Dynamics of Treatment Response to Faricimab for Diabetic Macular Edema
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Data Collection
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographics
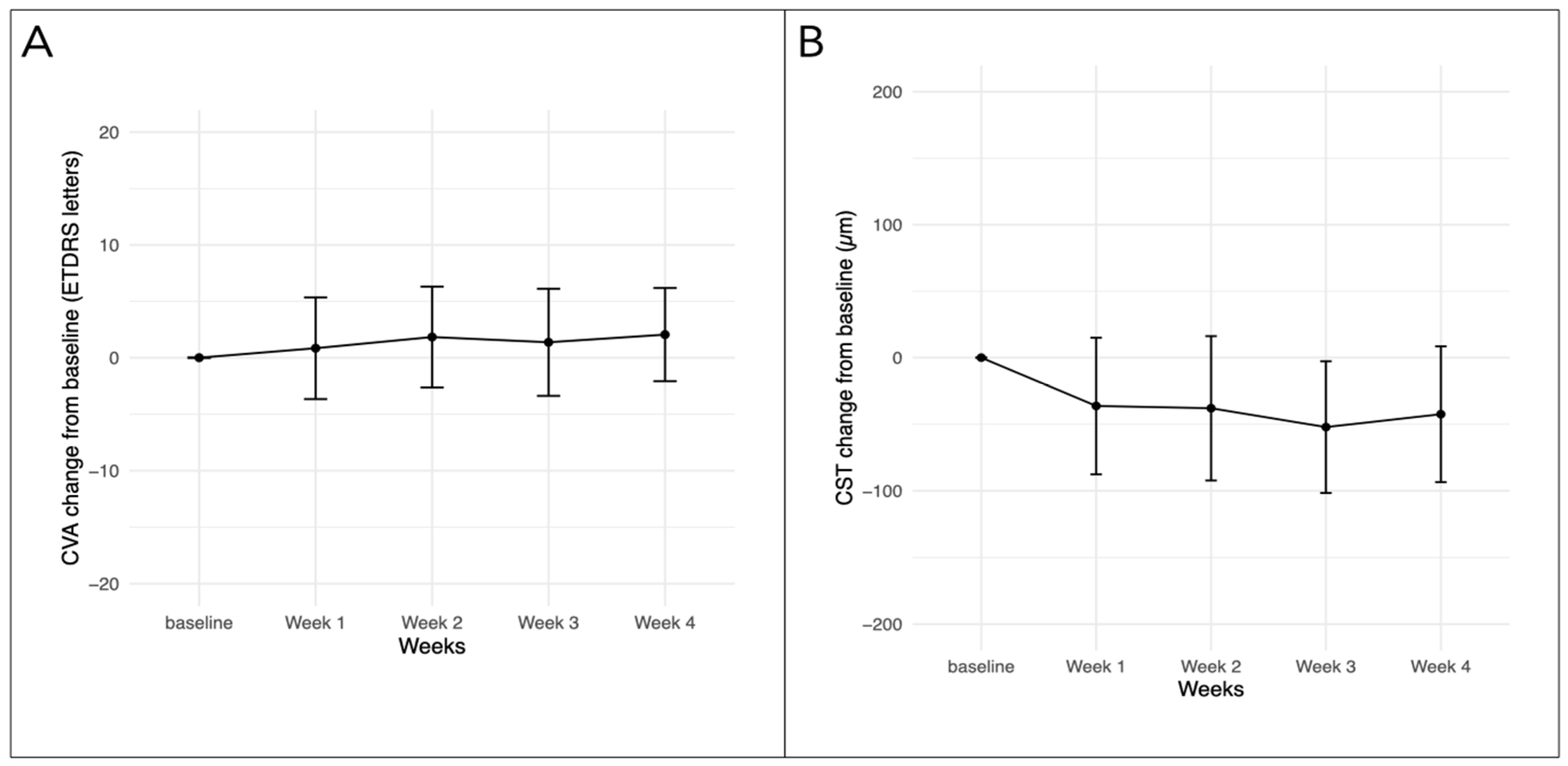
3.2. Efficacy
3.3. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, E.J.; Lin, W.V.; Rodriguez, S.M.; Chen, A.; Loya, A.; Weng, C.Y. Treatment of Diabetic Macular Edema. Curr. Diab. Rep. 2019, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B.; Brucker, A.J.; Ferris, F.L.; Hampton, G.R.; Jhaveri, C.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheema, A.A.; Cheema, H.R. Diabetic Macular Edema Management: A Review of Anti-Vascular Endothelial Growth Factor (VEGF) Therapies. Cureus 2024, 16, e52676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diabetic Retinopathy Clinical Research Network; Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Moja, L.; Lucenteforte, E.; Kwag, K.H.; Bertele, V.; Campomori, A.; Chakravarthy, U.; D’Amico, R.; Dickersin, K.; Kodjikian, L.; Lindsley, K.; et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2014, 9, CD011230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.M.; Woo, S.J. Immunogenicity and Potential for Intraocular Inflammation of Intravitreal Anti-VEGF Drugs. Curr. Ther. Res. Clin. Exp. 2024, 100, 100742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uludag, G.; Hassan, M.; Matsumiya, W.; Pham, B.H.; Chea, S.; Than, N.T.T.; Doan, H.L.; Akhavanrezayat, A.; Halim, M.S.; Do, D.V.; et al. Efficacy and safety of intravitreal anti-VEGF therapy in diabetic retinopathy: What we have learned and what should we learn further? Expert Opin. Biol. Ther. 2022, 22, 1275–1291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; A Eichenbaum, D.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.; Paulo, T.; Bührer, C.; Holekamp, N.M.; Bagijn, M. Comparative Efficacy, Durability and Safety of Faricimab in the Treatment of Diabetic Macular Edema: A Systematic Literature Review and Network Meta-Analysis. Adv. Ther. 2023, 40, 5204–5221, Erratum in Adv. Ther. 2024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Penha, F.M.; Masud, M.; Khanani, Z.A.; Thomas, M.; Fong, R.D.; Smith, K.; Chand, A.; Khan, M.; Gahn, G.; Melo, G.B.; et al. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int. J. Retin. Vitr. 2024, 10, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Chong, R.; Fung, A.T. Association of Occlusive Retinal Vasculitis with Intravitreal Faricimab. JAMA Ophthalmol. 2024, 142, 489. [Google Scholar] [CrossRef] [PubMed]
- Genentech. VABYSMO (Faricimab-Svoa): New Warnings and Precautions: Retinal Vasculitis and/or Retinal Vascular Occlusion. Available online: https://www.gene.com/download/pdf/Vabysmo_DHCP_Important_Drug_Warning_2023-11-03.pdf (accessed on 14 January 2024).
- Chen, X.; Wang, X.; Li, X. Intra-Ocular Inflammation and Occlusive Retinal Vasculitis Following Intravitreal Injections of Faricimab: A Case Report. Ocul. Immunol. Inflamm. 2024, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T.; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durrani, A.F.; Momenaei, B.; Wakabayashi, T.; Vemula, S.; Pandit, S.A.; Hsu, J.; Ho, A.C.; Spirn, M.J.; Klufas, M.A.; Garg, S.J.; et al. Conversion to faricimab after prior anti-vascular endothelial growth factor therapy for persistent diabetic macular oedema. Br. J. Ophthalmol. 2024, 108, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Quah, N.Q.X.; Javed, K.M.A.A.; Arbi, L.; Hanumunthadu, D. Real-World Outcomes of Faricimab Treatment for Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema. Clin. Ophthalmol. 2024, 18, 1479–1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muth, D.R.; Fasler, K.F.; Kvanta, A.; Rejdak, M.; Blaser, F.; Zweifel, S.A. Real-World Weekly Efficacy Analysis of Faricimab in Patients with Age-Related Macular Degeneration. Bioengineering 2024, 11, 478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Eyes, n | 43 |
| Patients, n | 31 |
| Women, n (%) | 12 (38.0) |
| Baseline age, years, median [IQR] | 66 [58.5–71.5] |
| Baseline CVA, ETDRS letters, median [IQR] | 72 [68.5–78.5] |
| Baseline CST, micrometers, median [IQR] | 325.0 [293.5–399.0] |
| Baseline IOP, mmHg, mean (±SD) | 15.5 (±3.1) |
| Diagnosis, n (%) | |
| non-proliferative DR | 24 (55.8) |
| proliferative DR | 19 (44.2) |
| Treatment-naïve eyes (%) | 8 (18.6) |
| Pretreated eyes (%) | 35 (81.4) |
| Median treatment interval, weeks, [min–max range] | 4 [4–6] |
| Previous number of IVTs, median [IQR] | 16 [6–28] |
| Previous anti-VEGF agent, eyes (%) | |
| Aflibercept | 27 (77.1) |
| Ranibizumab | 4 (11.4) |
| Aflibercept and ranibizumab | 4 (11.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasler, K.; Muth, D.R.; Cozzi, M.; Kvanta, A.; Rejdak, M.; Blaser, F.; Zweifel, S.A. Dynamics of Treatment Response to Faricimab for Diabetic Macular Edema. Bioengineering 2024, 11, 964. https://doi.org/10.3390/bioengineering11100964
Fasler K, Muth DR, Cozzi M, Kvanta A, Rejdak M, Blaser F, Zweifel SA. Dynamics of Treatment Response to Faricimab for Diabetic Macular Edema. Bioengineering. 2024; 11(10):964. https://doi.org/10.3390/bioengineering11100964
Chicago/Turabian StyleFasler, Katrin, Daniel R. Muth, Mariano Cozzi, Anders Kvanta, Magdalena Rejdak, Frank Blaser, and Sandrine A. Zweifel. 2024. "Dynamics of Treatment Response to Faricimab for Diabetic Macular Edema" Bioengineering 11, no. 10: 964. https://doi.org/10.3390/bioengineering11100964
APA StyleFasler, K., Muth, D. R., Cozzi, M., Kvanta, A., Rejdak, M., Blaser, F., & Zweifel, S. A. (2024). Dynamics of Treatment Response to Faricimab for Diabetic Macular Edema. Bioengineering, 11(10), 964. https://doi.org/10.3390/bioengineering11100964






