An In Vitro Comparative Analysis of Physico–Mechanical Properties of Commercial and Experimental Bioactive Endodontic Sealers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Teeth
2.2. Fracture Resistance Test
2.3. Sealer Penetration Test
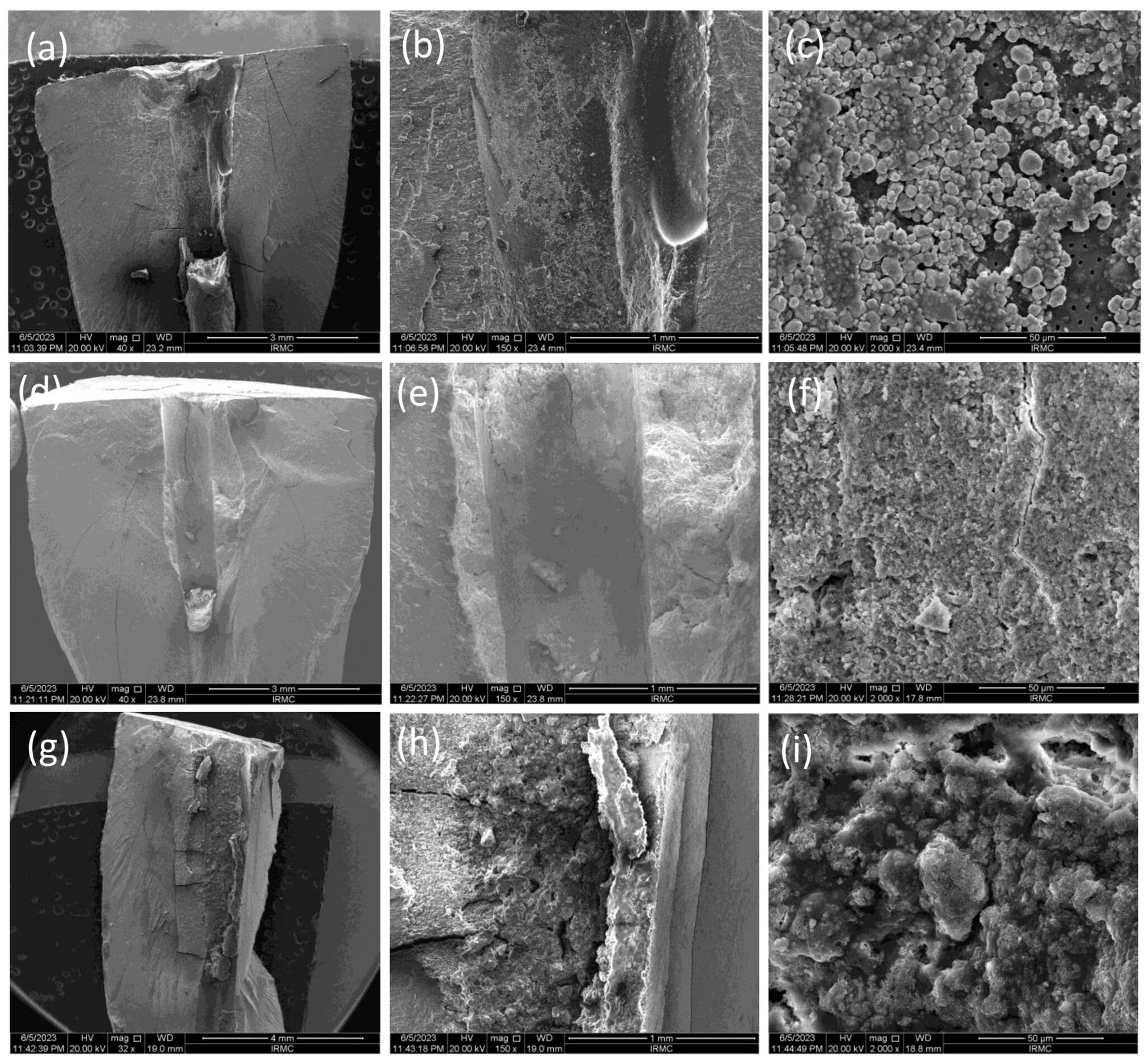
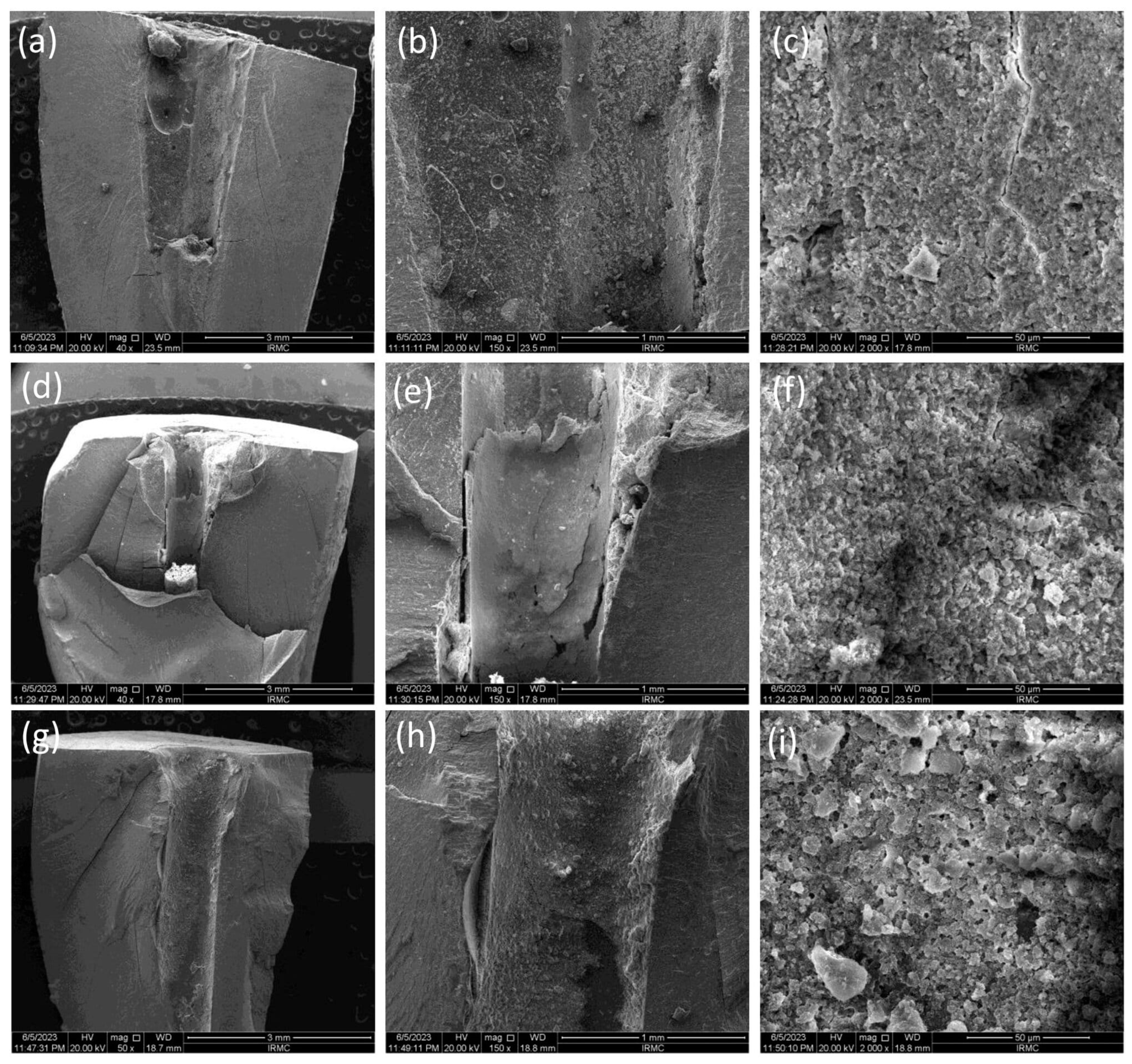
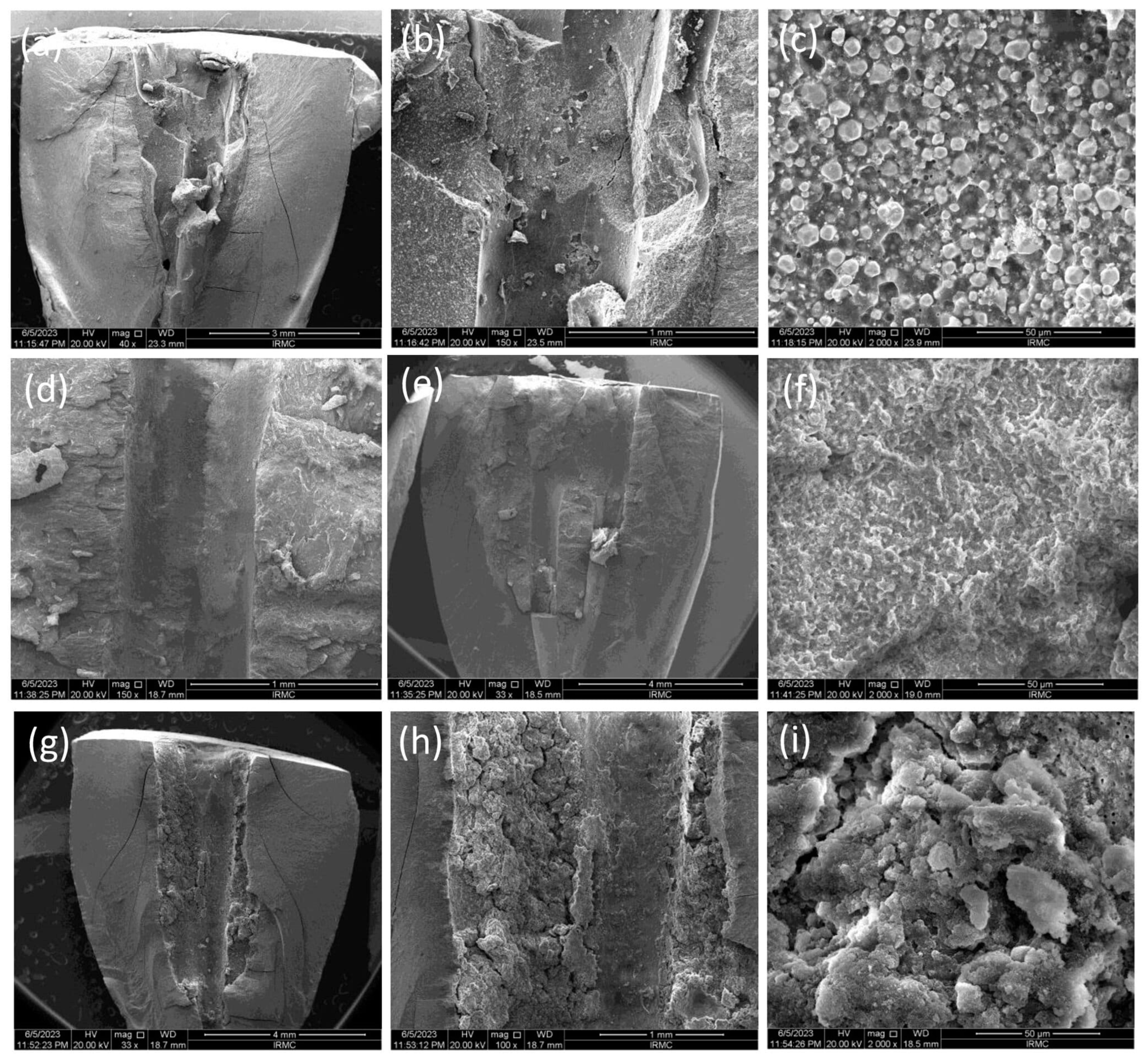
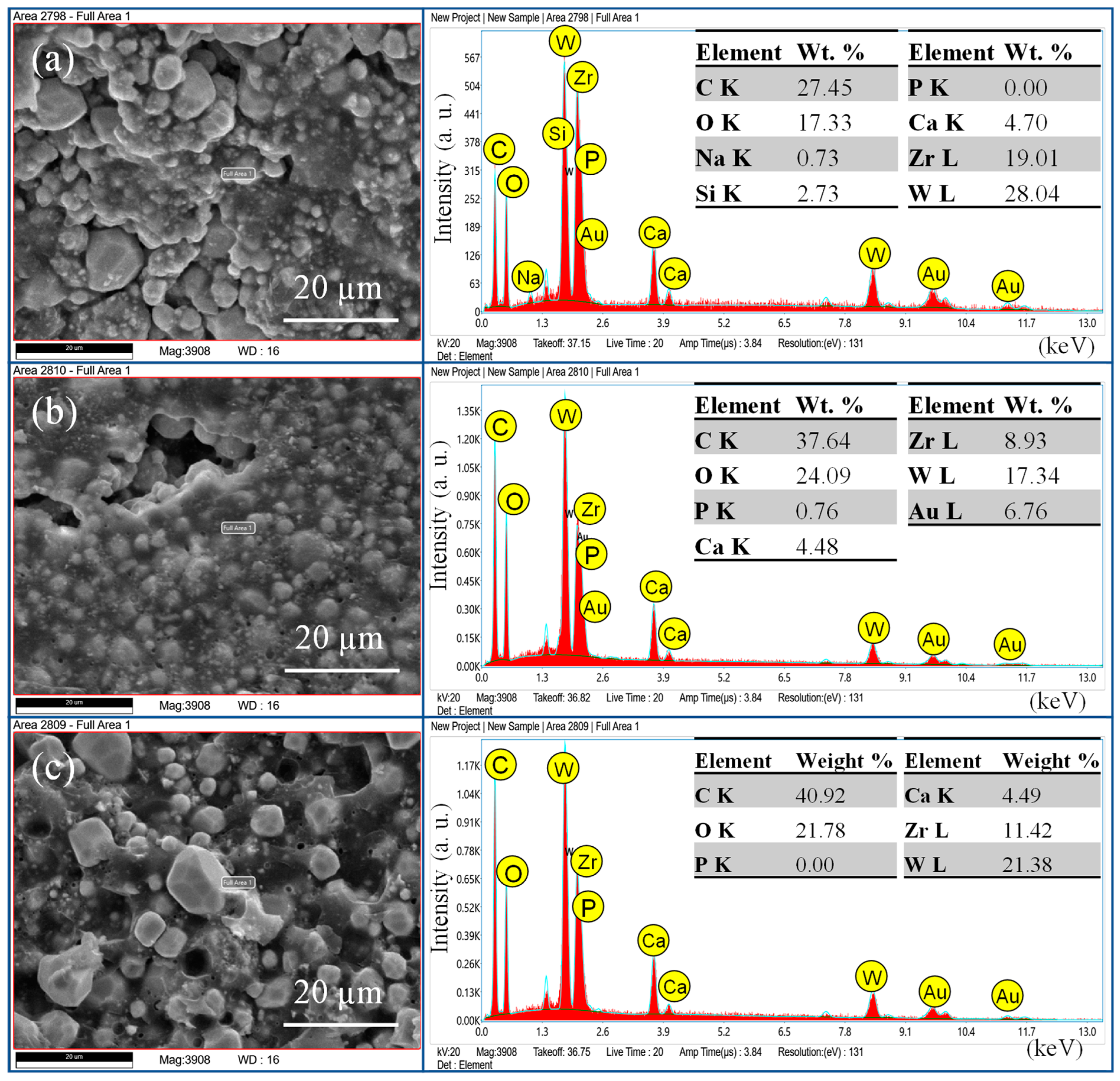
Confocal Laser Scanning Microscopic (CLSM) Analysis
2.4. Statistical Analysis
3. Results
3.1. Fracture Resistance Test
3.2. Sealer Penetration Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abusrewil, S.; Alshanta, O.A.; Albashaireh, K.; Alqahtani, S.; Nile, C.J.; Scott, J.A.; McLean, W. Detection, treatment and prevention of endodontic biofilm infections: What’s new in 2020? Crit. Rev. Microbiol. 2020, 46, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; AlJeaidi, Z.A.; AlQahtani, A.R.; Abuelqomsan, M.A.S.; Alofi, R.S.; Alghomlas, Z.I.; Abdullah AlOthman, T. Comparative analysis of fracture strength of remaining tooth structure after endodontic treatment with various access cavity preparation techniques. Open Dent. J. 2020, 14, 681–686. [Google Scholar] [CrossRef]
- Gulabivala, K.; Ng, Y.L. Factors that affect the outcomes of root canal treatment and retreatment—A reframing of the principles. Int. Endod. J. 2023, 56, 82–115. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Jiang, H.; Deng, Z.; Paranjpe, A.; Zhang, H.; Arola, D. Shrinkage Strains in the Dentin of Endodontically Treated Teeth with Water Loss. J. Endod. 2021, 47, 806–811. [Google Scholar] [CrossRef]
- Alkahtany, M.F.; Almadi, K.H.; Alahmad, F.A.; Alshehri, A.M.; AlSwayyed, A.A.; AlZahran, O.M.; AlHadan, A.; Almustafa, A.S.; Vohra, F.; Abduljabbar, T. Influence of Root Canal Sealers and Obturation Techniques on Vertical Root Fracture Resistance. An In Vitro Experiment. Appl. Sci. 2021, 11, 8022. [Google Scholar] [CrossRef]
- Girish, K.; Mandava, J.; Chandra, R.R.; Ravikumar, K.; Anwarullah, A.; Athaluri, M. Effect of obturating materials on fracture resistance of simulated immature teeth. J. Conserv. Dent. 2017, 20, 115–119. [Google Scholar] [CrossRef]
- Kanyılmaz, A.N.Ç.; Akman, M.; Şişmanoğlu, S.; Belli, S. The Effect of Different Fiber Post-Application Techniques on Fracture Resistance of Structurally Compromised Premolars with Flared Root Canals: An In Vitro Study. J. Adv. Oral Res. 2022, 13, 135–142. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Monoblocks in root canals: A hypothetical or a tangible goal. J. Endod. 2007, 33, 391–398. [Google Scholar] [CrossRef]
- Belli, S.; Eraslan, O.; Eskitascioglu, G.; Karbhari, V. Monoblocks in root canals: A finite elemental stress analysis study. Int. Endod. J. 2011, 44, 817–826. [Google Scholar] [CrossRef]
- Almanie, D.; Alaathy, S.; Almohaimede, E.A.A. Fracture resistance of roots filled with bio-ceramic and epoxy resin-based sealers: In vitro study. Eur. Endod. J. 2020, 5, 134. [Google Scholar]
- Atmeh, A.R.; Chong, E.Z.; Richard, G.; Festy, F.; Watson, T.F. Dentin-cement interfacial interaction: Calcium silicates and polyalkenoates. J. Dent. Res. 2012, 91, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Jung, C.; Shin, D.H.; Cho, Y.B.; Song, M. Calcium silicate-based root canal sealers: A literature review. Rest. Dent. Endod. 2020, 45, e35. [Google Scholar] [CrossRef] [PubMed]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive Glass-Based Endodontic Sealer as a Promising Root Canal Filling Material Without Semisolid Core Materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, O.S.; Meier, M.M.; Carvalho, E.M.; Ferreira, P.V.C.; Gavini, G.; Zago, P.M.W.; Grazziotin-Soares, R.; Menezes, A.S.d.; Carvalho, C.N.; Bauer, J. Synthesis and characterization of experimental endodontic sealers containing bioactive glasses particles of NbG or 45S5. J. Mech. Behav. Biomed. Mater. 2022, 125, 104971. [Google Scholar] [CrossRef]
- Mîrț, A.-L.; Ficai, D.; Oprea, O.-C.; Vasilievici, G.; Ficai, A. Current and Future Perspectives of Bioactive Glasses as Injectable Material. Nanomaterials 2024, 14, 1196. [Google Scholar] [CrossRef]
- Lee, J.K.; Kwak, S.W.; Ha, J.-H.; Lee, W.; Kim, H.-C. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg. Chem. Appl. 2017, 2017, 2582849. [Google Scholar] [CrossRef]
- El-Gamal, M.K.; fahim, m.m.; Elfaramawy, M.T. The effect of different obturation techniques on the fracture resistance of endodontically treated teeth obturated using bio-ceramic sealer. Egyp. Dent. J. 2024, 70, 1901–1909. [Google Scholar] [CrossRef]
- Santos, J.M.; Diogo, P.; Dias, S.; Marques, J.A.; Palma, P.J.; Ramos, J.C. Long-Term Outcome of Nonvital Immature Permanent Teeth Treated with Apexification and Corono-Radicular Adhesive Restoration: A Case Series. J. Endod. 2022, 48, 1191–1199. [Google Scholar] [CrossRef]
- Malik, Q.U.A.; Iftikhar, S.; Zahid, S.; Safi, S.Z.; Khan, A.F.; Nawshad, M.; Ghafoor, S.; Khan, A.S.; Tufail Shah, A. Smart injectable self-setting bioceramics for dental applications. Mater. Sci. Eng. C 2020, 113, 110956. [Google Scholar] [CrossRef]
- Wuersching, S.N.; Diegritz, C.; Hickel, R.; Huth, K.C.; Kollmuss, M. A comprehensive in vitro comparison of the biological and physicochemical properties of bioactive root canal sealers. Clin. Oral Investig. 2022, 26, 6209–6222. [Google Scholar] [CrossRef]
- Uzunoglu-Özyürek, E.; Küçükkaya Eren, S.; Karahan, S. Effect of root canal sealers on the fracture resistance of endodontically treated teeth: A systematic review of in vitro studies. Clin. Oral Investig. 2018, 22, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, E.U.; Türker, S.A. Evaluation of fracture resistance of roots-filled with various root canal sealers at different time periods. Eur. Oral Res. 2019, 53, 6–11. [Google Scholar]
- Sağsen, B.; Üstün, Y.; Pala, K.; Demirbuğa, S. Resistance to fracture of roots filled with different sealers. Dent. Mater. J. 2012, 31, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Kuah, H.G.; Lui, J.N.; Tseng, P.S.; Chen, N.N. The effect of EDTA with and without ultrasonics on removal of the smear layer. J. Endod. 2009, 35, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ye, Z.; Zhang, A.; Lin, F.; Fu, J.; Fok, A.S.L. Effects of concentration of sodium hypochlorite as an endodontic irrigant on the mechanical and structural properties of root dentine: A laboratory study. Int. Endod. J. 2022, 55, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Subbarao, C.; Subbarao, C.; De-Deus, G.; Zehnder, M. The impact of root dentine conditioning on sealing ability and push-out bond strength of an epoxy resin root canal sealer. Int. Endod. J. 2011, 44, 491–498. [Google Scholar] [CrossRef]
- Kok, D.; Duarte, M.A.H.; Da Rosa, R.A.; Wagner, M.H.; Pereira, J.R.; Só, M.V.R. Evaluation of epoxy resin sealer after three root canal filling techniques by confocal laser scanning microscopy. Microsc. Res. Tech. 2012, 75, 1277–1280. [Google Scholar] [CrossRef]
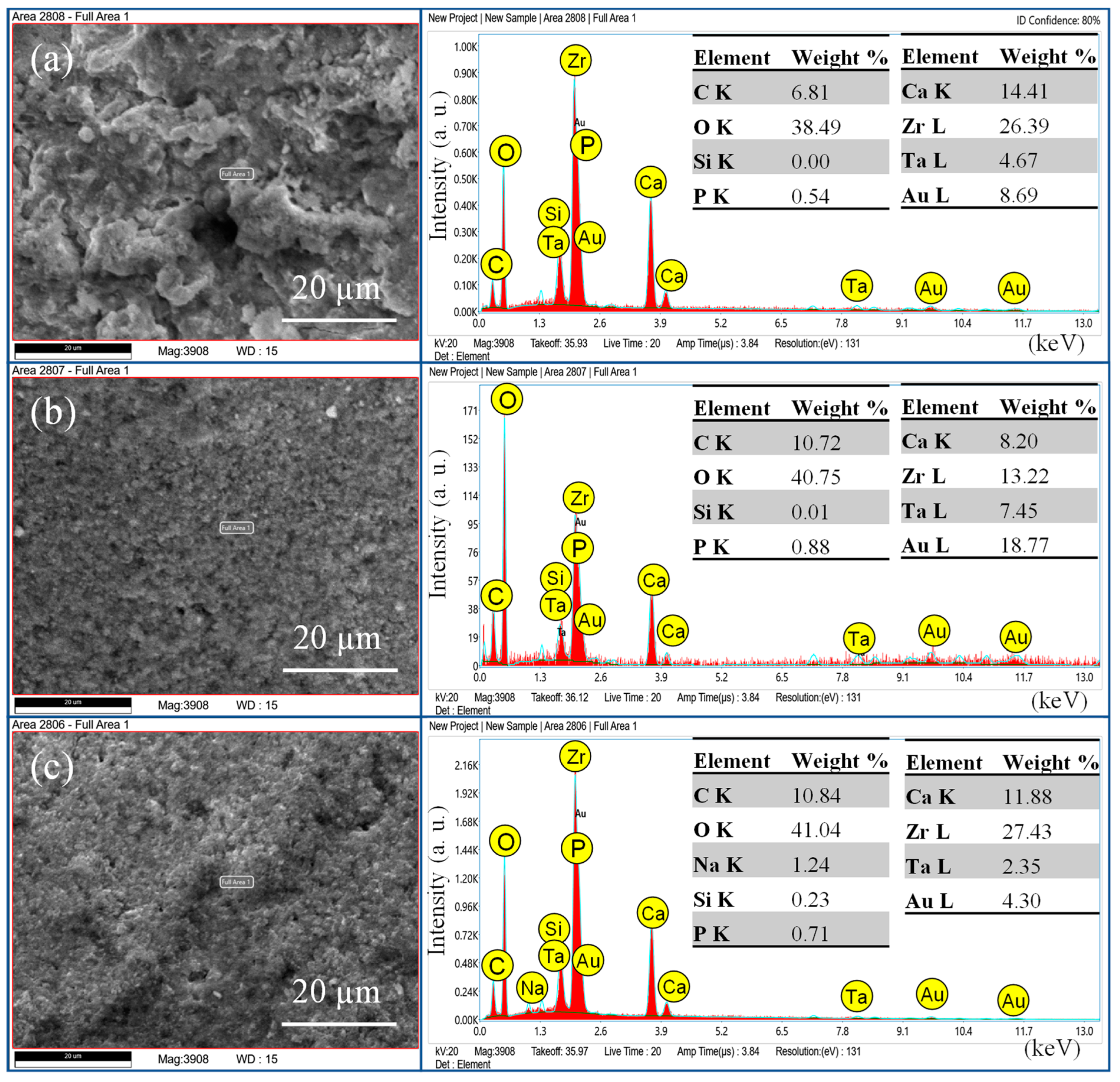



| Groups | Composition | Manufacturer |
|---|---|---|
| Total Fill BC Sealer Hiflow | Zirconium oxide, Tricalcium silicate, Dicalcium silicate, calcium hydroxide | FKG Dentaire SA Lashaudfon, Switzerland |
| AH plus | Bisphenol-A and F epoxy resin, calcium tungstate, zirconium oxide, silica, iron oxide dibenzyl diamine, aminoadamantane, tricyclodecane diamine, silicone oil. | Dentsply Sirona, Bensheim, Germany |
| Injectable Bioactive Glass | SiO2, CaO, Na2O, P2O5, F127 (Poloxamer 407), hydroxypropyl methylcellulose | Experimental 1 [19] |
| Days | +ve Control | −ve Control | AH + | TF BC | Exp.BG | p-Value |
|---|---|---|---|---|---|---|
| Day 7 | 457.67 ± 109.7 | 507.9 ± 104.5 | 476.07 ± 173.2 | 627.46 ± 188.14 | 517.93 ± 51.3 | 0.064 |
| Day 30 | 664.08 ± 138.8 | 594.17 ± 149.9 | 502.64 ± 76.23 | 0.002 | ||
| Day 90 | 493.38 ± 120.18 | 580.12 ± 149.74 | 581.26 ± 136.41 | 0.124 | ||
| p-value | 0.013 | 0.803 | 0.165 |
| Sections | Days | AH+ | TF BC | Exp.BG |
|---|---|---|---|---|
| Coronal Section | Day 7 | 347.6 ± 212 | 1362.5 ± 309 | 1583 ± 390 |
| Day 30 | 311 ± 183 | 1366 ± 186 | 1653.5 ± 348 | |
| Day 90 | 341 ± 278 | 1363 ± 281 | 1532.5 ± 461 | |
| Middle Section | Day 7 | 411 ± 167 | 821.5 ± 301 | 1901 ± 304 |
| Day 30 | 368.5 ± 122 | 817.5 ± 321 | 1902.5 ± 306 | |
| Day 90 | 393.5 ± 191 | 502.04 ± 358 | 1905 ± 309 | |
| Apical Section | Day 7 | 294.7 ± 92 | 613 ± 185 | 1118 ± 251 |
| Day 30 | 280.5 ± 101 | 566 ± 131 | 1110 ± 250 | |
| Day 90 | 295.2 ± 99 | 608.5 ± 184.5 | 1104 ± 240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashaf, A.; Alonaizan, F.; Almulhim, K.S.; Almohazey, D.; Alotaibi, D.A.; Akhtar, S.; Shetty, A.C.; Khan, A.S. An In Vitro Comparative Analysis of Physico–Mechanical Properties of Commercial and Experimental Bioactive Endodontic Sealers. Bioengineering 2024, 11, 1079. https://doi.org/10.3390/bioengineering11111079
Kashaf A, Alonaizan F, Almulhim KS, Almohazey D, Alotaibi DA, Akhtar S, Shetty AC, Khan AS. An In Vitro Comparative Analysis of Physico–Mechanical Properties of Commercial and Experimental Bioactive Endodontic Sealers. Bioengineering. 2024; 11(11):1079. https://doi.org/10.3390/bioengineering11111079
Chicago/Turabian StyleKashaf, Abdulmajeed, Faisal Alonaizan, Khalid S. Almulhim, Dana Almohazey, Deemah Abdullah Alotaibi, Sultan Akhtar, Ashwin C. Shetty, and Abdul Samad Khan. 2024. "An In Vitro Comparative Analysis of Physico–Mechanical Properties of Commercial and Experimental Bioactive Endodontic Sealers" Bioengineering 11, no. 11: 1079. https://doi.org/10.3390/bioengineering11111079
APA StyleKashaf, A., Alonaizan, F., Almulhim, K. S., Almohazey, D., Alotaibi, D. A., Akhtar, S., Shetty, A. C., & Khan, A. S. (2024). An In Vitro Comparative Analysis of Physico–Mechanical Properties of Commercial and Experimental Bioactive Endodontic Sealers. Bioengineering, 11(11), 1079. https://doi.org/10.3390/bioengineering11111079









