Abstract
Two of the greatest challenges in breast reconstruction are mastectomy skin flap necrosis (MSFN) and autologous flap failure. This review summarizes current evidence regarding the usage of indocyanine green angiography (ICGA) in breast reconstruction, identifies knowledge gaps, and provides directions for future studies. An umbrella review was conducted to identify related syntheses in Embase, Ovid Medline, Scopus, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the Clinical Trials databases. Data were extracted from systematic reviews (SRs) and meta-analyses (MAs) that discussed the use of ICGA in breast reconstruction. Sixteen syntheses were included (10 SRs and 6 MAs). Syntheses showed much evidence that ICGA usage typically reduces MSFN rates. However, it tends to overpredict necrosis and is best utilized in high-risk patients or those with an unclear clinical picture. ICGA is also useful in autologous breast reconstruction by reducing rates of breast fat necrosis (BFN), total flap loss, and reoperation. ICGA usage may also aid in perforator mapping and selection intraoperatively, with minimal complication risk. Most syntheses had moderate quality scores; however, they were small with significant heterogeneity in protocols and complication definitions. The use of ICGA in breast reconstruction is safe and useful in decreasing rates of MSFN, BFN, and reoperation after free flap reconstruction.
1. Introduction
Breast cancer is the most common cancer among women worldwide, making up 25% of all cancer cases [1,2]. As treatment and survival rates improve, additional women seek breast reconstruction post mastectomy [3]. One of the challenges faced by both general and plastic surgeons during the mastectomy and reconstruction period is complications related to poor tissue perfusion, mainly mastectomy skin flap necrosis (MSFN) and breast fat necrosis (BFN) after autologous reconstruction [4]. These have been reported in up to 30% and 11.3% of cases, respectively [5,6], and can significantly affect patient outcomes and potentially delay their oncological treatment.
The major surgical challenge when attempting to prevent MSFN remains to find the ideal superficial plane of dissection between subcutaneous fat and breast tissue, which is indistinct even microscopically in most cases [7]. Additional factors include patient-related factors such as higher body weight, smoking history, and previous radiation, as well as surgical factors, including the type of mastectomy and skin incision design [8]. Ultimately, MSFN can result in immediate or delayed wound healing problems and a higher risk of infection, which can have a significant impact on patient outcomes. On the other hand, BFN represents a more subtle problem that occurs due to adipocyte hypoperfusion, resulting in non-suppurative inflammation and cell death [6]. This results in cosmetically undesirable palpable nodules in the breast tissue, which can also be potentially suspicious for malignancy recurrence.
Several methods have been examined to accurately assess tissue perfusion during and after mastectomy and autologous breast reconstruction surgeries to accurately and promptly predict hypoperfusion-related complications. While clinical observation remains the traditional gold-standard method for intraoperative perfusion assessment, this can be unclear and misleading in certain cases due to several factors, including tissue edema, low blood pressure, and lack of surgeon experience [9]. Thus, additional adjunct modalities for objectively measuring intraoperative tissue perfusion have surfaced, such as indocyanine green angiography (ICGA) and fluorescein angiography (FA) with Wood’s lamp.
Indocyanine green (ICG) is an iodide dye that can be injected intravenously. It binds to plasma proteins and fluoresces when laser-excited between 705 and 805 nm, and this fluorescence can be captured in real time using a camera [10]. Intraoperatively, it has been used in various surgical fields to capture areas of poor tissue perfusion, as the level of fluorescence decreases with decreasing perfusion [11]. ICGA functions similarly to fluorescein dye angiography, in which fluorescein accumulates in the extracellular space and emits fluorescence in response to ultraviolet light [5]. ICGA is advantageous due to deep penetration and low leakage, which contribute to its superiority over fluorescein angiography due to the latter’s tendency to overestimate ischemia and longer half-life, which makes multiple evaluations within a single operation impractical [4].
In mastectomy and breast reconstruction surgeries, ICGA has been used to examine tissue perfusion both quantitatively and qualitatively [12]. An advantage of this technique is the identification of areas of hypoperfusion that require debridement and would otherwise be missed on clinical examination. However, drawbacks of ICGA include the tendency to overpredict ischemia as well as the potential for inter-observer variability, as no consensus yet exists for objectively assessing tissue viability [13].
Several studies have looked at the utility of ICGA in predicting MSFN and BFN after autologous reconstruction [4,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. These have been summarized in several systemic reviews and meta-analyses [5,10,13,30,31,32,33,34,35,36,37,38,39,40]. However, these studies lack consistency regarding study population and usage protocols, including ICG dosage and timing [36,38]. Another area of heterogeneity includes reported outcome measures [14,15,23,25,28,29]. The purpose of this umbrella review is to summarize these syntheses regarding the usage of ICGA in mastectomy and autologous breast reconstruction, as well as provide insights and directions for future studies.
2. Materials and Methods
2.1. Search Strategy
A literature search for records including the concepts of mastectomy and ICG angiography was carried out with the help of a specialized medical librarian (LHY). The search strategy was created using a combination of keywords and controlled vocabulary in Embase.com 1947-, Ovid Medline 1946-, Scopus 1823-, the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews (CDSR), and Clinicaltrials.gov 1997-. All search strategies were completed on 19 October 2023, with no added limits. A total of 2772 results were found; 1030 duplicate records were deleted using Covidence.org, resulting in a total of 1742 unique citations included in the project library. Fully reproducible search strategies for each database can be found in Appendix A.
2.2. Inclusion and Exclusion Criteria
Articles were included if they were systematic reviews or meta-analyses that synthesized data regarding the use of ICGA during mastectomy and/or breast reconstruction. Non-synthesis papers were excluded, as well as lone abstracts and non-English studies.
2.3. Article Screening and Data Extraction
Abstracts were screened separately by two reviewers (NF and FL), with discrepancies resolved by the senior author (SB). Full-text screening and data extraction were also performed in duplicate. For each article, the study type was recorded, as well as the patient population and types of procedures examined. Data were categorized into findings related to the use of the ICGA in predicting MSFN and BFN after mastectomy and autologous breast reconstruction.
2.4. Quality Assessment
A MeaSurement Tool to Assess Systematic Reviews-2 (AMSTAR-2) was used to assess the quality of included syntheses [41]. This is a 16-safeguard tool that allows methodological measurement of the quality of systematic reviews and meta-analyses.
3. Results
3.1. Study Selection and Quality Assessment
The search strategy resulted in 1726 studies after excluding 1034 duplicates. These studies were screened by title and abstract for eligibility, and 1699 studies were excluded for irrelevancy. The remaining 27 studies were screened by full text. Four studies were excluded due to being the wrong design, three were excluded for being abstracts without a full text, and six were excluded for using ICGA for the wrong intervention. This left a total of 14 syntheses: 8 systematic reviews (SRs) and 6 meta-analyses (MAs). A PRISMA flow diagram is shown in Figure 1.
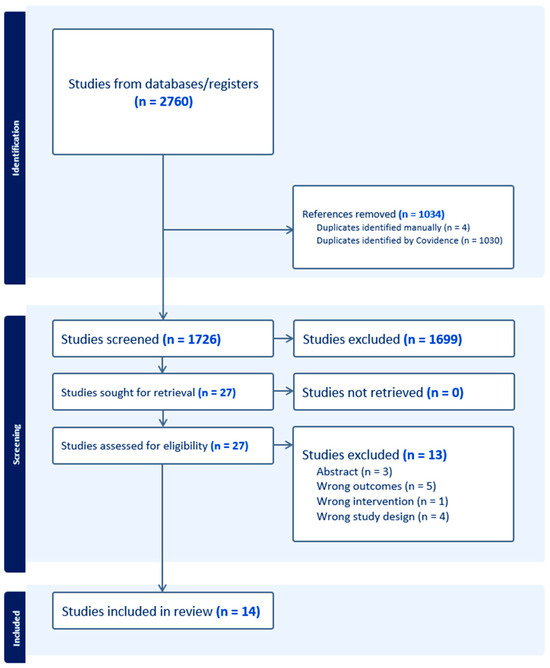
Figure 1.
PRISMA flow diagram.
The earliest review was conducted in 2017, and the latest in 2023. Thirteen studies reported outcome data on the use of ICGA in predicting MSFN, while seven studies reported on the usage of ICGA in predicting necrosis after autologous breast reconstruction. AMSTAR-2 scores are displayed in Figure 2. Quality assessment revealed that most syntheses were of moderate quality, with an average score of 8.3/16 and scores ranging from 1.5/16 to 16/16. The most common reasons for score deduction were failing to provide a list of excluded studies with reasoning and failing to provide sources of funding for included studies.
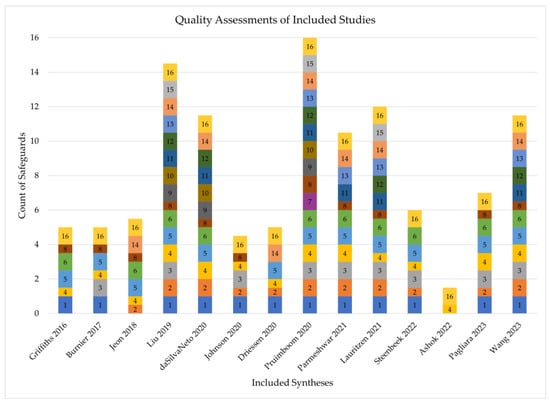
Figure 2.
AMSTAR-2 scores of included syntheses [5,10,13,30,31,32,33,34,35,36,37,38,39,40]. Each numbered section represents an AMSTAR-2 safeguard. A thick box indicates the synthesis contained the safeguard, a thin box indicates it was partially present, and missing numbers indicate the safeguard was not present.
3.2. The Utility of ICGA in Predicting MSFN
A total of eight SRs and five MAs that discussed the use of ICGA for preventing MSFN were identified (Table 1). Four meta-analyses reported a statistically significant reduction in incidence of MSFN with the use of ICGA [10,36,38,39] and one found no statistical benefit [34]. The greatest benefit reported was an odds ratio of 0.54 (95% CI 0.38–0.77) for MSFN development after mastectomy and reconstruction with intraoperative ICGA versus with clinical judgment alone [10]. Within systematic reviews examining the topic, a wide range of conclusions have been reported in the existing literature. The sensitivity and specificity of ICGA for detecting MSFN have ranged from 38 to 100% and 68 to 91%, respectively [5]. Reductions in MSFN rates after the use of ICGA have been reported as high as 84% [27], while other articles have found no statistical benefit [30]. Notably, ICGA has been found to possibly overpredict areas of skin flap necrosis by as high as 72% [23]. Syntheses recommend the use of ICGA in high-risk patients, such as those with high BMI and smokers, as well as when clinical evaluation of skin flaps is equivocal [37,40].

Table 1.
Syntheses that report incidence of MSFN with and without ICGA.
Based on these syntheses, ICGA has the potential to be useful for predicting areas of MSFN with high sensitivity. However, current conclusions are varied, and surgeons should be aware of the tendency for ICGA to overpredict areas of necrosis. It is challenging to draw definitive conclusions from the existing literature due to heterogeneity. Individual studies have differed in types of reconstructions included, ICG dosage and timing of administration, and classification of postoperative skin flap necrosis. More specifically, some classified necrosis by required intervention [14,15,26], others classified necrosis by affected/exposed tissue [23,25,28,29], and some did not explicitly state how necrosis was classified and what degree was included [16,22]. This makes it difficult to define and quantify the effect of ICGA on this outcome, as certain severities of necrosis included in one study may have been excluded in another. Further prospective studies with large, multi-institutional cohorts, standardized ICG protocols, and consistent necrosis definitions are needed to definitively quantify the benefits of ICGA for predicting MSFN.
3.3. The Utility of ICGA in Predicting BFN
Four SRs and four MAs examined the effect of ICGA on the incidence of fat necrosis in this review (Table 2). Of the four meta-analyses, three reported a significant reduction in incidence of fat necrosis with the use of ICGA [33,34,39] and one did not include fat necrosis as an outcome in pooled analysis [36]. The greatest reported benefit of ICGA when used for the prediction of breast flap necrosis was an odds ratio of 0.31 (p = 0.006) [33]. This benefit is likely due to the ability of ICGA to identify poorly perfused flap tissue intraoperatively and guide under perfused tissue resection [32]. No meta-analyses reported a statistically significant reduction in total flap loss with the use of ICGA, and one reported that ICGA reduced the risk of both major complications requiring operative intervention and minor complications managed conservatively (OR 0.62 and OR 0.53, respectively) [39]. A systematic review of the existing literature showed a wide variety of conclusions, with some studies reporting no statistically significant benefit of ICGA [14,24] and others reporting a nearly 100% reduction in postoperative fat necrosis when intraoperative ICGA was used [31].

Table 2.
Syntheses that report incidence of BFN with and without ICGA.
Many prior studies and syntheses have reported a statistically significant benefit of ICGA in reducing postoperative breast flap necrosis. However, like MSFN, the generalizability of these conclusions is limited due to varying ICGA protocols, small sample sizes, and heterogenous definitions of fat necrosis. Fat necrosis was sometimes classified by the size of a palpable mass within the breast [17], some was further classified based on severity [18,21], and other studies [19,20] used a classification system based on a review performed in 2013 by Lie et al. [42]. Large, multi-center prospective trials with consistent ICGA protocols, a follow-up time of at least one year, and standardized inclusion and exclusion criteria for diagnoses of breast fat necrosis are needed.
4. Discussion
Several studies [4,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] have looked at the usage of ICGA in breast reconstruction. However, these studies have been criticized for their small sample size, retrospective study design, significant heterogeneity [36], short-term follow-up [33], and inconsistent usage protocols [36,38]. This umbrella review summarizes 14 syntheses examining evidence on the benefits of ICGA during mastectomy and breast reconstruction. Overall, ICGA appears promising as a tool to reduce the risk of MSFN and BFN in patients undergoing breast reconstruction as well as reduce the risk of returning to the operating room. It has been shown to reduce rates of MSFN by up to 84% and rates of BFN by up to 69%. However, current evidence for these claims is severely limited by significant heterogeneity in studies’ patient populations, ICGA protocols, and definitions of ischemic complications.
To address the abovementioned challenges, a large, multi-center, prospective randomized control trial should be designed with consistent ICGA doses and protocols. Based on current recommendations in the literature, for such a trial we recommend an intravenous ICG dose of 2.5 mg/mL followed by a 10 mL normal saline flush 90 s prior to perfusion assessment, a working distance of 20 cm from the skin, turning off all room lights during assessment, and the avoidance of epinephrine-containing tumescent solution for study patients [43]. Mastectomy skin flap necrosis should be grouped into necrosis requiring debridement or necrosis managed non-surgically, and breast flap fat necrosis should be stratified by palpability, visibility, and presence of symptoms [44]. Follow-up for each patient should be at least one year to adequately capture the incidence of fat necrosis.
Another important focus point for future studies would be the standardization of outcome measures. There is a wide variety of methods for identification and stratification of MSFN and fat necrosis, and this makes it difficult to compare and generalize the findings of different studies examining these outcomes [33,39]. While classifications of MSFN exist, a consensus on a standardized definition has yet to be reached [45]. Reaching such a consensus would be an important step in the ability to compare the outcomes of separate studies examining outcomes of ICGA on breast reconstruction.
While the use of ICGA shows great promise in improving outcomes of breast reconstruction, other modalities for measuring perfusion are also being studied for this purpose. One such modality is hyperspectral imaging (HSI), in which tissue is illuminated with a broadband light source and a hyperspectral camera captures the reflection of a large number of wavelengths across the electromagnetic spectrum [46]. This data can then be used to provide information on oxyhemoglobin and deoxyhemoglobin levels, tissue oxygenation, and near-infrared perfusion [47]. HSI is non-invasive, radiation-free, and requires no dye or medication. Recent research has shown that data from HSI on tissue oxygenation and near-infrared perfusion have correlated to areas of necrosis of both mastectomy skin flaps and free flaps [48]. Additionally, HSI has been used in conjunction with artificial intelligence and deep learning models to assist with achieving negative margins in breast-conserving surgery, as AI excels at the interpretation of the large volume of data HSI can generate [46]. However, current applications of HSI technology are physically large and expensive, making them difficult to integrate into surgical workflows [49]. Future work will need to focus on making HSI faster, more portable, and more accessible, as well as the standardization of protocols and image interpretation.
Another promising modality for the prediction of MSFN and BFN is laser speckle contrast imaging (LSCI). In this technique, tissue is illuminated with 785 nm laser light, and a camera is used to analyze the scatter, or speckle, pattern to quantify perfusion using “perfusion units” (PU) [50]. LSCI can be performed in only a few seconds, requires no contrast dye, and is cheaper per case than ICGA [51]. It has been successfully applied in animal studies of free flap perfusion to predict flap necrosis, and its perfusion measurements have also been shown to correlate with those from ICGA [52,53]. In human studies, LSCI has been able to display reductions in DIEP flap perfusion that correlate with Holm classifications, as well as predict areas of DIEP flap necrosis based on a 30 PU threshold [51]. While quite promising, LSCI suffers from similar drawbacks as ICGA in that its application and interpretation must still be standardized to truly understand its benefit. LSCI data are also quite sensitive to motion artifacts and can only display perfusion data to a depth of 300 μm, meaning it may miss deeper fat necrosis [51].
Lastly, photoacoustic imaging (PAI) is a modality that has seen use in many other medical disciplines that may prove useful in preventing MSFN and BFN. PAI is a hybrid imaging modality that uses nanosecond pulses of laser light that heat tissues, which generate acoustic waves via thermoelastic expansion that are detected using ultrasound [54]. Due to their differences in intrinsic optical absorption, PAI can differentiate between deoxyhemoglobin and hemoglobin to measure oxygenation and perfusion. It can be used with or without contrast dye, such as ICG, and is effective up to a depth of several centimeters. In a mouse model, PAI has been shown to be able to map perforators for flap harvest and identify reduced perfusion in areas that go on to develop necrosis [55]. However, PAI technology must become much more portable and requires much more clinical testing in breast reconstruction to become standard for improving outcomes. Ultimately, there is a large volume of research being conducted to develop a cheap, safe, and reliable imaging modality that allows surgeons to identify and excise tissue at high risk of necrosis in breast reconstruction. With each of these techniques, improvements in artificial intelligence and deep learning models may allow for an even greater predictive accuracy and account for patient factors such as BMI, smoking, and medical comorbidities.
5. Conclusions
The use of ICGA in mastectomy and breast reconstruction is useful in reducing perfusion-related complications such as mastectomy skin flap necrosis, fat necrosis, and partial flap loss. Further studies are needed to identify an optimal standardized ICGA protocol and outcome measures.
Author Contributions
Conceptualization, S.B. and N.F.; methodology, S.B., S.A.S. and L.Y.; formal analysis, N.F., F.L., E.O. and S.B.; investigation, N.F., F.L., E.O. and S.B.; writing—original draft preparation, N.F. and S.B.; writing—review and editing, N.F., F.L., E.O., M.D.W., X.L., J.M.S. and S.B.; supervision, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Full Search Strategy
Embase
Date Searched: 19 October 2023
Applied Database Supplied Limits: none
Number of Results: 808
Full Search Strategy:
(‘mastectomy’/exp OR ‘nipple-sparing mastectomy’/exp OR ‘subcutaneous mastectomy’/exp OR ‘skin-sparing mastectomy’/exp OR ‘breast reconstruction’/exp OR (mastectom* OR NACSM OR ‘breast amputation*’ OR ‘breast resection*’ OR mammectom* OR ‘breast reconstruction’ OR mammoplast* OR mastoplast*):ti,ab,kw) AND (‘indocyanine green’/exp OR ‘fluorescence’/de OR ‘fluorescence angiography’/de OR (‘indocyanine green’ OR ‘ICG-based fluorescence’ OR ‘cardio green’ OR ‘cardio-green’ OR cardiogreen OR diagnogreen OR ‘fox green’ OR ICG OR ‘indocyanide green’ OR ‘indocyanin green’ OR ‘spy agent green’ OR ‘tricarbocyanine ii’ OR ujoviridin OR vofaverdin OR wofaferdin OR wofaverdin OR fluorescence OR ‘fluorescent method’ OR ‘fluorescein angiography’ OR ‘fluorescein photography’ OR ‘fluorescence angiophotography’ OR ‘fluorescent angiography’ OR ‘fluorescin angiography’ OR ‘fluoroangiography’ OR ‘fluorescence angiography’):ti,ab,kw)
Ovid Medline
Date Searched: 19 October 2023
Applied Database Supplied Limits: none
Number of Results: 420
Full Search Strategy:
(exp Mastectomy, Subcutaneous/ OR exp Mastectomy/ OR exp Mammaplasty/ OR (mastectom* OR NACSM OR breast amputation* OR breast resection* OR mammectom* OR breast reconstruction OR mammoplast* OR mastoplast*).ti,ab,kf.) AND (exp Indocyanine Green/ OR *Fluorescence/ OR *Fluorescein Angiography/ OR (indocyanine green OR ICG-based fluorescence OR cardio green OR cardio-green OR cardiogreen OR diagnogreen OR fox green OR ICG OR indocyanide green OR indocyanin green OR spy agent green OR tricarbocyanine ii OR ujoviridin OR vofaverdin OR wofaferdin OR wofaverdin OR fluorescence OR fluorescent method OR fluorescein angiography OR fluorescein photography OR fluorescence angiophotography OR fluorescent angiography OR fluorescin angiography OR fluoroangiography OR fluorescence angiography).ti,ab,kf.)
Scopus
Date Searched: 19 October 2023
Applied Database Supplied Limits: none
Number of Results: 1477
Full Search Strategy:
((TITLE-ABS-KEY(mastectom* OR NACSM OR “breast amputation*” OR “breast resection*” OR mammectom* OR “breast reconstruction” OR mammoplast* OR mastoplast*))) AND ((TITLE-ABS-KEY(“indocyanine green” OR “ICG-based fluorescence” OR “cardio green” OR “cardio-green” OR cardiogreen OR diagnogreen OR “fox green” OR ICG OR “indocyanide green” OR “indocyanin green” OR “spy agent green” OR “tricarbocyanine ii” OR ujoviridin OR vofaverdin OR wofaferdin OR wofaverdin OR fluorescence OR “fluorescent method” OR “fluorescein angiography” OR “fluorescein photography” OR “fluorescence angiophotography” OR “fluorescent angiography” OR “fluorescin angiography” OR “fluoroangiography” OR “fluorescence angiography”)))
The Cochrane Library
Date Searched: 19 October 2023
Applied Database Supplied Limits: none
Number of Results
CENTRAL: 54
CDSR: 1
Full Search Strategy:
([mh “Mastectomy, Subcutaneous”] OR [mh “Mastectomy”] OR [mh “Mammaplasty”] OR (mastectom* OR NACSM OR “breast amputation*” OR “breast resection*” OR mammectom* OR “breast reconstruction” OR mammoplast* OR mastoplast*):ti,ab,kw) AND ([mh “Indocyanine Green”] OR [mh “Fluorescence”] OR [mh “Fluorescein Angiography”] OR (“indocyanine green” OR “ICG based fluorescence” OR “cardio green” OR “cardio green” OR cardiogreen OR diagnogreen OR “fox green” OR ICG OR “indocyanide green” OR “indocyanin green” OR “spy agent green” OR “tricarbocyanine ii” OR ujoviridin OR vofaverdin OR wofaferdin OR wofaverdin OR fluorescence OR “fluorescent method” OR “fluorescein angiography” OR “fluorescein photography” OR “fluorescence angiophotography” OR “fluorescent angiography” OR “fluorescin angiography” OR “fluoroangiography” OR “fluorescence angiography”):ti,ab,kw)
ClinicalTrials.gov
Date Searched: 19 October 2023
Number of Results: 12
Full Search Strategy:
mastectomy AND (“Indocyanine Green” OR fluorescence)
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Kronowitz, S.J.; Meric-Bernstam, F.; Feig, B.W.; Symmans, W.F.; Lucci, A.; I Ross, M.; Babiera, G.V.; Kuerer, H.M.; Hunt, K.K. Local, regional, and systemic recurrence rates in patients undergoing skin-sparing mastectomy compared with conventional mastectomy. Cancer 2011, 117, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Komorowska-Timek, E.; Gurtner, G.C. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast. Reconstr. Surg. 2010, 125, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Jeon, F.H.K.; Varghese, J.; Griffin, M.; Butler, P.E.; Ghosh, D.; Mosahebi, A. Systematic review of methodologies used to assess mastectomy flap viability. BJS Open 2018, 2, 175–184. [Google Scholar] [CrossRef]
- Khansa, I.; Momoh, A.O.; Patel, P.P.; Nguyen, J.T.; Miller, M.J.; Lee, B.T. Fat necrosis in autologous abdomen-based breast reconstruction: A systematic review. Plast. Reconstr. Surg. 2013, 131, 443–452. [Google Scholar] [CrossRef]
- Beer, G.M.; Varga, Z.; Budi, S.; Seifert, B.; Meyer, V.E. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer 2002, 94, 1619–1625. [Google Scholar] [CrossRef]
- Robertson, S.A.; Jeevaratnam, J.A.; Agrawal, A.; Cutress, R.I. Mastectomy skin flap necrosis: Challenges and solutions. Breast Cancer Targets Ther. 2017, 9, 141–152. [Google Scholar] [CrossRef]
- Singer, R.; Lewis, C.M.; Franklin, J.D.; Lynch, J.B. Fluorescein Test for Prediction of Flap Viability during Breast Reconstructions. Plast. Reconstr. Surg. 1978, 61, 371–375. [Google Scholar] [CrossRef]
- Neto, E.d.S.; Figueiredo, P.H.M.; Moro, M.G.; Assumpção, C.B.; Perina, A.L.F.; da Costa, F.P.P.; Faria, E.P.; de Oliveira, A.C.V.; Prates, R.A. Use of laser-assisted indocyanine green angiography in breast reconstruction: Systematic review and meta-analysis. J. Surg. Oncol. 2020, 121, 759–765. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green. Surg. Innov. 2015, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Pruimboom, T.; Van Kuijk, S.M.J.; Qiu, S.S.; van den Bos, J.; Wieringa, F.; Van Der Hulst, R.R.W.J.; Schols, R.M. Optimizing Indocyanine Green Fluorescence Angiography in Reconstructive Flap Surgery: A Systematic Review and Ex Vivo Experiments. Surg. Innov. 2020, 27, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Chae, M.P.; Rozen, W.M. Indocyanine green-based fluorescent angiography in breast reconstruction. Gland. Surg. 2016, 5, 133. [Google Scholar] [CrossRef]
- Duggal, C.S.; Madni, T.; Losken, A. An Outcome Analysis of Intraoperative Angiography for Postmastectomy Breast Reconstruction. Aesthetic Surg. J. 2014, 34, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Diep, G.K.; Hui, J.Y.C.; Marmor, S.; Cunningham, B.L.; Choudry, U.; Portschy, P.R.; Tuttle, T.M. Postmastectomy Reconstruction Outcomes After Intraoperative Evaluation with Indocyanine Green Angiography versus Clinical Assessment. Ann. Surg. Oncol. 2016, 23, 4080–4085. [Google Scholar] [CrossRef]
- Sood, M.; Glat, P. Potential of the SPY intraoperative perfusion assessment system to reduce ischemic complications in immediate postmastectomy breast reconstruction. Ann. Surg. Innov. Res. 2013, 7, 9. [Google Scholar] [CrossRef]
- Hembd, A.S.; Yan, J.; Zhu, H.; Haddock, N.T.; Teotia, S.S. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast. Reconstr. Surg. 2020, 146, 1E–10E. [Google Scholar] [CrossRef]
- Malagón-López, P.; Vilà, J.; Carrasco-López, C.; García-Senosiain, O.; Priego, D.; Ibañez, J.F.J.; Higueras-Suñe, C. Intraoperative Indocyanine Green Angiography for Fat Necrosis Reduction in the Deep Inferior Epigastric Perforator (DIEP) Flap. Aesthetic Surg. J. 2019, 39, NP45–NP54. [Google Scholar] [CrossRef]
- Michi, M.; Verduijn, P.S.; Corion, L.U.M.; Vahrmeijer, A.L.; Mulder, B.G.S. Assessment of deep inferior epigastric perforator flap perfusion with near-infrared fluorescence: A pilot study and description of a standardized working protocol. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1171–1178. [Google Scholar] [CrossRef]
- Varela, R.; Casado-Sanchez, C.; Zarbakhsh, S.; Diez, J.; Hernandez-Godoy, J.; Landin, L. Outcomes of DIEP Flap and Fluorescent Angiography: A Randomized Controlled Clinical Trial. Plast. Reconstr. Surg. 2020, 145, 1–10. [Google Scholar] [CrossRef]
- Yoo, A.; Palines, P.A.; Mayo, J.L.; Bartow, M.J.; Danos, D.M.; Hilaire, H.M.S.; Wise, M.W.; Stalder, M.W. The Impact of Indocyanine Green Angiography on Fat Necrosis in Deep Inferior Epigastric Perforator Flap Breast Reconstruction. Ann. Plast. Surg. 2022, 88, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Wapnir, I.; Dua, M.; Kieryn, A.; Paro, J.; Morrison, D.; Kahn, D.; Meyer, S.; Gurtner, G. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann. Surg. Oncol. 2014, 21, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.T.; Lanier, S.T.; Conkling, N.; Wang, E.D.; Dagum, A.B.; Ganz, J.C.; Khan, S.U.; Bui, D.T. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: Results of a prospective trial. Plast. Reconstr. Surg. 2012, 129, 778e–788e. [Google Scholar] [CrossRef] [PubMed]
- Alstrup, T.; Christensen, B.O.; Damsgaard, T.E. ICG angiography in immediate and delayed autologous breast reconstructions: Peroperative evaluation and postoperative outcomes. J. Plast. Surg. Hand Surg. 2018, 52, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Gorai, K.; Inoue, K.; Saegusa, N.; Shimamoto, R.; Takeishi, M.; Okazaki, M.; Nakagawa, M. Prediction of Skin Necrosis after Mastectomy for Breast Cancer Using Indocyanine Green Angiography Imaging. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1321. [Google Scholar] [CrossRef] [PubMed]
- Hammer-Hansen, N.; Juhl, A.A.; Damsgaard, T.E. Laser-assisted indocyanine green angiography in implant-based immediate breast reconstruction: A retrospective study. J. Plast. Surg. Hand Surg. 2018, 52, 158–162. [Google Scholar] [CrossRef]
- Harless, C.A.; Jacobson, S.R. Tailoring through Technology: A Retrospective Review of a Single Surgeon’s Experience with Implant-Based Breast Reconstruction before and after Implementation of Laser-Assisted Indocyanine Green Angiography. Breast J. 2016, 22, 274–281. [Google Scholar] [CrossRef]
- Mirhaidari, S.J.; Beddell, G.M.; Orlando, M.V.; Parker, M.G.; Pedersen, J.C.; Wagner, D.S. A Prospective Study of Immediate Breast Reconstruction with Laser-Assisted Indocyanine Green Angiography. Plast. Reconstr. Surg. Glob. Open. 2018, 6, e1774. [Google Scholar] [CrossRef]
- Rinker, B. A Comparison of Methods to Assess Mastectomy Flap Viability in Skin-Sparing Mastectomy and Immediate Reconstruction: A Prospective Cohort Study. Plast. Reconstr. Surg. 2016, 137, 395–401. [Google Scholar] [CrossRef]
- Driessen, C.; Arnardottir, T.H.; Lorenzo, A.R.; Mani, M.R. How should indocyanine green dye angiography be assessed to best predict mastectomy skin flap necrosis? A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1031–1042. [Google Scholar] [CrossRef]
- Burnier, P.; Niddam, J.; Bosc, R.; Hersant, B.; Meningaud, J.P. Indocyanine green applications in plastic surgery: A review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B.C.; Kabilan, H.K.; Anantheswar, Y.N.; Srikanth, V.; Somashekar, S.P.; Prasad, A. Role of Indocyanine Green in Breast Surgery. Indian. J. Surg. 2022, 84, 592–601. [Google Scholar] [CrossRef]
- Parmeshwar, N.; Sultan, S.M.; Kim, E.A.; Piper, M.L. A Systematic Review of the Utility of Indocyanine Angiography in Autologous Breast Reconstruction. Ann. Plast. Surg. 2021, 86, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiao, L.; Chen, S.; Li, Z.; Xiao, Y.; Du, F.; Huang, J.; Long, X. Flap perfusion assessment with indocyanine green angiography in deep inferior epigastric perforator flap breast reconstruction: A systematic review and meta-analysis. Microsurgery 2023, 43, 627–638. [Google Scholar] [CrossRef]
- Steenbeek, L.M.; Peperkamp, K.; Ulrich, D.J.O.; Hummelink, S. Alternative imaging technologies for perforator mapping in free flap breast reconstructive surgery—A comprehensive overview of the current literature. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 4074–4084. [Google Scholar] [CrossRef]
- Pruimboom, T.; Schols, R.M.; Van Kuijk, S.M.J.; Van der Hulst, R.R.W.J.; Qiu, S.S. Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction. Cochrane Database Syst. Rev. 2020, 2020, CD013280. [Google Scholar] [CrossRef]
- Pagliara, D.; Schiavone, L.; Garganese, G.; Bove, S.; Montella, R.A.; Costantini, M.; Rinaldi, P.M.; Bottosso, S.; Grieco, F.; Rubino, C.; et al. Predicting Mastectomy Skin Flap Necrosis: A Systematic Review of Preoperative and Intraoperative Assessment Techniques. Clin. Breast Cancer 2023, 23, 249–254. [Google Scholar] [CrossRef]
- Liu, E.H.; Zhu, S.L.; Hu, J.; Wong, N.; Farrokhyar, F.; Thoma, A. Intraoperative SPY Reduces Post-mastectomy Skin Flap Complications: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg—Glob. Open 2019, 7, e2060. [Google Scholar] [CrossRef]
- Lauritzen, E.; Damsgaard, T.E. Use of Indocyanine Green Angiography decreases the risk of complications in autologous- and implant-based breast reconstruction: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 1703–1717. [Google Scholar] [CrossRef]
- Johnson, A.C.; Colakoglu, S.; Chong, T.W.; Mathes, D.W. Indocyanine green angiography in breast reconstruction: Utility, limitations, and search for standardization. Plast. Reconstr. Surg—Glob. Open 2020, 8, e2694. [Google Scholar] [CrossRef]
- Shea, B.J.; Grimshaw, J.M.; A Wells, G.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Lie, K.H.; Barker, A.S.; Ashton, M.W. A classification system for partial and complete diep flap necrosis based on a review of 17,096 DIEP flaps in 693 articles including analysis of 152 total flap failures. Plast. Reconstr. Surg. 2013, 132, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Nakagawa, T.; Oda, G.; Hosoya, T.; Hayashi, K.; Yoshino, M.; Mori, H.; Uemura, N.; Fujioka, T.; Mori, M.; et al. Study of the protocol used to evaluate skin-flap perfusion in mastectomy based on the characteristics of indocyanine green. Photodiagnosis Photodyn. Ther. 2021, 35, 102401. [Google Scholar] [CrossRef]
- Wagner, I.J.; Tong, W.M.; Halvorson, E.G. A classification system for fat necrosis in autologous breast reconstruction. Ann. Plast. Surg. 2013, 70, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Oleck, N.C.; Gu, C.; Pyfer, B.J.; Phillips, B.T. Defining Mastectomy Skin Flap Necrosis: A Systematic Review of the Literature and a Call for Standardization. Plast. Reconstr. Surg. 2022, 149, 858E–866E. [Google Scholar] [CrossRef]
- Jong, L.J.S.; Appelman, J.G.C.; Sterenborg, H.J.C.M.; Ruers, T.J.M.; Dashtbozorg, B. Spatial and Spectral Reconstruction of Breast Lumpectomy Hyperspectral Images. Sensors 2024, 24, 1567. [Google Scholar] [CrossRef]
- Thiem, D.G.E.; Frick, R.W.; Goetze, E.; Gielisch, M.; Al-Nawas, B.; Kämmerer, P.W. Hyperspectral analysis for perioperative perfusion monitoring—A clinical feasibility study on free and pedicled flaps. Clin. Oral. Investig. 2021, 25, 933–945. [Google Scholar] [CrossRef]
- Pruimboom, T.; Lindelauf, A.A.M.A.; Felli, E.; Sawor, J.H.; Deliaert, A.E.K.; van der Hulst, R.R.W.J.; Al-Taher, M.; Diana, M.; Schols, R.M. Perioperative Hyperspectral Imaging to Assess Mastectomy Skin Flap and DIEP Flap Perfusion in Immediate Autologous Breast Reconstruction: A Pilot Study. Diagnostics 2022, 12, 184. [Google Scholar] [CrossRef]
- Shapey, J.; Xie, Y.; Nabavi, E.; Bradford, R.; Saeed, S.R.; Ourselin, S.; Vercauteren, T. Intraoperative multispectral and hyperspectral label-free imaging: A systematic review of in vivo clinical studies. J. Biophotonics 2019, 12, e201800455. [Google Scholar] [CrossRef]
- Zötterman, J.; Bergkvist, M.; Iredahl, F.; Tesselaar, E.; Farnebo, S. Monitoring of partial and full venous outflow obstruction in a porcine flap model using laser speckle contrast imaging. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 936–943. [Google Scholar] [CrossRef]
- Zötterman, J.; Opsomer, D.; Farnebo, S.; Blondeel, P.; Monstrey, S.; Tesselaar, E. Intraoperative Laser Speckle Contrast Imaging in DIEP Breast Reconstruction: A Prospective Case Series Study. Plast. Reconstr. Surg—Glob. Open 2020, 8, E2529. [Google Scholar] [CrossRef] [PubMed]
- Zötterman, J.; Tesselaar, E.; Farnebo, S. The use of laser speckle contrast imaging to predict flap necrosis: An experimental study in a porcine flap model. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Zötterman, J.; Tesselaar, E.; Elawa, S.; Farnebo, S. Correlation between Indocyanine Green Fluorescence Angiography and Laser Speckle Contrast Imaging in a Flap Model. Plast. Reconstr. Surg—Glob. Open 2023, 11, E5187. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, H.; Hu, X.; Yu, A. Photoacoustic microscopy: A novel approach for studying perforator skin flap in a mouse model. Quant. Imaging Med. Surg. 2021, 11, 4365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).