. Microbiol. 2023, 13, 1061645. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, M.; Zheng, J.; Song, Q.; Yin, W.; Li, J.; Niu, W. Relationship of biofilm formation and gelE gene expression in Enterococcus faecalis recovered from root canals in patients requiring endodontic retreatment. J. Endod. 2011, 37, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Jayaraman, J.; Suresh, A.; Kalyanasundaram, S.; Neelakantan, P. Effectiveness of ultrasonically activated irrigation on root canal disinfection: A systematic review of in vitro studies. Clin. Oral. Investig. 2018, 22, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Niavarzi, S.; Pourhajibagher, M.; Khedmat, S.; Ghabraei, S.; Chiniforush, N.; Bahador, A. Effect of ultrasonic activation on the efficacy of antimicrobial photodynamic therapy: Evaluation of penetration depth of photosensitizer and elimination of Enterococcus faecalis biofilms. Photodiagn. Photodyn. Ther. 2019, 27, 362–366. [Google Scholar] [CrossRef]
- Bao, P.; Shen, Y.; Lin, J.; Haapasalo, M. In Vitro Efficacy of XP-endo Finisher with 2 Different Protocols on Biofilm Removal from Apical Root Canals. J. Endod. 2017, 43, 321–325. [Google Scholar] [CrossRef]
- Yang, S.; Meng, X.; Zhen, Y.; Baima, Q.; Wang, Y.; Jiang, X.; Xu, Z. Strategies and mechanisms targeting Enterococcus faecalis biofilms associated with endodontic infections: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 1433313. [Google Scholar] [CrossRef]
- Delboni, M.G.; Gomes, B.P.; Francisco, P.A.; Teixeira, F.B.; Drake, D. Diversity of Enterococcus faecalis Genotypes from Multiple Oral Sites Associated with Endodontic Failure Using Repetitive Sequence-based Polymerase Chain Reaction and Arbitrarily Primed Polymerase Chain Reaction. J. Endod. 2017, 43, 377–382. [Google Scholar] [CrossRef]
- Ramsey, M.; Hartke, A.; Huycke, M. The Physiology and Metabolism of Enterococci. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; NCBI: Bethesda, MD, USA, 2014. [Google Scholar]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic irrigants: Different methods to improve efficacy and related problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef]
- Pacheco-Yanes, J.; Provenzano, J.C.; Marceliano-Alves, M.F.; Gazzaneo, I.; Pérez, A.R.; Gonçalves, L.S.; Siqueira, J.F., Jr. Distribution of sodium hypochlorite throughout the mesial root canal system of mandibular molars after adjunctive irrigant activation procedures: A micro-computed tomographic study. Clin. Oral Investig. 2020, 24, 907–914. [Google Scholar] [CrossRef]
- Ahangari, Z.; Asnaashari, M.; Akbarian Rad, N.; Shokri, M.; Azari-Marhabi, S.; Asnaashari, N. Investigating the Antibacterial Effect of Passive Ultrasonic Irrigation, Photodynamic Therapy and Their Combination on Root Canal Disinfection. J. Lasers Med. Sci. 2021, 12, 81–87. [Google Scholar] [CrossRef]
- Gu, Y.; Perinpanayagam, H.; Kum, D.J.; Yoo, Y.J.; Jeong, J.S.; Lim, S.M.; Chang, S.W.; Baek, S.H.; Zhu, Q.; Kum, K.Y. Efect of diferent agitation techniques on the penetration of irrigant and sealer into dentinal tubules. Photomed. Laser Surg. 2017, 35, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sheetal, R.; Barsha, S.; Sultan, A.; Omar, K.; Alfred, T.; Amr, S.F. Evaluation of Antibacterial Efficacy of High-Intensity Focused Ultrasound Versus Photodynamic Therapy Against Enterococcus faecalis–Infected Root Canals. Ultrasound Med. Biol. 2023, 8, 1875–1881. [Google Scholar]
- Duque, J.A.; Duarte, M.A.H.; Canali, L.C.F.; Zancan, R.F.; Vivan, R.R.; Bernardes, R.A.; Bramante, C. Comparative Effectiveness of New Mechanical Irrigant Agitating Devices for Debris Removal from the Canal and Isthmus of Mesial Roots of Mandibular Molars. J. Endod. 2017, 43, 326–331. [Google Scholar] [CrossRef]
- Van Der Sluis, L.W.M.; Versluis, M.; Wu, M.K.; Wesselink, P.R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 2007, 40, 415–426. [Google Scholar] [CrossRef]
- Sasanakul, P.; Ampornaramveth, R.S.; Chivatxaranukul, P. Influence of Adjuncts to Irrigation in the Disinfection of Large Root Canals. J. Endod. 2019, 45, 332–337. [Google Scholar] [CrossRef]
- Leonardi, D.P.; Dds, N.M.G.; Tomazinho, F.S.F.; Marques-Da-Silva, B.; Gonzaga, C.C.; Filho, F.B.; Plotino, G. Influence of activation mode and preheating on intracanal irrigant temperature. Aust. Endod. J. 2019, 45, 373–377. [Google Scholar] [CrossRef]
- Elnaghy, A.M.; Mandorah, A.; Elsaka, S.E. Effectiveness of XP-endo Finisher, EndoActivator, and File agitation on debris and smear layer removal in curved root canals: A comparative study. Odontology 2017, 105, 178–183. [Google Scholar] [CrossRef]
- Mathew, D.M.; Durvasulu, A.; Shanmugam, S.; Pradeepkumar, A.R. Evaluation of Different Agitation Techniques on Smear Layer Formation and Dentine Erosions—An In Vitro Study. Eur. Endod. J. 2023, 8, 72–78. [Google Scholar]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions—Irrigants and irrigation methods. Int. Endod. J. 2022, 55, 588–612. [Google Scholar] [CrossRef]
- Bulacio, M.D.L.Á.; Galván, L.R.; Gaudioso, C.; Cangemi, R.; Erimbaue, M.I. Enterococcus faecalis Biofilm. Formation and Development in Vitro Observed by Scanning Electron Microscopy. Acta Odontol. Latinoam. 2015, 28, 210–214. [Google Scholar]
- Leoni, G.B.; Versiani, M.A.; Silva-Sousa, Y.T.; Bruniera, J.F.; Pécora, J.D.; Sousa-Neto, M.D. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int. Endod. J. 2017, 50, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Căpută, P.E.; Retsas, A.; Kuijk, L.; Chávez de Paz, L.E.; Boutsioukis, C. Ultrasonic Irrigant Activation during Root Canal Treatment: A Systematic Review. J. Endod. 2019, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
| Treatment | m | s | Med | Min | Max | p | |
|---|---|---|---|---|---|---|---|
| CNI | Before | 3.75 × 107 | 4.72 × 107 | 2.30 × 107 | 1.13 × 106 | 1.32 × 108 | 0.001 |
| After | 9.82 × 104 | 9.80 × 104 | 8.38 × 104 | 6.70 × 103 | 3.45 × 105 | ||
| PUI | Before | 1.35 × 107 | 1.25 × 107 | 8.94 × 106 | 4.50 × 105 | 3.48 × 107 | 0.001 |
| After | 5.86 × 104 | 4.39 × 104 | 4.15 × 104 | 1.11 × 104 | 1.46 × 105 | ||
| XPF | Before | 3.07 × 105 | 7.09 × 105 | 1.03 × 105 | 1.82 × 104 | 2.55 × 106 | 0.001 |
| After | 7.82 × 103 | 9.81 × 103 | 3.93 × 103 | 7.90 × 102 | 3.58 × 104 | ||
| Control | Before | 1.74 × 107 | 3.50 × 107 | 1.72 × 106 | 6.30 × 105 | 8.00 × 107 | 0.001 |
| After | 4.10 × 106 | 7.78 × 106 | 2.26 × 105 | 1.40 × 105 | 1.80 × 107 | ||
| Treatment | m | s | Med | Min | Max | p | |
|---|---|---|---|---|---|---|---|
| CNI | Before | 1.50 × 106 | 4.93 × 105 | 1.80 × 106 | 7.80 × 105 | 1.98 × 106 | 0.002 |
| After | 1.70 × 105 | 1.90 × 105 | 1.05 × 105 | 5.00 × 101 | 6.80 × 105 | ||
| PUI | Before | 1.07 × 106 | 2.76 × 105 | 1.00 × 106 | 7.00 × 105 | 1.50 × 106 | 0.002 |
| After | 2.34 × 105 | 2.40 × 105 | 1.80 × 105 | 8.00 × 101 | 7.60 × 105 | ||
| XPF | Before | 1.96 × 105 | 1.15 × 105 | 1.85 × 105 | 4.00 × 104 | 4.00 × 105 | 0.002 |
| After | 2.33 × 103 | 2.67 × 103 | 2.00 × 103 | 0.00. × 100 | 9.00 × 103 | ||
| Control | Before | 1.66 × 106 | 3.05 × 105 | 1.55 × 106 | 1.30 × 106 | 2.00 × 106 | 0.043 |
| After | 9.60 × 105 | 1.52 × 105 | 8.70 × 105 | 8.30 × 105 | 1.15 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, R.; Ilić, J.; Šubarić, L.; Vlahović, Z.; Simić, S.; Arsić, Z.; Radunović, M.; Popović, B. Investigating the Antibacterial Ability of Sodium Hypochlorite Solution Activated with PUI and XPF File Against Enterococcus faecalis Using CFU, RT-PCR, and SEM. Bioengineering 2024, 11, 1086. https://doi.org/10.3390/bioengineering11111086
Jovanović R, Ilić J, Šubarić L, Vlahović Z, Simić S, Arsić Z, Radunović M, Popović B. Investigating the Antibacterial Ability of Sodium Hypochlorite Solution Activated with PUI and XPF File Against Enterococcus faecalis Using CFU, RT-PCR, and SEM. Bioengineering. 2024; 11(11):1086. https://doi.org/10.3390/bioengineering11111086
Chicago/Turabian StyleJovanović, Radovan, Jugoslav Ilić, Ljiljana Šubarić, Zoran Vlahović, Sanja Simić, Zoran Arsić, Milena Radunović, and Branka Popović. 2024. "Investigating the Antibacterial Ability of Sodium Hypochlorite Solution Activated with PUI and XPF File Against Enterococcus faecalis Using CFU, RT-PCR, and SEM" Bioengineering 11, no. 11: 1086. https://doi.org/10.3390/bioengineering11111086
APA StyleJovanović, R., Ilić, J., Šubarić, L., Vlahović, Z., Simić, S., Arsić, Z., Radunović, M., & Popović, B. (2024). Investigating the Antibacterial Ability of Sodium Hypochlorite Solution Activated with PUI and XPF File Against Enterococcus faecalis Using CFU, RT-PCR, and SEM. Bioengineering, 11(11), 1086. https://doi.org/10.3390/bioengineering11111086








