Diseased Tendon Models Demonstrate Influence of Extracellular Matrix Alterations on Extracellular Vesicle Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospinning
2.2. Scaffold Characterization
2.3. Cell Culture
2.4. EV Characterization
2.5. Cellular Activity Assessment
2.6. Statistical Analysis
3. Results
3.1. Nanofibrous Scaffold Characterization
3.2. Characterization of Fibroblast-EVs
3.3. Assessment of Cellular Activity
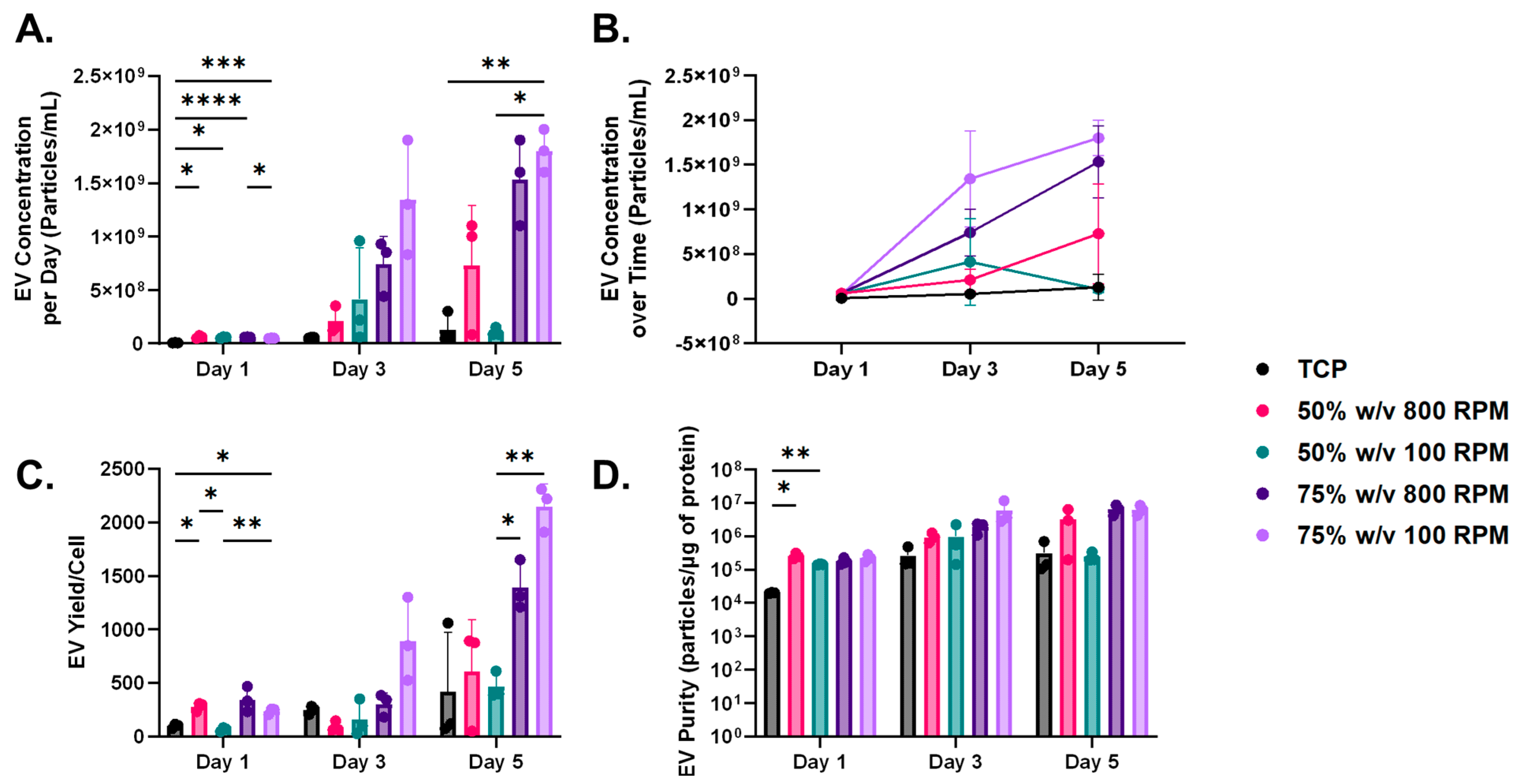
3.4. Influence of Nanofibrous Scaffolds on EV Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thorpe, C.T.; Screen, H.R. Tendon Structure and Composition. Adv. Exp. Med. Biol. 2016, 920, 3–10. [Google Scholar] [PubMed]
- Screen, H.R.C.; Birk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Labrador-Rached, C.J.; Domingues, R.M.; Gomes, M.E. The tendon microenvironment: Engineered in vitro models to study cellular crosstalk. Adv. Drug Deliv. Rev. 2022, 185, 114299. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; El Khatib, M.; Prencipe, G.; Citeroni, M.R.; Faydaver, M.; Mauro, A.; Berardinelli, P.; Cerveró-Varona, A.; Haidar-Montes, A.A.; Turriani, M.; et al. Tendon Immune Regeneration: Insights on the Synergetic Role of Stem and Immune Cells during Tendon Regeneration. Cells 2022, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell GA, C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, T.; Liu, M.; Yin, Z. Chapter 5—Therapies related to mesenchymal stem cells for cartilage, joint, and bone diseases. In Joint and Bone; Jiang, D., El-Hashash, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 3, pp. 79–116. [Google Scholar]
- Konar, S.; Bolam, S.M.; Coleman, B.; Dalbeth, N.; McGlashan, S.R.; Leung, S.; Cornish, J.; Naot, D.; Musson, D.S. Changes in Physiological Tendon Substrate Stiffness Have Moderate Effects on Tendon-Derived Cell Growth and Immune Cell Activation. Front. Bioeng. Biotechnol. 2022, 10, 800748. [Google Scholar] [CrossRef]
- Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef]
- Sánchez, G.B.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular vesicles: Mediators of intercellular communication in tissue injury and disease. Cell Commun. Signal. 2021, 19, 104. [Google Scholar] [CrossRef]
- Petroni, D.; Fabbri, C.; Babboni, S.; Menichetti, L.; Basta, G.; Del Turco, S. Extracellular Vesicles and Intercellular Communication: Challenges for In Vivo Molecular Imaging and Tracking. Pharmaceutics 2023, 15, 1639. [Google Scholar] [CrossRef]
- Taverna, S.; Pucci, M.; Alessandro, R. Extracellular vesicles: Small bricks for tissue repair/regeneration. Ann. Transl. Med. 2017, 5, 83. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Chapter One—Extracellular vesicles in heart failure. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 119, pp. 1–32. [Google Scholar]
- Chang, W.-H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. In Cancer Cell Signaling; Robles-Flores, M., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2174, pp. 143–170. [Google Scholar] [CrossRef]
- Zanirati, G.; dos Santos, P.G.; Alcará, A.M.; Bruzzo, F.; Ghilardi, I.M.; Wietholter, V.; Xavier, F.A.; Gonçalves, J.I.; Marinowic, D.; Shetty, A.K.; et al. Extracellular Vesicles: The Next Generation of Biomarkers and Treatment for Central Nervous System Diseases. Int. J. Mol. Sci. 2024, 25, 7371. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Bussolati, B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022, 18, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, S.; Yang, D.; Xu, W.; Qian, H. Extracellular vesicles: Emerging roles, biomarkers and therapeutic strategies in fibrotic diseases. J. Nanobiotechnol. 2023, 21, 164. [Google Scholar] [CrossRef]
- Martin-Medina, A.; Lehmann, M.; Burgy, O.; Hermann, S.; Baarsma, H.A.; Wagner, D.E.; De Santis, M.M.; Ciolek, F.; Hofer, T.P.; Frankenberger, M.; et al. Increased Extracellular Vesicles Mediate WNT5A Signaling in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y. Extracellular vesicles in idiopathic pulmonary fibrosis: Pathogenesis and therapeutics. Inflamm. Regen. 2022, 42, 23. [Google Scholar] [CrossRef]
- Tang, T.-T.; Lv, L.-L.; Lan, H.-Y.; Liu, B.-C. Extracellular Vesicles: Opportunities and Challenges for the Treatment of Renal Diseases. Front. Physiol. 2019, 10, 226. [Google Scholar] [CrossRef]
- Sadovska, L.; Zayakin, P.; Bajo-Santos, C.; Endzeliņš, E.; Auders, J.; Keiša, L.; Jansons, J.; Lietuvietis, V.; Linē, A. Effects of urinary extracellular vesicles from prostate cancer patients on the transcriptomes of cancer-associated and normal fibroblasts. BMC Cancer 2022, 22, 1055. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Liao, Y.; Hosseinifard, H.; Imani, S.; Wen, Q.-L. Diagnostic Role of Extracellular Vesicles in Cancer: A Comprehensive Systematic Review and Meta-Analysis. Front. Cell Dev. Biol. 2021, 9, 705791. [Google Scholar] [CrossRef]
- Ishihara, S.; Haga, H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers 2022, 14, 1049. [Google Scholar] [CrossRef]
- Wu, B.; Liu, D.-A.; Guan, L.; Myint, P.K.; Chin, L.; Dang, H.; Xu, Y.; Ren, J.; Li, T.; Yu, Z.; et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat. Cell Biol. 2023, 25, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.; Moulin, V.J. Extracellular vesicles on the move: Traversing the complex matrix of tissues. Eur. J. Cell Biol. 2023, 102, 151372. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.J.; Ashraf, A.; Chung, E.J. Extracellular Vesicles as Regulators of the Extracellular Matrix. Bioengineering 2023, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Al Halawani, A.; Mithieux, S.M.; Yeo, G.C.; Hosseini-Beheshti, E.; Weiss, A.S. Extracellular Vesicles: Interplay with the Extracellular Matrix and Modulated Cell Responses. Int. J. Mol. Sci. 2022, 23, 3389. [Google Scholar] [CrossRef] [PubMed]
- Hast, M.W.; Zuskov, A.; Soslowsky, L.J. The role of animal models in tendon research. Bone Jt. Res. 2014, 3, 193–202. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Legerlotz, K.; Demirci, T.; Klemt, C.; Riley, G.P.; Screen, H.R. Functionally distinct tendon fascicles exhibit different creep and stress relaxation behaviour. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 228, 49–59. [Google Scholar] [CrossRef]
- Brehm, M.A.; Shultz, L.D.; Luban, J.; Greiner, D.L. Overcoming Current Limitations in Humanized Mouse Research. J. Infect. Dis. 2013, 208, S125–S130. [Google Scholar] [CrossRef]
- Riley, G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 2004, 43, 131–142. [Google Scholar] [CrossRef]
- Biagini, V.; Busi, F.; Anelli, V.; Kerschbamer, E.; Baghini, M.; Gurrieri, E.; Notarangelo, M.; Pesce, I.; van Niel, G.; D’Agostino, V.G.; et al. Zebrafish Melanoma-Derived Interstitial EVs Are Carriers of ncRNAs That Induce Inflammation. Int. J. Mol. Sci. 2022, 23, 5510. [Google Scholar] [CrossRef]
- Cicero, A.L.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef]
- Shin, H.; Han, C.; Labuz, J.M.; Kim, J.; Kim, J.; Cho, S.; Gho, Y.S.; Takayama, S.; Park, J. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci. Rep. 2015, 5, srep13103. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-Z.; Zhou, Y.-C.; Huang, L.-H.; Lu, H.-B. Development of biodegradable polycaprolactone film as an internal fixation material to enhance tendon repair: An in vitro study. BMC Musculoskelet. Disord. 2013, 14, 246. [Google Scholar] [CrossRef]
- Andric, T.; Wright, L.D.; Taylor, B.L.; Freeman, J.W. Fabrication and characterization of three-dimensional electrospun scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2012, 100, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Ligasová, A.; Koberna, K. Quantification of fixed adherent cells using a strong enhancer of the fluorescence of DNA dyes. Sci. Rep. 2019, 9, 8701. [Google Scholar] [CrossRef]
- Itoi, E.; Berglund, L.J.; Grabowski, J.J.; Schultz, F.M.; Growney, E.S.; Morrey, B.F.; An, K.N. Tensile properties of the supraspinatus tendon. J. Orthop. Res. 1995, 13, 578–584. [Google Scholar] [CrossRef]
- Moffat, K.L.; Kwei, A.S.; Spalazzi, J.P.; Doty, S.B.; Levine, W.N.; Lu, H.H. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng. Part A 2009, 15, 115–126. [Google Scholar] [CrossRef]
- Helland, C.; Bojsen-Møller, J.; Raastad, T.; Seynnes, O.R.; Moltubakk, M.M.; Jakobsen, V.; Visnes, H.; Bahr, R. Mechanical properties of the patellar tendon in elite volleyball players with and without patellar tendinopathy. Br. J. Sports Med. 2013, 47, 862–868. [Google Scholar] [CrossRef]
- Arya, S.; Kulig, K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J. Appl. Physiol. 2010, 108, 670–675. [Google Scholar] [CrossRef]
- Luyet, P.P.; Falguieres, T.; Pons, V.; Pattnaik, A.K.; Gruenberg, J. The ESCRT-I subunit TSG101 controls endosome-to-cytosol release of viral RNA. Traffic 2008, 9, 2279–2290. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zieren, R.C.; Horie, K.; Kim, C.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M.D.; Green, J.; Amend, S.R.; et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracell. Vesicles 2020, 10, e12044. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, S.; Koh, J.; Longo, U.G.; Stelitano, G.; Amirouche, F.; Denaro, V. Tendon Biomechanics-Structure and Composition. In Orthopaedic Biomechanics in Sports Medicine; Koh, J., Zaffagnini, S., Kuroda, R., Longo, U.G., Amirouche, F., Eds.; Springer International Publishing: Cham, The Netherlands, 2021; pp. 81–90. [Google Scholar]
- Chartier, C.; ElHawary, H.; Baradaran, A.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Tendon: Principles of Healing and Repair. Semin. Plast. Surg. 2021, 35, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.J.F.; Franklin, S.L.; Carr, A.J. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Jt. Res. 2012, 1, 158–166. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Kim, M.; Yun, H.-W.; Park, D.Y.; Choi, B.H.; Min, B.-H. Three-Dimensional Spheroid Culture Increases Exosome Secretion from Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 427–436. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Mimpen, J.Y.; Cribbs, A.P.; Stace, E.; Philpott, M.; Dakin, S.G.; Carr, A.J.; Snelling, S.J. Electrospun Scaffold Micro-Architecture Induces an Activated Transcriptional Phenotype within Tendon Fibroblasts. Front. Bioeng. Biotechnol. 2022, 9, 795748. [Google Scholar] [CrossRef]
- Sooriyaarachchi, D.; Minière, H.J.; Maharubin, S.; Tan, G.Z. Hybrid Additive Microfabrication Scaffold Incorporated with Highly Aligned Nanofibers for Musculoskeletal Tissues. Tissue Eng. Regen. Med. 2019, 16, 29–38. [Google Scholar] [CrossRef]
- Bereiter-Hahn, J.; Münnich, A.; Woiteneck, P. Dependence of energy metabolism on the density of cells in culture. Cell Struct. Funct. 1998, 23, 85–93. [Google Scholar] [CrossRef][Green Version]
- Mignon, C.; Uzunbajakava, N.E.; Raafs, B.; Botchkareva, N.V.; Tobin, D.A.-O. Photobiomodulation of human dermal fibroblasts in vitro: Decisive role of cell culture conditions and treatment protocols on experimental outcome. Sci. Rep. 2017, 7, 2797. [Google Scholar] [CrossRef] [PubMed]
- Quent, V.M.; Loessner, D.; Friis, T.; Reichert, J.C.; Hutmacher, D.W. Discrepancies between metabolic activity and DNA content as tool to assess cell proliferation in cancer research. J. Cell. Mol. Med. 2010, 14, 1003–1013. [Google Scholar] [CrossRef]
- Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.C.; Bayik, D.; Srivatsan, A.; Bergamaschi, C.; Valentin, A.; Niu, G.; Bear, J.; Monninger, M.; Sun, M.; Morales-Kastresana, A.; et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 2016, 105, 195–205. [Google Scholar] [CrossRef]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; Bissonnette, F.S.-D.; Johnston, M.J.W.; et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef]
- Jenkins, T.L.; Little, D. Synthetic scaffolds for musculoskeletal tissue engineering: Cellular responses to fiber parameters. NPJ Regen. Med. 2019, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-C.; Baji, A.; Leng, S. Effect of fiber diameter on tensile properties of electrospun poly(ɛ-caprolactone). Polymer 2008, 49, 4713–4722. [Google Scholar] [CrossRef]
- Rajeev, M.; Helms, C.C. A Study of the Relationship between Polymer Solution Entanglement and Electrospun PCL Fiber Mechanics. Polymers 2023, 15, 4555. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Li, Y.; Xu, S.; Wang, Y.; Zhu, Y.; Jiang, C.; Wang, K.; Zhang, Y.; Wang, Y. ECM stiffness affects cargo sorting into MSC-EVs to regulate their secretion and uptake behaviors. J. Nanobiotechnol. 2024, 22, 124. [Google Scholar] [CrossRef]
- Sneider, A.; Liu, Y.; Starich, B.; Du, W.; Nair, P.R.; Marar, C.; Faqih, N.; Ciotti, G.E.; Kim, J.H.; Krishnan, S.; et al. Small Extracellular Vesicles Promote Stiffness-mediated Metastasis. Cancer Res. Commun. 2024, 4, 1240–1252. [Google Scholar] [CrossRef]
- Lenzini, S.; Bargi, R.; Chung, G.; Shin, J.-W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 2020, 15, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Rippe, B. Water transport across the peritoneal membrane. Kidney Int. 2014, 85, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-F.; Li, W.-J.; Hu, K.-S.; Gao, J.; Zhai, W.-L.; Yang, J.-H.; Zhang, S.-J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Abdel, A.A. Mechanisms of Extracellular Vesicle Biogenesis, Cargo Loading, and Release. In Extracellular Vesicles; Manash, K.P., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar]
- Debbi, L.; Guo, S.; Safina, D.; Levenberg, S. Boosting extracellular vesicle secretion. Biotechnol. Adv. 2022, 59, 107983. [Google Scholar] [CrossRef] [PubMed]
- Zubkova, E.; Kalinin, A.; Bolotskaya, A.; Beloglazova, I.; Menshikov, M. Autophagy-Dependent Secretion: Crosstalk between Autophagy and Exosome Biogenesis. Curr. Issues Mol. Biol. 2024, 46, 2209–2235. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef]
- Gilboa, T.; Ter-Ovanesyan, D.; Wang, S.-C.; Whiteman, S.; Kannarkat, G.T.; Church, G.M.; Chen-Plotkin, A.S.; Walt, D.R. Assessment of extracellular vesicle protein cargo as neurodegenerative disease biomarkers. bioRxiv 2023, 2023.11.02.565137. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- DiIorio, S.E.; Young, B.; Parker, J.B.; Griffin, M.F.; Longaker, M.T. Understanding Tendon Fibroblast Biology and Heterogeneity. Biomedicines 2024, 12, 859. [Google Scholar] [CrossRef]
- Rahimi, A.M.; Cai, M.; Hoyer-Fender, S. Heterogeneity of the NIH3T3 Fibroblast Cell Line. Cells 2022, 11, 2677. [Google Scholar] [CrossRef]
- O’neill, C.P.; Gilligan, K.E.; Dwyer, R.M. Role of Extracellular Vesicles (EVs) in Cell Stress Response and Resistance to Cancer Therapy. Cancers 2019, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.; Mahadik, P.; Shetty, O.; Sen, S. ECM stiffness-tuned exosomes drive breast cancer motility through thrombospondin-1. Biomaterials 2021, 279, 121185. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shama, K.A.; Greenberg, Z.F.; Tammame, C.; He, M.; Taylor, B.L. Diseased Tendon Models Demonstrate Influence of Extracellular Matrix Alterations on Extracellular Vesicle Profile. Bioengineering 2024, 11, 1019. https://doi.org/10.3390/bioengineering11101019
Shama KA, Greenberg ZF, Tammame C, He M, Taylor BL. Diseased Tendon Models Demonstrate Influence of Extracellular Matrix Alterations on Extracellular Vesicle Profile. Bioengineering. 2024; 11(10):1019. https://doi.org/10.3390/bioengineering11101019
Chicago/Turabian StyleShama, Kariman A., Zachary Franklin Greenberg, Chadine Tammame, Mei He, and Brittany L. Taylor. 2024. "Diseased Tendon Models Demonstrate Influence of Extracellular Matrix Alterations on Extracellular Vesicle Profile" Bioengineering 11, no. 10: 1019. https://doi.org/10.3390/bioengineering11101019
APA StyleShama, K. A., Greenberg, Z. F., Tammame, C., He, M., & Taylor, B. L. (2024). Diseased Tendon Models Demonstrate Influence of Extracellular Matrix Alterations on Extracellular Vesicle Profile. Bioengineering, 11(10), 1019. https://doi.org/10.3390/bioengineering11101019







