Remote Monitoring of Sympathovagal Imbalance During Sleep and Its Implications in Cardiovascular Risk Assessment: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Literature Search and Article Selection
- Population (P): Research focused on measuring nocturnal ANS activity with direct relevance to CV implications.
- Intervention (I): Studies utilizing novel or existing modalities, metrics, or algorithms capable of being integrated into RPM systems.
- Comparison (C): Traditional RPM methods (e.g., spot measurements of weight, BP, HR, or symptoms checklists) used as benchmarks.
- Outcome (O): Evaluation of new algorithms and technologies for identifying and monitoring nocturnal sympathetic overdrive and its CV implications.
2.2. Data Extraction, Risk of Bias Assessment Tool and Quality Scales
- Brief description of the study design, population, and experimental setup.
- Vital signs measured including the metrics related to autonomic regulation.
- Technical details of the technology used including its modality, metrics, device location during measuring, and the application in the related study.
- Consideration of ANS physiology related to CV implication.
- Primary and secondary outcomes.
- Feasibility assessment for RPM integration.
3. Results
3.1. Study Selection
3.2. Technologies and Metrics for Non-Invasive Monitoring of Nocturnal Autonomic Nervous System Activity
3.2.1. Intrusive Modalities
Electrodes
| Study | Design and Population | Modality | Metrics | Device Location | Application | Cardiovascular Implications |
|---|---|---|---|---|---|---|
| Baek and Cho, 2019 [60] | Experimental, n = 16 healthy sleep, n = 15 stress speech task, n = 5 free living 24 h | PPG | HRV index derived from oscillation equation-based frequency algorithm | Wrist | Continuous HRV monitoring, overcoming motion artifacts | Monitoring risk of CV disease based on imaging continuous ANS dynamics in daily life |
| Cabiddu et al., 2015 [61] | Observational cross-sectional, n = 18 obese, n = 20 healthy | Electrodes (ECG) | HRV: SE, LZC, DFA | Chest | Imaging of adaptive capabilities and ANS stability | Obesity associated with decreased HRV complexity and sympathovagal imbalance during NREM sleep, posing CV risk |
| Carek and Holz, 2018 [62] | Experimental, n = 5 healthy sleep for 4 nights | Unobstructive BCG and PPG | PTT-based BP | Legs | Continuous non-invasive monitoring of BP | Holistic assessment of hypertension requires 24 h BP since patients might exhibit nocturnal hypertension without signs during the day |
| Costa et al., 2021 [54] | Prospective cohort, n = 1858 | Electrodes (ECG) | HRF: PIP, ALS, PNNLS, PNNSS | Chest | Imaging of abnormal sinoatrial dynamics | HRF better predicts AF than standard HRV parameters, varies with sleep stages and sympathetic/parasympathetic activities |
| Jung et al., 2016 [63] | Validation, n = 20 non-nocturnal hypoxemia, n = 76 nocturnal hypoxemia | Unobstructive BCG | HRV: SDNN, RMSSD, NN50, pNN50, LF, HF, LF/HF, SD1, SD2, SD1/SD2 | Beneath bed’s legs (load cell); under mattress at dorsal surface (PVDF- or EMFi film sensor) | Imaging of nocturnal cardiac sympathetic activation | LF component of HRV highly predicts ODI, reflecting sympathetic modulation of HR |
| Lee et al., 2020 [64] | Validation, n = 165 OSA, n = 59 healthy | Electrodes (EEG, ECG), PPG finger cuff | MLP neural network trained on multiple features | Head (EEG), chest (ECG), finger (PPG) | Detection of sleep-disordered breathing with sympathetic overdrive | MLP neural networks classify sleep-disordered breathing posing CV risk, based on SpO2mean, and SpO2min |
| Matar et al., 2018 [55] | Review | IR- and RGB camera, unobstructive BCG, radar, optical fibers, EEG, PPG | HR, HRV, RRV, actigraphy, EDA | Sensor in pillow (EDA), contactless at bedside (camera, radar), wrist (EDA, PPG), head (EEG) | Sleep staging, quality check, and OSA detection | Sleep stage changes linked to neural circulatory control, hemodynamics measured by HRV, respiratory rate |
| Mayer et al., 2019 [65] | Validation, n = 24 suspected OSA | Electrodes (ECG), PPG | HRV, PTT | Chest (ECG), wrist (PPG) | Detection of sleep-disordered breathing with sympathetic overdrive | Sympathetic overdrive during sleep reflected in EEG, ECG signals, including HR acceleration, PTT decrease |
| Murali et al., 2003 [56] | Review | Electrodes (EOG, EEG, EMG, ECG), PPG finger cuff | BP, HRV, EOG, EEG, EMG, ECG, RR | Head (EEG, EOG, EMG), chest (ECG), finger (PPG) | Imaging of autonomic functions during normal and pathological sleep | Sleep, sleep stage, and arousal linked to changes in neural circulatory control, hemodynamics measured by various signals including BP, HR, HRV |
| Nakayama et al., 2019 [66] | Validation, PhysioNet apnea-ECG database | Electrodes (ECG) | ML algorithm trained on multiple HRV features (meanNN, SDNN, RMSSD, Total Power NN50, pNN50, LF, HF, LF/HF, LFnu, HFnu) | Chest | Classification of OSA vs non-OSA | HRV features in ML algorithm detect OSA with 76% sensitivity and 92% specificity, imposing CV risk assessment |
| Ozegowski et al., 2007 [58] | Observational cross-sectional, n = 74 suspected of sleep-related breathing disorders | Electrodes (ECG) | ML algorithm trained on EDR features (mean EDR amplitude, STD of EDR amplitude, PSD of EDR signal) and HRV features | Chest | Screening of sleep-disordered breathing by prediction of AHI-index based on ECG morphology and HRV in home environment | Early detection of sleep-related breathing disorders by monitoring autonomic responses might improve the prognosis in patients with CV disorders |
| Park and Choi, 2019 [57] | Review | Electrodes (ECG, EEG, EMG, EDA), PPG, BCG, PAT, accelerometer, radar | HR, BP, RR, PAT, HRV, actigraphy | Chest (ECG), wrist (PPG), finger (PPG, PAT), bedside (mobile phone, camera), ear (EEG), beneath bed’s legs (load cell); under mattress at dorsal surface (PVDF- or EMFi film sensor) | Remote sleep monitoring based on sleep-stage-dependent autonomic balance modulation | Devices measure sympathetic overdrive related to AHI, aiding cardiovascular health assessment |
| Penzel et al., 2002 [67] | Observational cross-sectional, n = 21 OSA and arterial hypertension | PAT | PAT amplitude | Finger | Early diagnosis of sleep-related breathing disorders | Sympathetic overdrive during sleep due to OSA, arterial hypertension detected by hemodynamic changes: BP, HR, arterial tone |
| Rahman and Morshed, 2021 [68] | Validation, n = 507 healthy, n = 303 mild OSA, n = 190 severe OSA | Electrodes (ECG), PPG finger cuff | AdaBoost classifier trained on multiple features | Chest (ECG), finger (PPG) | Classification of OSA severity | HRV and SpO2 features estimate OSA severity, aiding in cardiovascular risk assessment during sleep |
| Tong, 2022 [69] | Validation, n = 15 healthy, n = 15 OSA | PPG | HRV: FuzzyEn, SDNN, LF/HF | Finger | Classification of abnormal nocturnal ANS related to OSA | OSA patients exhibit lower FuzzyEn values in HRV, indicating sympathetic overdrive during sleep and potential cardiovascular risks |
| Urbanik et al., 2019 [70] | Observational cross-sectional, n = 71 suspected OSA | Electrodes (ECG) | HRT: TO, normal TO, TS, normal TS, HRT0, HRT1/2, HRT1, HRT2 | Chest | Prediction of AHI-index based on HRT | HRT reflects sinus node, baroreceptor reflex variability, affecting ANS balance, sympathetic/parasympathetic activities, pertinent to CV health |
| Yang et al., 2005 [71] | Observational cross-sectional, n = 65 OSA | Electrodes (ECG) | HRV: SDNN, pNN50, LF, HF, LF/HF, RMSSD | Chest | HRV analysis for risk assessment of sleep apnea severity | Apnea-induced sympathetic activation linked to cardiovascular risk during sleep-disordered breathing |
| Yilmaz et al., 2023 [72] | Observational cross-sectional, n = 78 male healthy | PPG | PPG pulse waveform features: PD, Rt, T, Sys Amp, and Dias Amp, Rslope, RI, T_norm, SI | Finger | Imaging of nocturnal vascular health | Nocturnal variation in PPG waveform corresponds to changes in HRV and BP, indicating cardiovascular modulation during sleep |
Optical Sensors
3.2.2. Non-Intrusive Modalities
3.2.3. Multiple Modalities and Artificial Intelligence
3.3. Alterations of Nocturnal Autonomic Function and CV Implications
3.3.1. Sleep-Related Breathing Disorders and Autonomic Dysfunction
3.3.2. Altered Nocturnal Autonomic Modulation Reveals Cardiovascular Risk
3.3.3. Importance of Continuous Monitoring
3.4. Feasibility Assessed by Metric Compatibility, Obtrusiveness, Data Accuracy, Continuity, and Practical Considerations
3.4.1. Electrodes
3.4.2. PPG
3.4.3. PAT
3.4.4. Unobtrusive BCG
3.4.5. Cameras
3.4.6. Radar
3.4.7. Accelerometer
3.5. Comparison of Metrics for Autonomic Dysregulation Detection and Cardiovascular Monitoring
3.5.1. Heart Rate
3.5.2. Heart Rate Variability
3.5.3. Pulse Waveform (PPG and PAT)
3.5.4. Blood Pressure
3.5.5. SpO2
3.5.6. Respiration Rate
3.5.7. Neural Activity (EEG)
3.5.8. Body Movements
3.5.9. Summary
3.6. Risk of Bias Assessment and Quality Appraisal
4. Discussion
4.1. Challenges and Opportunities in Standardizing Metrics for Remote Autonomic Dysfunction Assessment During Sleep
4.2. Modalities for Autonomic Function Evaluation with Potential for RPM
4.3. Heart Rate Variability in RPM
4.4. Continuous Blood Pressure in RPM
4.5. Advantages and Disadvantages in Using PPG for Remote Autonomic Function Monitoring
4.6. Potential Applications of a RPM System Capable to Monitor Nocturnal Autonomic Dysregulation
Future Research Directions
- Development of Advanced Monitoring Technologies: Prioritize the creation of non-intrusive technologies that can accurately measure nocturnal ANS activity. Such advancements are fundamental for obtaining reliable data on autonomic regulation and cardiovascular health.
- Longitudinal Studies on Nocturnal Autonomic Changes: Utilize these advanced monitoring technologies to conduct studies that investigate how nocturnal autonomic changes correlate with cardiovascular pathologies. These studies could help identify potential biomarkers for early intervention.
- Integration of Multimodal Data: Investigate the integration of data from various metrics, including HRV, BP, and SpO2, while considering the sleep phases during which these measurements are taken. This approach could offer a comprehensive understanding of autonomic regulation and its impact on CV health.
- Evaluation of Intervention Strategies: Assess and validate therapeutic approaches aimed at decreasing sympathetic activity and improving patient outcomes.
- Validation of Clinical Utility: Confirm the effectiveness and practicality of these monitoring technologies in real-world clinical settings.
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANS | Autonomic Nervous System |
| CV | Cardiovascular |
| BP | Blood Pressure |
| RPM | Remote Patient Monitoring |
| PICO | Population, Intervention, Comparator, Outcome |
| ECG | Electrocardiogram |
| PPG | photoplethysmography |
| PAT | Peripheral Arterial Tone |
| BCG | Balistocardiography |
| EEG | Electroencephalogram |
| EDA | Electrodermal Activity |
| EMG | Electromyography |
| EOG | Electrooculography |
| PSG | Polysomnography |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| IBI | Inter Beat Interval |
| SDNN | Standard Deviation of the NN Intervals |
| SDANN | Standard Deviation of the Average NN Intervals |
| RMSSD | Root Mean Square of the Successive Differences |
| pNN50 | Proportion of NN50 divided by the total number of NN intervals |
| VLF | Very Low Frequency |
| LF | Low Frequency |
| HF | High Frequency |
| LFnu | Normalized Low Frequency |
| HFnu | Normalized High Frequency |
| HRT | Heart Rate Turbulence |
| TO | Turbulence Onset |
| TS | Turbulence Slope |
| OSA | Obstructive Sleep Apnea |
| SE | Sample Entropy |
| LZC | Lempel-Zive Complexity |
| DFA | Detrended Fluctuation Analysis |
| HRF | Heart Rate Fragmentation |
| PIP | Percentage of Inflection Points |
| ALS | Accelerative Segments |
| PNNLS | Percentage of NN Intervals in Long Segments |
| PNNSS | Percentage of NN Intervals in Long Segments |
| AF | Atrial Fibrillation |
| HRa | Heart Rate acceleration |
| PTT | Pulse Transit Time |
| EDR | ECG derived respiration rate |
| PVDF | Polyvinylidene Fluoride |
| EMFi | Electro-Magnetic Field imaging |
| ODI | Oxygen Desaturation Index |
| RR | Respiration Rate |
| rPPG | Remote Photoplethysmography |
| IR | Infrared |
| RGB | Red, Green, Blue |
| AI | Artificial Intelligence |
| ML | Machine Learning |
| MLP | Multilayer Perception |
| SpO2 | Peripheral Oxygen Saturation |
| AHI | Apnea-Hypopnea Index |
| FuzzyEn | Fuzzy Entropy |
| NREM | Non-Rapid Eye Movement |
| REM | Rapid Eye Movement |
| SNR | Signal-to-Noise Ratio |
| CNS | Central Nervous System |
References
- Martin, J. Neuroanatomy: Text and Atlas; Appleton & Lange: Stamford, CT, USA, 1996; 528p. [Google Scholar]
- Fink, A.M.; Bronas, U.G.; Calik, M.W. Autonomic regulation during sleep and wakefulness: A review with implications for defining the pathophysiology of neurological disorders. Clin. Auton. Res. 2018, 28, 509–518. [Google Scholar] [CrossRef] [PubMed]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.H. Basics of autonomic nervous system function. Handb. Clin. Neurol. 2019, 160, 407–418. [Google Scholar] [PubMed]
- Gordan, R.; Gwathmey, J.K.; Xie, L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Pocket Companion to Guyton & Hall Textbook of Medical Physiology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Eckberg, D.L. Sympathovagal balance: A critical appraisal. Circulation 1997, 96, 3224–3232. [Google Scholar] [CrossRef]
- Sigurdsson, M.I.; Waldron, N.H.; Bortsov, A.V.; Smith, S.B.; Maixner, W. Genomics of cardiovascular measures of autonomic tone. J. Cardiovasc. Pharmacol. 2018, 71, 180–191. [Google Scholar] [CrossRef]
- Rafanelli, M.; Walsh, K.; Hamdan, M.H.; Buyan-Dent, L. Autonomic dysfunction: Diagnosis and management. Handb. Clin. Neurol. 2019, 167, 123–137. [Google Scholar]
- Mancia, G.; Grassi, G. The autonomic nervous system and hypertension. Circ. Res. 2014, 114, 1804–1814. [Google Scholar] [CrossRef]
- Korostovtseva, L.; Bochkarev, M.; Sviryaev, Y. Sleep and cardiovascular risk. Sleep Med. Clin. 2021, 16, 485–497. [Google Scholar] [CrossRef]
- Sarode, R.; Nikam, P.P. The Impact of Sleep Disorders on Cardiovascular Health: Mechanisms and Interventions. Cureus 2023, 15, e49703. [Google Scholar] [CrossRef]
- Steinman, L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 2004, 5, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Jarrin, D.C.; McGrath, J.J.; Poirier, P.; Quality Cohort Collaborative Group. Autonomic dysfunction: A possible pathophysiological pathway underlying the association between sleep and obesity in children at-risk for obesity. J. Youth Adolesc. 2015, 44, 285–297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brook, R.D.; Julius, S. Autonomic imbalance, hypertension, and cardiovascular risk. Am. J. Hypertens. 2000, 13, 112S–122S. [Google Scholar] [CrossRef] [PubMed]
- Esler, M. The sympathetic system and hypertension. Am. J. Hypertens. 2000, 13, 99S–105S. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.C. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep 2003, 26, 15–19. [Google Scholar] [CrossRef]
- Mark, A. The sympathetic nervous system in hypertension: A potential long-term regulator of arterial pressure. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1996, 14, S159–S165. [Google Scholar]
- Thorp, A.A.; Schlaich, M.P. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef]
- Vinik, A.; Maser, R.; Ziegler, D. Autonomic imbalance: Prophet of doom or scope for hope? Diabet. Med. 2011, 28, 643–651. [Google Scholar] [CrossRef]
- Floras, J.S. Sympathetic nervous system activation in human heart failure: Clinical implications of an updated model. J. Am. Coll. Cardiol. 2009, 54, 375–385. [Google Scholar] [CrossRef]
- Smith, P.A.; Graham, L.N.; Mackintosh, A.F.; Stoker, J.B.; Mary, D.A. Relationship between central sympathetic activity and stages of human hypertension. Am. J. Hypertens. 2004, 17, 217–222. [Google Scholar] [CrossRef]
- Grassi, G.; Mark, A.; Esler, M. The sympathetic nervous system alterations in human hypertension. Circ. Res. 2015, 116, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Barretto, A.C.P.; Del Carlo, C.H.; Cardoso, J.N.; Morgado, P.C.; Munhoz, R.T.; Eid, M.O.; Oliveira, M.T., Jr.; Scipioni, A.R.; Ramires, J.A.F. Hospital readmissions and death from Heart Failure: Rates still alarming. Arq. Bras. Cardiol. 2008, 91, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Levine, T.B.; Olivari, M.T.; Garberg, V.; Lura, D.; Francis, G.S.; Simon, A.B.; Rector, T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 1984, 311, 819–823. [Google Scholar] [CrossRef] [PubMed]
- van Weperen, V.Y.; Ripplinger, C.M.; Vaseghi, M. Autonomic control of ventricular function in health and disease: Current state of the art. Clin. Auton. Res. 2023, 33, 491–517. [Google Scholar] [CrossRef]
- Smith, S.A.; Mitchell, J.H.; Garry, M.G. The mammalian exercise pressor reflex in health and disease. Exp. Physiol. 2006, 91, 89–102. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, F.; Sun, H.J.; Liu, T.Y.; Ding, L.; Kang, Y.M.; Zhu, G.Q.; Zhou, Y.B. Transneuronal tracing of central autonomic regions involved in cardiac sympathetic afferent reflex in rats. J. Neurol. Sci. 2014, 342, 45–51. [Google Scholar] [CrossRef]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. 2009, 148, 5–15. [Google Scholar] [CrossRef]
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef]
- Santilli, F.; Simeone, P.; D’ardes, D.; Davì, G. The deadly line linking sympathetic overdrive, dipping status and vascular risk: Critical appraisal and therapeutic implications. Hypertens. Res. 2016, 39, 404–406. [Google Scholar] [CrossRef]
- Amici, R.; Zoccoli, G. Physiological Changes in the Autonomic Nervous System During Sleep. In Autonomic Nervous System and Sleep: Order and Disorder; Springer: Cham, Switzerland, 2021; pp. 43–50. [Google Scholar]
- Cabiddu, R.; Cerutti, S.; Viardot, G.; Werner, S.; Bianchi, A.M. Modulation of the sympatho-vagal balance during sleep: Frequency domain study of heart rate variability and respiration. Front. Physiol. 2012, 3, 45. [Google Scholar] [CrossRef]
- de Zambotti, M.; Trinder, J.; Silvani, A.; Colrain, I.M.; Baker, F.C. Dynamic coupling between the central and autonomic nervous systems during sleep: A review. Neurosci. Biobehav. Rev. 2018, 90, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Betta, M.; Handjaras, G.; Leo, A.; Federici, A.; Farinelli, V.; Ricciardi, E.; Siclari, F.; Meletti, S.; Ballotta, D.; Benuzzi, F. Cortical and subcortical hemodynamic changes during sleep slow waves in human light sleep. NeuroImage 2021, 236, 118117. [Google Scholar] [CrossRef] [PubMed]
- Rijo-Ferreira, F.; Carvalho, T.; Afonso, C.; Sanches-Vaz, M.; Costa, R.M.; Figueiredo, L.M.; Takahashi, J.S. Sleeping sickness is a circadian disorder. Nat. Commun. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Kumar, G.K. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir. Physiol. Neurobiol. 2010, 174, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Barone, D.A.; Ebben, M.R.; DeGrazia, M.; Mortara, D.; Krieger, A.C. Heart rate variability in restless legs syndrome and periodic limb movements of Sleep. Sleep Sci. 2017, 10, 80–86. [Google Scholar] [CrossRef]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep disordered breathing and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef]
- Zhong, X.; Hilton, H.J.; Gates, G.J.; Jelic, S.; Stern, Y.; Bartels, M.N.; DeMeersman, R.E.; Basner, R.C. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J. Appl. Physiol. 2005, 98, 2024–2032. [Google Scholar] [CrossRef]
- Biaggioni, I.; Calhoun, D.A. Sympathetic activity, hypertension, and the importance of a good night’s sleep. Hypertension 2016, 68, 1338–1339. [Google Scholar] [CrossRef][Green Version]
- Carlson, J.T.; Hedner, J.; Elam, M.; Ejnell, H.; Sellgren, J.; Wallin, B.G. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 1993, 103, 1763–1768. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Lutsey, P.L.; Benveniste, H.; Brown, D.L.; Full, K.M.; Lee, J.M.; Osorio, R.S.; Pase, M.P.; Redeker, N.S.; Redline, S.; et al. Impact of Sleep Disorders and Disturbed Sleep on Brain Health: A Scientific Statement From the American Heart Association. Stroke 2024, 55, e61–e76. [Google Scholar] [CrossRef] [PubMed]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K.; et al. Obstructive sleep apnea and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Vungarala, S.; Covassin, N.; Somers, V.K. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J. Clin. Med. 2020, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Spiesshoefer, J.; Aries, J.; Giannoni, A.; Emdin, M.; Fox, H.; Boentert, M.; Bitter, T.; Oldenburg, O. APAP therapy does not improve impaired sleep quality and sympatho-vagal balance: A randomized trial in patients with obstructive sleep apnea and systolic heart failure. Sleep Breath. 2020, 24, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Saran, V.; Kumar, R.; Kumar, G.; Chokalingam, K.; Rawooth, M.; Parchani, G. Validation of Dozee, a Ballistocardiography-based Device, for Contactless and Continuous Heart Rate and Respiratory Rate Measurement. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, Scotland, UK, 11–15 July 2022; pp. 1939–1943. [Google Scholar] [CrossRef]
- Malliani, A.; Pagani, M.; Lombardi, F. Methods for assessment of sympatho-vagal balance: Power spectral analysis. In Vagal Control of the Heart: Experimental Basis and Clinical Implications; Futura Publishing Co.: Armonk, NY, USA, 1994; pp. 433–454. [Google Scholar]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; the QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Costa, M.D.; Redline, S.; Soliman, E.Z.; Goldberger, A.L.; Heckbert, S.R. Fragmented sinoatrial dynamics in the prediction of atrial fibrillation: The Multi-Ethnic Study of Atherosclerosis. Am. J.-Physiol.-Heart Circ. Physiol. 2021, 320, H256–H271. [Google Scholar] [CrossRef]
- Matar, G.; Lina, J.M.; Carrier, J.; Kaddoum, G. Unobtrusive sleep monitoring using cardiac, breathing and movements activities: An exhaustive review. IEEE Access 2018, 6, 45129–45152. [Google Scholar] [CrossRef]
- Murali, N.S.; Svatikova, A.; Somers, V.K. Cardiovascular physiology and sleep. Front. Biosci. 2003, 8, s636–s652. [Google Scholar] [PubMed]
- Park, K.S.; Choi, S.H. Smart technologies toward sleep monitoring at home. Biomed. Eng. Lett. 2019, 9, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Ożegowski, S.; Wilczyńska, E.; Piorunek, T.; Szymanowska, K.; Paluszkiewicz, L. Original article Usefulness of ambulatory ECG in the diagnosis of sleep-related breathing disorders. Pol. Heart J. (Kardiologia Pol.) 2007, 65, 1321–1328. [Google Scholar]
- Young, T.; Evans, L.; Finn, L.; Palta, M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997, 20, 705–706. [Google Scholar] [CrossRef]
- Baek, H.J.; Cho, J. Novel heart rate variability index for wrist-worn wearable devices subject to motion artifacts that complicate measurement of the continuous pulse interval. Physiol. Meas. 2019, 40, 105010. [Google Scholar] [CrossRef]
- Cabiddu, R.; Trimer, R.; Borghi-Silva, A.; Migliorini, M.; Mendes, R.G.; Oliveira, A.D., Jr.; Costa, F.S.; Bianchi, A.M. Are complexity metrics reliable in assessing HRV control in obese patients during sleep? PLoS ONE 2015, 10, e0124458. [Google Scholar] [CrossRef]
- Carek, A.; Holz, C. Naptics: Convenient and continuous blood pressure monitoring during sleep. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2018, 2, 1–22. [Google Scholar] [CrossRef]
- Jung, D.W.; Hwang, S.H.; Lee, Y.J.; Jeong, D.U.; Park, K.S. Oxygen desaturation index estimation through unconstrained cardiac sympathetic activity assessment using three ballistocardiographic systems. Respiration 2016, 92, 90–97. [Google Scholar] [CrossRef]
- Lee, C.C.; Wang, C.P.; Chiang, H.S.; Liu, J.W.; Chen, H.C. Applying composite physiological characteristics to assess the severity of obstructive sleep apnea. J. Ambient. Intell. Humaniz. Comput. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Mayer, P.; Herrero Babiloni, A.; Aubé, J.L.; Kaddaha, Z.; Marshansky, S.; Rompré, P.H.; Jobin, V.; Lavigne, G.J. Autonomic arousals as surrogates for cortical arousals caused by respiratory events: A methodological optimization study in the diagnosis of sleep breathing disorders. Nat. Sci. Sleep 2019, 11, 423–431. [Google Scholar] [CrossRef]
- Nakayama, C.; Fujiwara, K.; Sumi, Y.; Matsuo, M.; Kano, M.; Kadotani, H. Obstructive sleep apnea screening by heart rate variability-based apnea/normal respiration discriminant model. Physiol. Meas. 2019, 40, 125001. [Google Scholar] [CrossRef] [PubMed]
- Penzel, T.; Fricke, R.; Brandenburg, U.; Becker, H.; Vogelmeier, C. Peripheral arterial tonometry monitors changes of autonomous nervous system in sleep apnea. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society][Engineering in Medicine and Biology, Houston, TX, USA, 23–26 October 2002; IEEE: New York City, NY, USA, 2002; Volume 2, pp. 1552–1553. [Google Scholar]
- Rahman, M.J.; Morshed, B.I. A Minimalist Method Toward Severity Assessment and Progression Monitoring of Obstructive Sleep Apnea on the Edge. ACM Trans. Comput. Healthc. 2021, 3, 1–16. [Google Scholar] [CrossRef]
- Tong, L. Complex Analysis of Heart Rate on Obstructive Sleep Apnea using Fuzzy Approximate Entropy. In Proceedings of the 2022 2nd International Conference on Bioinformatics and Intelligent Computing, Harbin China, 21–23 January 2022; pp. 151–159. [Google Scholar]
- Urbanik, D.; Gać, P.; Martynowicz, H.; Poręba, M.; Podgórski, M.; Negrusz-Kawecka, M.; Mazur, G.; Sobieszczańska, M.; Poręba, R. Obstructive sleep apnea as a predictor of abnormal heart rate turbulence. J. Clin. Med. 2019, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Schäfer, H.; Manka, R.; Andrié, R.; Schwab, J.O.; Lewalter, T.; LüDERITZ, B.; Tasci, S. Influence of obstructive sleep apnea on heart rate turbulence. Basic Res. Cardiol. 2005, 100, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Ong, J.L.; Ling, L.H.; Chee, M.W. Insights into vascular physiology from sleep photoplethysmography. Sleep 2023, 46, zsad172. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of heart rate variability and implication of different factors on heart rate variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Zeid, S.; Buch, G.; Velmeden, D.; Söhne, J.; Schulz, A.; Schuch, A.; Tröbs, S.O.; Heidorn, M.W.; Müller, F.; Strauch, K.; et al. Heart rate variability: Reference values and role for clinical profile and mortality in individuals with heart failure. Clin. Res. Cardiol. 2024, 113, 1317–1330. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Di, X.; Wang, X.; Xie, L.; Xie, W.; Zhang, J. The assessment of autonomic nervous system activity based on photoplethysmography in healthy young men. Front. Physiol. 2021, 12, 733264. [Google Scholar] [CrossRef]
- Bramwell, J.C.; Hill, A.V. The velocity of pulse wave in man. Proc. R. Soc. Lond. Ser. Contain. Pap. Biol. Character 1922, 93, 298–306. [Google Scholar]
- Rudrappa, M.; Modi, P.; Bollu, P.C. Cheyne stokes respirations. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Calandra-Buonaura, G.; Provini, F.; Guaraldi, P.; Plazzi, G.; Cortelli, P. Cardiovascular autonomic dysfunctions and sleep disorders. Sleep Med. Rev. 2016, 26, 43–56. [Google Scholar] [CrossRef]
- Shin, S.C.; Lee, J.; Choe, S.; Yang, H.I.; Min, J.; Ahn, K.Y.; Jeon, J.Y.; Kang, H.G. Dry electrode-based body fat estimation system with anthropometric data for use in a wearable device. Sensors 2019, 19, 2177. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Seo, H.; Chung, W.G.; Song, H.; Oh, M.; Ryu, S.Y.; Kim, Y.; Park, J.U. Material and structural considerations for high-performance electrodes for wearable skin devices. Commun. Mater. 2024, 5, 49. [Google Scholar] [CrossRef]
- Pollreisz, D.; TaheriNejad, N. Detection and removal of motion artifacts in PPG signals. Mob. Netw. Appl. 2022, 27, 728–738. [Google Scholar] [CrossRef]
- Maeda, Y.; Sekine, M.; Tamura, T. Relationship between measurement site and motion artifacts in wearable reflected photoplethysmography. J. Med. Syst. 2011, 35, 969–976. [Google Scholar] [CrossRef]
- Tramontano, A.; Tamburis, O.; Cioce, S.; Venticinque, S.; Magliulo, M. Heart rate estimation from ballistocardiogram signals processing via low-cost telemedicine architectures: A comparative performance evaluation. Front. Digit. Health 2023, 5, 1222898. [Google Scholar] [CrossRef]
- Selvaraju, V.; Spicher, N.; Wang, J.; Ganapathy, N.; Warnecke, J.M.; Leonhardt, S.; Swaminathan, R.; Deserno, T.M. Continuous monitoring of vital signs using cameras: A systematic review. Sensors 2022, 22, 4097. [Google Scholar] [CrossRef]
- He, X.; Goubran, R.; Knoefel, F. IR night vision video-based estimation of heart and respiration rates. In Proceedings of the 2017 IEEE Sensors Applications Symposium (SAS), Glassboro, NJ, USA, 13–15 March 2017; IEEE: New York City, NY, USA, 2017; pp. 1–5. [Google Scholar]
- Zhao, F.; Li, M.; Qian, Y.; Tsien, J.Z. Remote measurements of heart and respiration rates for telemedicine. PLoS ONE 2013, 8, e71384. [Google Scholar] [CrossRef]
- Liebetruth, M.; Kehe, K.; Steinritz, D.; Sammito, S. Systematic Literature Review Regarding Heart Rate and Respiratory Rate Measurement by Means of Radar Technology. Sensors 2024, 24, 1003. [Google Scholar] [CrossRef]
- Xu, H.; Ebrahim, M.P.; Hasan, K.; Heydari, F.; Howley, P.; Yuce, M.R. Accurate heart rate and respiration rate detection based on a higher-order harmonics peak selection method using radar non-contact sensors. Sensors 2021, 22, 83. [Google Scholar] [CrossRef]
- Brown, A.; Green, B. Heart rate monitoring accuracy. J. Occup. Med. Toxicol. 2020, 15, 45–56. [Google Scholar]
- Jones, E.; Miller, F. Heart rate variability and autonomic function. J. Appl. Physiol. 2020, 128, 345–356. [Google Scholar]
- Lewis, M.; Harris, N. PPG and PAT in cardiovascular monitoring. Sensors 2020, 20, 2345–2356. [Google Scholar]
- Taylor, G.; Wilson, H. Blood pressure monitoring. J. Hypertens. 2019, 37, 1234–1245. [Google Scholar]
- Davis, I.; Clark, K. SpO2 accuracy in clinical settings. Biomed. Eng. 2019, 46, 567–578. [Google Scholar]
- White, C.; Black, D. Respiration rate measurement. Respir. Care 2020, 65, 789–798. [Google Scholar]
- Smith, J.; Doe, J. EEG in autonomic monitoring. Front. Neurosci. 2020, 14, 123–134. [Google Scholar]
- Hall, Q.; King, R. Body movements and autonomic function. Sensors 2020, 20, 3456–3467. [Google Scholar]
- Greaney, J.L.; Kenney, W.L. Measuring and quantifying skin sympathetic nervous system activity in humans. J. Neurophysiol. 2017, 118, 2181–2193. [Google Scholar] [CrossRef]
- Carter, J.R. Microneurography and sympathetic nerve activity: A decade-by-decade journey across 50 years. J. Neurophysiol. 2019, 121, 1183–1194. [Google Scholar] [CrossRef]
- Heusser, K.; Tank, J.; Engeli, S.; Diedrich, A.; Menne, J.; Eckert, S.; Peters, T.; Sweep, F.C.; Haller, H.; Pichlmaier, A.M.; et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 2010, 55, 619–626. [Google Scholar] [CrossRef]
- Furlan, R.; Porta, A.; Costa, F.; Tank, J.; Baker, L.; Schiavi, R.; Robertson, D.; Malliani, A.; Mosqueda-Garcia, R. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation 2000, 101, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Montano, N.; Porta, A.; Malliani, A.; Abboud, F.M.; Birkett, C.; Somers, V.K. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 1997, 95, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.G.; Kim, S.E.; Kim, N.H.; Suh, K.H.; Lee, E.C. Pulse rate variability analysis using remote photoplethysmography signals. Sensors 2021, 21, 6241. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.; Orini, M.; Bailon, R.; Vergara, J.M.; Mainardi, L.; Laguna, P. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol. Meas. 2010, 31, 1271. [Google Scholar] [CrossRef] [PubMed]
- van Es, V.A.; Lopata, R.G.; Scilingo, E.P.; Nardelli, M. Contactless cardiovascular assessment by imaging photoplethysmography: A comparison with wearable monitoring. Sensors 2023, 23, 1505. [Google Scholar] [CrossRef] [PubMed]
- Baquero, G.A.; Banchs, J.E.; Ahmed, S.; Naccarelli, G.V.; Luck, J.C. Surface 12 lead electrocardiogram recordings using smart phone technology. J. Electrocardiol. 2015, 48, 1–7. [Google Scholar] [CrossRef]
- Desteghe, L.; Raymaekers, Z.; Lutin, M.; Vijgen, J.; Dilling-Boer, D.; Koopman, P.; Schurmans, J.; Vanduynhoven, P.; Dendale, P.; Heidbuchel, H. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. EP Eur. 2017, 19, 29–39. [Google Scholar]
- Samol, A.; Masin, M.; Gellner, R.; Otte, B.; Pavenstädt, H.J.; Ringelstein, E.B.; Reinecke, H.; Waltenberger, J.; Kirchhof, P. Prevalence of unknown atrial fibrillation in patients with risk factors. Europace 2013, 15, 657–662. [Google Scholar] [CrossRef]
- Nigolian, A.; Dayal, N.; Nigolian, H.; Stettler, C.; Burri, H. Diagnostic accuracy of multi-lead ECGs obtained using a pocket-sized bipolar handheld event recorder. J. Electrocardiol. 2018, 51, 278–281. [Google Scholar] [CrossRef]
- Foster, K.R.; Torous, J. The opportunity and obstacles for smartwatches and wearable sensors. IEEE Pulse 2019, 10, 22–25. [Google Scholar] [CrossRef]
- Avila, C.O. Novel use of Apple Watch 4 to obtain 3-lead electrocardiogram and detect cardiac ischemia. Perm. J. 2019, 23, 19-025. [Google Scholar] [CrossRef] [PubMed]
- Samol, A.; Bischof, K.; Luani, B.; Pascut, D.; Wiemer, M.; Kaese, S. Single-lead ECG recordings including Einthoven and Wilson leads by a smartwatch: A new era of patient directed early ECG differential diagnosis of cardiac diseases? Sensors 2019, 19, 4377. [Google Scholar] [CrossRef] [PubMed]
- Mohamoud, A.; Jensen, J.; Buda, K.G. Consumer-grade wearable cardiac monitors: What they do well, and what needs work. Clevel. Clin. J. Med. 2024, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.K.; Veeraraghavan, A.; Sabharwal, A. PPGMotion: Model-based detection of motion artifacts in photoplethysmography signals. Biomed. Signal Process. Control 2022, 75, 103632. [Google Scholar] [CrossRef]
- Afandizadeh Zargari, A.H.; Aqajari, S.A.H.; Khodabandeh, H.; Rahmani, A.; Kurdahi, F. An Accurate Non-accelerometer-based PPG Motion Artifact Removal Technique using CycleGAN. ACM Trans. Comput. Healthc. 2023, 4, 1–14. [Google Scholar] [CrossRef]
- Sawangjai, P.; Wilaiprasitporn, T. PPGANet: Removal of Motion Artifacts from the PPG Signal Using Generative Adversarial Networks. In Proceedings of the 2023 IEEE Biomedical Circuits and Systems Conference (BioCAS), Toronto, ON, Canada, 19–21 October 2023; pp. 1–5. [Google Scholar] [CrossRef]
- Ghamari, M. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195. [Google Scholar] [CrossRef]
- Elgendi, M.; Liang, Y.; Ward, R. Toward Generating More Diagnostic Features from Photoplethysmogram Waveforms. Diseases 2018, 6, 20. [Google Scholar] [CrossRef]
- Solelhac, G.; la Torre, M.S.D.; Blanchard, M.; Berger, M.; Hirotsu, C.; Imler, T.; la Torre, A.S.D.; Haba-Rubio, J.; Marchi, N.A.; Bayon, V.; et al. Pulse Wave Amplitude Drops Index: A Biomarker of Cardiovascular Risk in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2023, 207, 1620–1632. [Google Scholar] [CrossRef]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-Ananta, T.; Ajmal; Ramella-Roman, J.C.; McShane, M.J.; Coté, G.L. Sources of inaccuracy in photoplethysmography for continuous cardiovascular monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef]
- Aurora, R.N.; Patil, S.P.; Punjabi, N.M. Portable Sleep Monitoring for Diagnosing Sleep Apnea in Hospitalized Patients with Heart Failure. Chest 2018, 154, 91–98. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009, 6, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Logan, A.G.; Kimoff, R.J.; Sériès, F.; Morrison, D.; Ferguson, K.; Belenkie, I.; Pfeifer, M.; Fleetham, J.; Hanly, P.; et al. Continuous Positive Airway Pressure for Central Sleep Apnea and Heart Failure. N. Engl. J. Med. 2005, 353, 2025–2033. Available online: https://www.nejm.org/doi/pdf/10.1056/NEJMoa051001 (accessed on 1 January 2020). [CrossRef] [PubMed]
- Cowie, M.R.; Woehrle, H.; Wegscheider, K.; Angermann, C.; d’Ortho, M.P.; Erdmann, E.; Levy, P.; Simonds, A.K.; Somers, V.K.; Zannad, F.; et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N. Engl. J. Med. 2015, 373, 1095–1105. [Google Scholar] [CrossRef]
- Patel, S.R.; Sykes, A.V.; Malhotra, A. Sleep apnoea in congestive heart failure: One step forwards. Lancet Respir. Med. 2024, 12, 94–96. [Google Scholar] [CrossRef]
- Alharbi, S.H.; Alzahrani, A.M.; Syed, T.A.; Alqahtany, S.S. Integrity and Privacy Assurance Framework for Remote Healthcare Monitoring Based on IoT. Computers 2024, 13, 64. [Google Scholar] [CrossRef]
- Yoon, H.; Choi, S.H. Technologies for sleep monitoring at home: Wearables and nearables. Biomed. Eng. Lett. 2023, 13, 313–327. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Yeo, W.H. Recent advances in wearable sensors and portable electronics for sleep monitoring. iScience 2021, 24, 102461. [Google Scholar] [CrossRef]
- Klum, M.; Urban, M.; Tigges, T.; Pielmus, A.G.; Feldheiser, A.; Schmitt, T.; Orglmeister, R. Wearable Cardiorespiratory Monitoring Employing a Multimodal Digital Patch Stethoscope: Estimation of ECG, PEP, LVET and Respiration Using a 55 mm Single-Lead ECG and Phonocardiogram. Sensors 2020, 20, 2003. [Google Scholar] [CrossRef]
- Schneider, J.; Schroth, M.; Ottenbacher, J.; Stork, W. High-accuracy pulse wave estimation using impedance plethysmography: A wearable approachA Novel Wearable Sensor Device for Continuous Monitoring of Cardiac Activity During Sleep. In Proceedings of the 2018 IEEE Sensors Applications Symposium (SAS), Seoul, Republic of Korea, 12–14 March 2018. [Google Scholar] [CrossRef]
- Weng, C.-K.; Chen, J.-W.; Lee, P.-Y.; Huang, C.-C. Implementation of a Wearable Ultrasound Device for the Overnight Monitoring of Tongue Base Deformation during Obstructive Sleep Apnea Events. Ultrasound Med. Biol. 2017, 43, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Alomri, R.M.; Kennedy, G.A.; Wali, S.O.; Alhejaili, F.; Robinson, S.R. Association between Nocturnal Activity of the Sympathetic Nervous System and Cognitive Dysfunction in Obstructive Sleep Apnea. Sci. Rep. 2021, 11, 11990. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, N.; Jeanneret, F.; Nilchian, M.; Albertoni, P.; Ha, P.; Millet, G.P. Sleep Deprivation Deteriorates Heart Rate Variability and Photoplethysmography. Front. Neurosci. 2021, 15, 642548. [Google Scholar] [CrossRef] [PubMed]


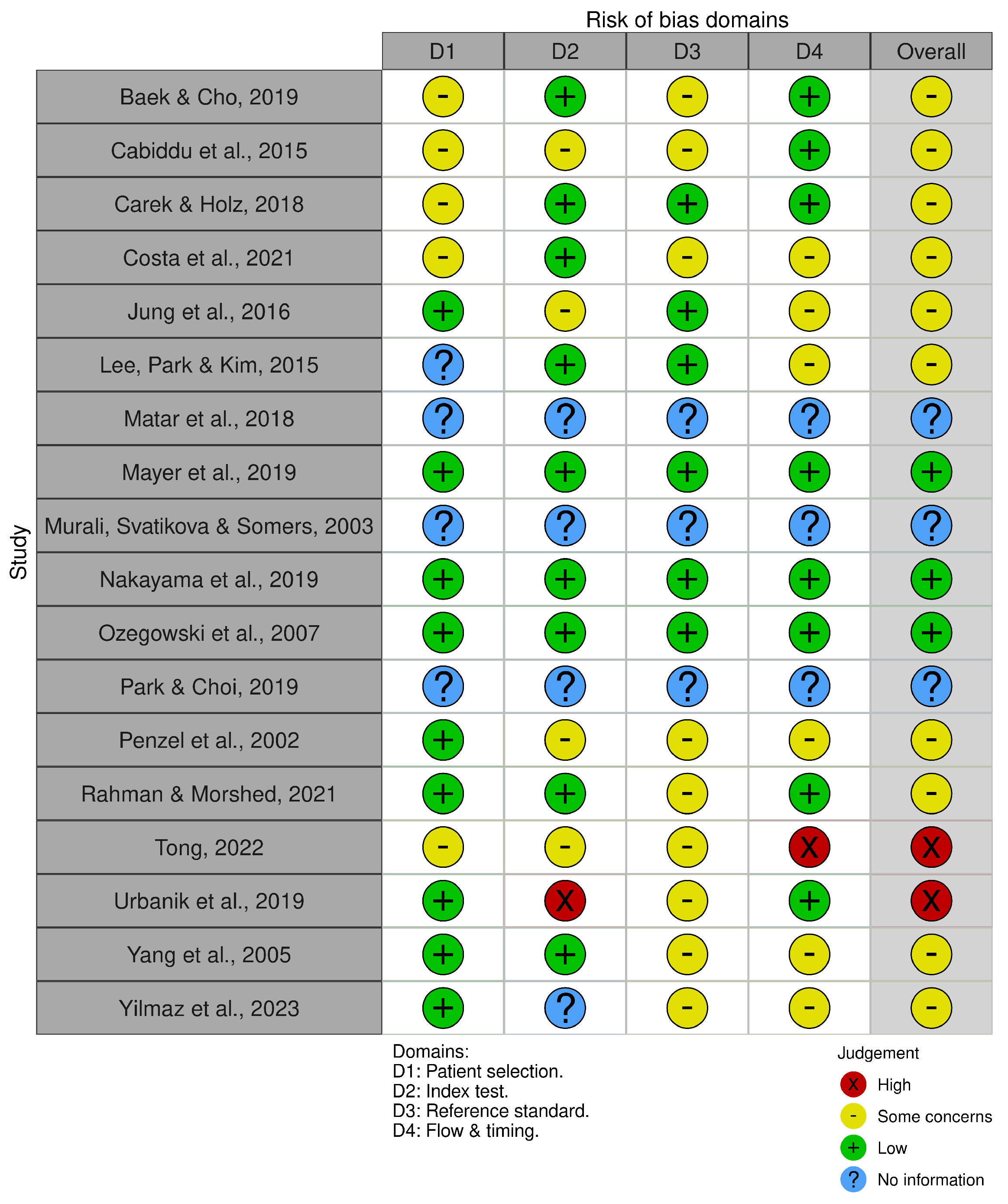
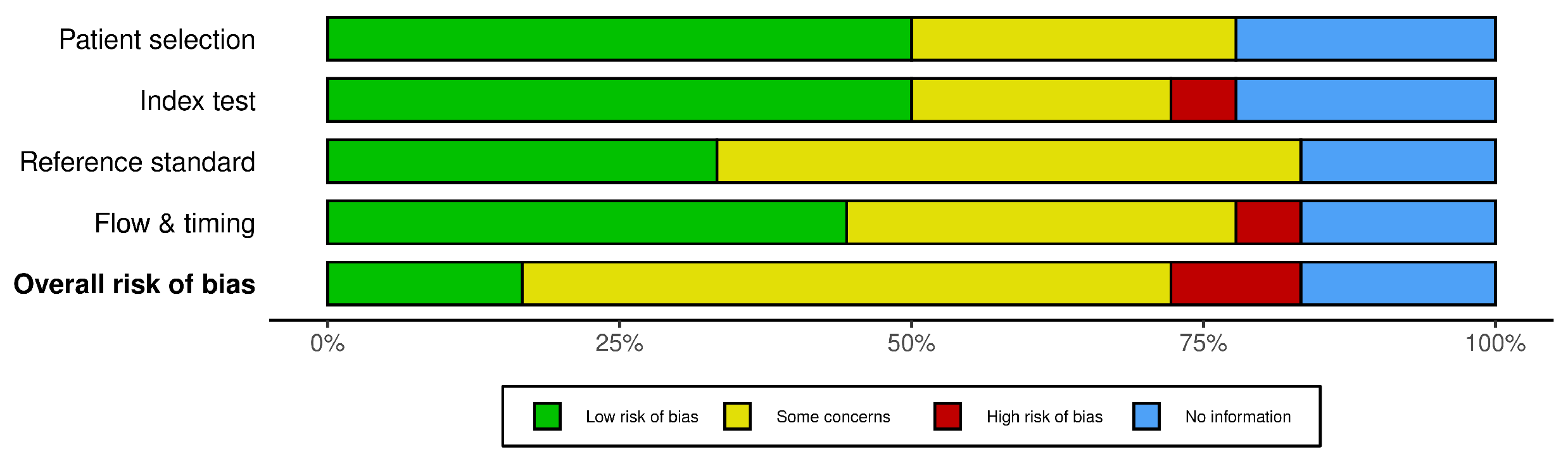
| Modality | Compatible Metrics | Obtrusiveness | Data Accuracy | Continuity | Patient Comfort and Compliance | Economic Cost |
|---|---|---|---|---|---|---|
| Electrodes | HRV, skin conductance, neural activity | High (skin contact) | High | Continuous | Low (discomfort, skin irritation) | Moderate (for consumables) |
| PPG sensor | HRV, SpO2, pulse waveform | Low (wrist or finger contact) | Moderate to high (prone to motion artifacts) | Continuous | High (wearable, non-invasive) | Low (widely available) |
| PAT | HRV, PAT amplitude | Moderate (cuff pressure) | Moderate to high | Interval | Low (pressure discomfort) | High (specialized equipment) |
| BCG | HR, RR, body movements | Low (no contact) | Moderate (prone to artifacts) | Interval | High (embedded in environment) | High (infrastructure cost) |
| RGB camera | HRV, RR, body movements | None (contactless) | Moderate (lighting conditions, artifacts) | Interval | High (contactless, non-invasive) | Moderate to high (equipment) |
| IR camera | HR, RR, body movements | None (contactless) | Low (limited by single-channel processing) | Interval | High (contactless, non-invasive) | Moderate to high (equipment) |
| Radar | HR, RR, body movements | None (contactless) | Moderate (affected by motion, interference) | Interval | High (contactless, non-invasive) | High (advanced technology) |
| Accelerometer | Body movements | Low (wearable) | Moderate | Continuous | High (integrated in wearables) | Low (widely available) |
| Metric | Accuracy | Reliability | Autonomic Relevance |
|---|---|---|---|
| HR | High (ECG), Moderate (PPG, motion-sensitive) | High (Clinically reliable for CV health) | Moderate (Reflects autonomic shifts, but limited without HRV analysis) |
| HRV | Gold standard (ECG), moderate (PPG) | High (Reliable for ANS shifts) | High (Strong indicator of autonomic modulation, especially sympathetic overdrive) |
| Pulse Waveform (PPG/PAT) | Accurate (PPG for HR, SpO2), Moderate (PAT for BP) | Moderate (Affected by motion artifacts) | Moderate (Reflects vascular tone and BP regulation) |
| BP | High (Cuff-based), Moderate (Cuff-less) | High (Cornerstone for CV monitoring) | High (BP dipping patterns strongly reflect autonomic activity) |
| SpO2 | High (PPG, but motion-sensitive) | High (Critical for respiratory disorders like OSA) | Moderate (Desaturation events linked to autonomic dysregulation) |
| RR | High (PPG, PAT, or accelerometers) | Moderate (Affected by body movement) | Moderate (Linked to autonomic control of respiratory and CV systems) |
| Neural Activity (EEG) | Moderate ( 85% sensitivity for autonomic dysfunctions) | Moderate (Reliable but affected by artifacts) | Moderate (Tracks brain-autonomic links in conditions like epilepsy, sleep disorders) |
| Body Movements | High (Accelerometers/gyroscopes, 95%) | High (Depends on sensor calibration/placement) | Low (Indirect insights into autonomic function through activity levels and sleep) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Es, V.A.A.; de Lathauwer, I.L.J.; Kemps, H.M.C.; Handjaras, G.; Betta, M. Remote Monitoring of Sympathovagal Imbalance During Sleep and Its Implications in Cardiovascular Risk Assessment: A Systematic Review. Bioengineering 2024, 11, 1045. https://doi.org/10.3390/bioengineering11101045
van Es VAA, de Lathauwer ILJ, Kemps HMC, Handjaras G, Betta M. Remote Monitoring of Sympathovagal Imbalance During Sleep and Its Implications in Cardiovascular Risk Assessment: A Systematic Review. Bioengineering. 2024; 11(10):1045. https://doi.org/10.3390/bioengineering11101045
Chicago/Turabian Stylevan Es, Valerie A. A., Ignace L. J. de Lathauwer, Hareld M. C. Kemps, Giacomo Handjaras, and Monica Betta. 2024. "Remote Monitoring of Sympathovagal Imbalance During Sleep and Its Implications in Cardiovascular Risk Assessment: A Systematic Review" Bioengineering 11, no. 10: 1045. https://doi.org/10.3390/bioengineering11101045
APA Stylevan Es, V. A. A., de Lathauwer, I. L. J., Kemps, H. M. C., Handjaras, G., & Betta, M. (2024). Remote Monitoring of Sympathovagal Imbalance During Sleep and Its Implications in Cardiovascular Risk Assessment: A Systematic Review. Bioengineering, 11(10), 1045. https://doi.org/10.3390/bioengineering11101045







