Integrating Electroencephalography Source Localization and Residual Convolutional Neural Network for Advanced Stroke Rehabilitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Description
2.2. System Description
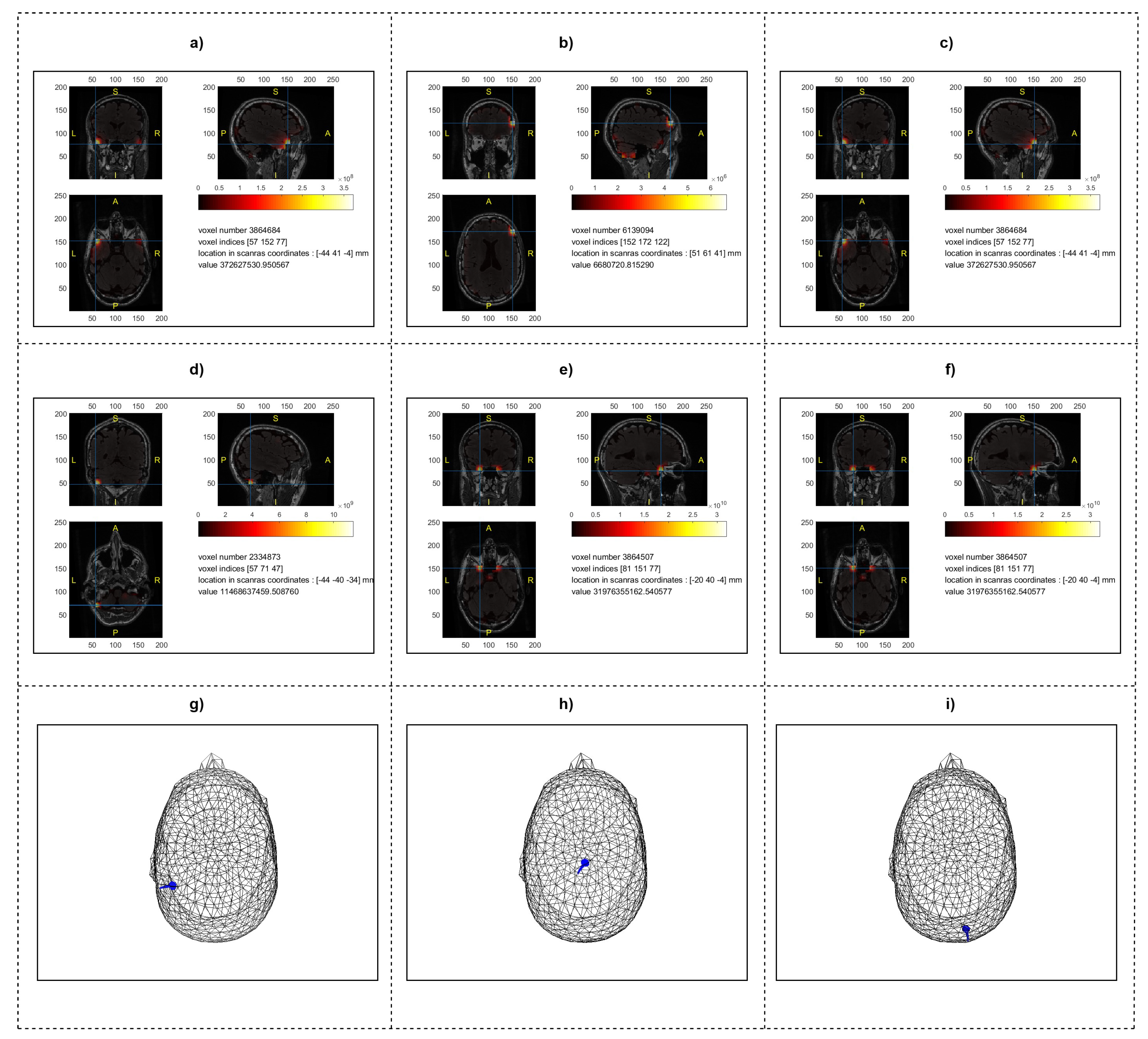
2.3. Source Localization and Inverse Problem
2.4. Residual Convolutional Neural Network Architecture
2.5. System Setup
2.6. Source Localization Analysis
3. Results
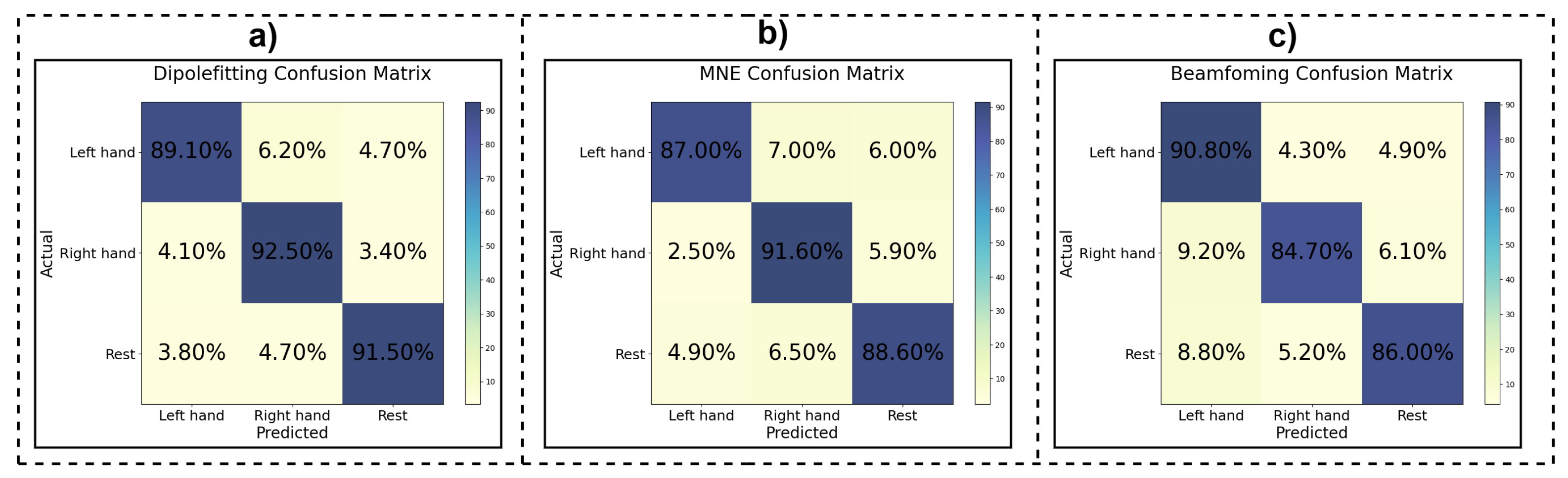
3.1. Per-Subject Classification Performance Analysis
3.1.1. Beamforming Method
3.1.2. MNE Method
3.1.3. Dipole Fitting Method
3.2. Overall Classification Performance across All Participants
4. Discussion
4.1. Comparative Performance Analysis
4.2. Detailed Results and Implications
4.3. Applications in BCI and Neurorehabilitation
4.4. Strengths and Limitations
4.5. Future Directions
5. Conclusions
- Enhanced classification accuracy: source localization before CNN analysis improved classification accuracy, demonstrating more precise and reliable BCIs for clinical applications.
- Advanced health technology integration: the integration of advanced preprocessing, source localization, and deep learning supports personalized therapies for stroke patients by accurately interpreting brain signals.
- Innovative use of source localization with CNN: combining source localization techniques with ResNet enabled the effective capture of EEG spatial patterns, improving classification performance and advancing research in BCI and neurorehabilitation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Rho, E.; Lee, H.; Lee, Y.; Lee, K.-D.; Mun, J.; Kim, M.; Kim, D.; Park, H.-S.; Jo, S. Multiple hand posture rehabilitation system using vision-based intention detection and soft-robotic glove. IEEE Trans. Ind. Inform. 2024, 20, 6499–6509. [Google Scholar] [CrossRef]
- Takahashi, C.D.; Der-Yeghiaian, L.; Le, V.; Motiwala, R.R.; Cramer, S.C. Robot-based hand motor therapy after stroke. Brain 2008, 131, 425–437. [Google Scholar] [CrossRef]
- Takeuchi, N.; Izumi, S.-I. Rehabilitation with poststroke motor recovery: A review with a focus on neural plasticity. Stroke Res. Treat. 2013, 2013, 128641. [Google Scholar] [CrossRef]
- Lebedev, M. Brain-machine interfaces: An overview. Transl. Neurosci. 2014, 5, 99–110. [Google Scholar] [CrossRef]
- Slutzky, M.W. Brain-machine interfaces: Powerful tools for clinical treatment and neuroscientific investigations. Neuroscientist 2019, 25, 139–154. [Google Scholar] [CrossRef]
- Zeng, H.; Song, A. Optimizing single-trial EEG classification by stationary matrix logistic regression in brain–computer interface. IEEE Trans. Neural Netw. Learn. Syst. 2015, 27, 2301–2313. [Google Scholar] [CrossRef]
- Biasiucci, A.; Franceschiello, B.; Murray, M.M. Electroencephalography. Curr. Biol. 2019, 29, R80–R85. [Google Scholar] [CrossRef]
- Lotte, F.; Congedo, M.; Lécuyer, A.; Lamarche, F.; Arnaldi, B. A review of classification algorithms for EEG-based brain–computer interfaces. J. Neural Eng. 2007, 4, R1. [Google Scholar] [CrossRef]
- López-Larraz, E.; Sarasola-Sanz, A.; Irastorza-Landa, N.; Birbaumer, N.; Ramos-Murguialday, A. Brain-machine interfaces for rehabilitation in stroke: A review. NeuroRehabilitation 2018, 43, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.; Chouhan, T.; Guan, C. BCI for stroke rehabilitation: Motor and beyond. J. Neural Eng. 2020, 17, 041001. [Google Scholar] [CrossRef]
- Sohrabpour, A.; He, B. Exploring the extent of source imaging: Recent advances in noninvasive electromagnetic brain imaging. Curr. Opin. Biomed. Eng. 2021, 18, 100277. [Google Scholar] [CrossRef] [PubMed]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F.C. A novel explainable machine learning approach for EEG-based brain-computer interface systems. Neural Comput. Appl. 2022, 34, 11347–11360. [Google Scholar] [CrossRef]
- Liu, H.; Wei, P.; Wang, H.; Lv, X.; Duan, W.; Li, M.; Zhao, Y.; Wang, Q.; Chen, X.; Shi, G.; et al. An EEG motor imagery dataset for brain computer interface in acute stroke patients. Sci. Data 2024, 11, 131. [Google Scholar] [CrossRef]
- Michel, C.M.; He, B. EEG source localization. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 85–101. [Google Scholar]
- Cuffin, B.N. EEG dipole source localization. IEEE Eng. Med. Biol. Mag. 1998, 17, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neuroinform. 2013, 7, 267. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE software for processing MEG and EEG data. NeuroImage 2014, 86, 446–460. [Google Scholar] [CrossRef]
- Grech, R.; Cassar, T.; Muscat, J.; Camilleri, K.P.; Fabri, S.G.; Zervakis, M.; Xanthopoulos, P.; Sakkalis, V.; Vanrumste, B. Review on solving the inverse problem in EEG source analysis. J. Neuroeng. Rehabil. 2008, 5, 1–33. [Google Scholar] [CrossRef]
- Asadzadeh, S.; Rezaii, T.Y.; Beheshti, S.; Delpak, A.; Meshgini, S. A systematic review of EEG source localization techniques and their applications on diagnosis of brain abnormalities. J. Neurosci. Methods 2020, 339, 108740. [Google Scholar] [CrossRef]
- Grosse-Wentrup, M.; Liefhold, C.; Gramann, K.; Buss, M. Beamforming in noninvasive brain–computer interfaces. IEEE Trans. Biomed. Eng. 2009, 56, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Huang, Y.; Parra, L.C. A highly detailed FEM volume conductor model based on the ICBM152 average head template for EEG source imaging and TCS targeting. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 5744–5747. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Szegedy, C.; Ioffe, S.; Vanhoucke, V.; Alemi, A. Inception-v4, inception-resnet and the impact of residual connections on learning. In Proceedings of the AAAI Conference on Artificial Intelligence, San Francisco, CA, USA, 4–9 February 2017; Volume 31. [Google Scholar]
- Niu, Z.; Zhong, G.; Yu, H. A review on the attention mechanism of deep learning. Neurocomputing 2021, 452, 48–62. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Biasiucci, A.; Franceschiello, B.; Murray, M.M. Neuroplasticity in Stroke Rehabilitation through Motor Imagery-based Brain–Computer Interfaces. J. Neural Eng. 2018, 15, 041001. [Google Scholar]
- Naros, G.; Gharabaghi, A. Reinforcement Learning of Motor Imagery-Based Brain–Computer Interface Control in Chronic Stroke. J. Neural Eng. 2015, 12, 066003. [Google Scholar]


| Participant | Accuracy (%) | Kappa | Precision | Sensitivity |
|---|---|---|---|---|
| participant 1 | 98.33 | 0.975 | 0.984 | 0.983 |
| participant 2 | 96.67 | 0.950 | 0.967 | 0.967 |
| participant 3 | 98.33 | 0.975 | 0.984 | 0.983 |
| participant 4 | 98.33 | 0.975 | 0.984 | 0.983 |
| participant 5 | 96.67 | 0.950 | 0.970 | 0.967 |
| participant 6 | 91.67 | 0.875 | 0.920 | 0.917 |
| participant 7 | 96.67 | 0.950 | 0.968 | 0.967 |
| participant 8 | 98.33 | 0.975 | 0.984 | 0.983 |
| participant 9 | 96.67 | 0.950 | 0.970 | 0.967 |
| participant 10 | 98.33 | 0.975 | 0.984 | 0.983 |
| Participant | Accuracy (%) | Kappa | Precision | Sensitivity |
|---|---|---|---|---|
| participant 1 | 91.67 | 0.875 | 0.926 | 0.917 |
| participant 2 | 93.33 | 0.900 | 0.944 | 0.933 |
| participant 3 | 96.67 | 0.950 | 0.970 | 0.967 |
| participant 4 | 98.33 | 0.975 | 0.984 | 0.983 |
| participant 5 | 98.33 | 0.975 | 0.984 | 0.983 |
| participant 6 | 88.33 | 0.825 | 0.914 | 0.883 |
| participant 7 | 95.00 | 0.925 | 0.957 | 0.950 |
| participant 8 | 83.33 | 0.750 | 0.873 | 0.833 |
| participant 9 | 88.33 | 0.825 | 0.914 | 0.883 |
| participant 10 | 93.33 | 0.900 | 0.944 | 0.933 |
| Participant | Accuracy (%) | Kappa | Precision | Sensitivity |
|---|---|---|---|---|
| participant 1 | 88.33 | 0.825 | 0.888 | 0.883 |
| participant 2 | 93.33 | 0.900 | 0.944 | 0.933 |
| participant 3 | 83.33 | 0.750 | 0.853 | 0.833 |
| participant 4 | 91.67 | 0.875 | 0.924 | 0.917 |
| participant 5 | 95.00 | 0.925 | 0.951 | 0.950 |
| participant 6 | 93.33 | 0.900 | 0.935 | 0.933 |
| participant 7 | 88.33 | 0.825 | 0.892 | 0.883 |
| participant 8 | 93.33 | 0.900 | 0.939 | 0.933 |
| participant 9 | 80.00 | 0.700 | 0.829 | 0.800 |
| participant 10 | 98.33 | 0.975 | 0.984 | 0.983 |
| Method | Average Accuracy (%) | Kappa | Precision | Sensitivity |
|---|---|---|---|---|
| CSP + LDA [15] | 55.57 | 0.1114 | 0.5619 | 0.5707 |
| FBCSP + SVM [15] | 57.57 | 0.1514 | 0.5690 | 0.5668 |
| TSLDA + DGFMDRM [15] | 61.20 | 0.2240 | 0.6160 | 0.6111 |
| TWFB + DGFMDRM [15] | 72.21 | 0.4442 | 0.7543 | 0.7845 |
| Dipole fitting | 91.03 | 0.8655 | 0.9106 | 0.9103 |
| MNE | 89.07 | 0.8360 | 0.8916 | 0.8907 |
| Beamforming | 87.17 | 0.8075 | 0.8734 | 0.8717 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaviri, S.M.; Vinjamuri, R. Integrating Electroencephalography Source Localization and Residual Convolutional Neural Network for Advanced Stroke Rehabilitation. Bioengineering 2024, 11, 967. https://doi.org/10.3390/bioengineering11100967
Kaviri SM, Vinjamuri R. Integrating Electroencephalography Source Localization and Residual Convolutional Neural Network for Advanced Stroke Rehabilitation. Bioengineering. 2024; 11(10):967. https://doi.org/10.3390/bioengineering11100967
Chicago/Turabian StyleKaviri, Sina Makhdoomi, and Ramana Vinjamuri. 2024. "Integrating Electroencephalography Source Localization and Residual Convolutional Neural Network for Advanced Stroke Rehabilitation" Bioengineering 11, no. 10: 967. https://doi.org/10.3390/bioengineering11100967
APA StyleKaviri, S. M., & Vinjamuri, R. (2024). Integrating Electroencephalography Source Localization and Residual Convolutional Neural Network for Advanced Stroke Rehabilitation. Bioengineering, 11(10), 967. https://doi.org/10.3390/bioengineering11100967








