Body Composition and Demographic Features Do Not Affect the Diagnostic Accuracy of Shear Wave Elastography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
2.4. Assessments
2.4.1. Demographic and Body Composition Features
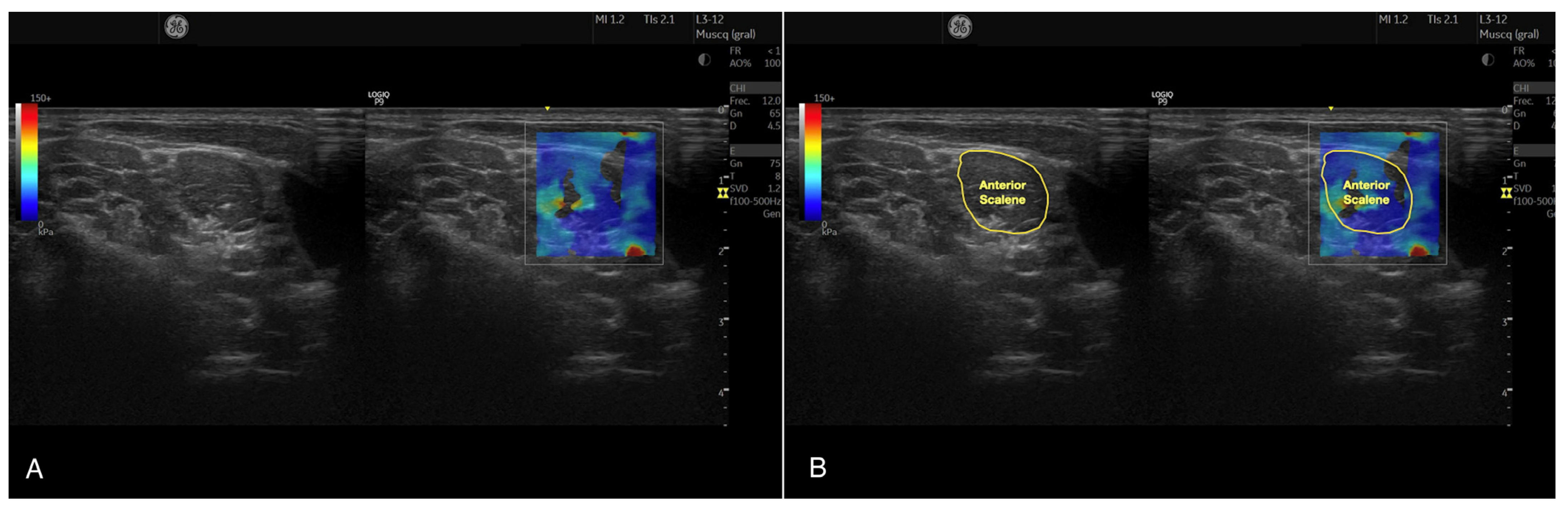
2.4.2. Anterior Scalene Muscle Stiffness
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, T.; Ying, M.; Sau Lan Tsang, C.; Huang, M.; Pang, M.Y.C. Reliability and Validity of Ultrasound Elastography for Evaluating Muscle Stiffness in Neurological Populations: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzaa188. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, R.; Galán-Del-Río, F.; Cantalapiedra, J.A.; Flórez-García, M.T.; Martínez-Martín, J.; Álvaro-Meca, A.; Koppenhaver, S.L.; Fernández-de-Las-Peñas, C. Reliability and discriminative validity of real-time ultrasound elastography in the assessment of tissue stiffness after calf muscle injury. J. Bodyw. Mov. Ther. 2021, 28, 463–469. [Google Scholar] [CrossRef]
- Gharaibeh, B.; Chun-Lansinger, Y.; Hagen, T.; Ingham, S.J.M.; Wright, V.; Fu, F.; Huard, J. Biological approaches to improve skeletal muscle healing after injury and disease. Birth Defects Res. Part C Embryo Today 2012, 96, 82–94. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Sánchez-Jorge, S.; Buffet-García, J.; Varol, U.; Gallego-Sendarrubias, G.M.; Álvarez-González, J. Is Shear-Wave Elastography a Clinical Severity Indicator of Myofascial Pain Syndrome? An Observational Study. J. Clin. Med. 2021, 10, 2895. [Google Scholar] [CrossRef]
- Janczyk, E.M.; Champigny, N.; Michel, E.; Raffaelli, C.; Annweiler, C.; Zory, R.; Guérin, O.; Sacco, G. Sonoelastography to Assess Muscular Stiffness among Older Adults and its Use for the Diagnosis of Sarcopenia: A Systematic Review. Ultraschall Med. 2021, 42, 634–642. [Google Scholar] [CrossRef]
- Yoshida, K.; Itoigawa, Y.; Maruyama, Y.; Kaneko, K. Healing Process of Gastrocnemius Muscle Injury on Ultrasonography Using B-Mode Imaging, Power Doppler Imaging, and Shear Wave Elastography. J. Ultrasound Med. 2019, 38, 3239–3246. [Google Scholar] [CrossRef]
- Snoj, Ž.; Wu, C.H.; Taljanovic, M.S.; Dumić-Čule, I.; Drakonaki, E.E.; Klauser, A.S. Ultrasound Elastography in Musculoskeletal Radiology: Past, Present, and Future. Semin. Musculoskelet. Radiol. 2020, 24, 156–166. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Fernández-de-las-Peñas, C.; Fernández-Rodríguez, T.; Arias-Buría, J.L.; Varol, U.; Gallego-Sendarrubias, G.M. Influence of Examiners’ Experience and Region of Interest Location on Semiquantitative Elastography Validity and Reliability. Appl. Sci. 2021, 11, 9247. [Google Scholar] [CrossRef]
- Koo, T.K.; Guo, J.Y.; Cohen, J.H.; Parker, K.J. Quantifying the passive stretching response of human tibialis anterior muscle using shear wave elastography. Clin. Biomech. 2014, 29, 33–39. [Google Scholar] [CrossRef]
- Koppenhaver, S.; Kniss, J.; Lilley, D.; Oates, M.; Fernández-de-Las-Peñas, C.; Maher, R.; Croy, T.; Shinohara, M. Reliability of ultrasound shear-wave elastography in assessing low back musculature elasticity in asymptomatic individuals. J. Electromyogr. Kinesiol. 2018, 39, 49–57. [Google Scholar] [CrossRef]
- Rosskopf, A.B.; Ehrmann, C.; Buck, F.M.; Gerber, C.; Flück, M.; Pfirrmann, C.W. Quantitative Shear-Wave US Elastography of the Supraspinatus Muscle: Reliability of the Method and Relation to Tendon Integrity and Muscle Quality. Radiology 2016, 278, 465–474. [Google Scholar] [CrossRef]
- Deng, W.; Lin, M.; Yu, S.; Liang, H.; Zhang, Z.; Liu, C. Quantifying Region-Specific Elastic Properties of Distal Femoral Articular Cartilage: A Shear-Wave Elastography Study. Appl. Bionics Biomech. 2022, 2022, 9406863. [Google Scholar] [CrossRef]
- DeJong, H.; Abbott, S.; Zelesco, M.; Spilsbury, K.; Martin, L.; Sanderson, R.; Ziman, M.; Kennedy, B.F.; Wood, F.M. A Novel, Reliable Protocol to Objectively Assess Scar Stiffness Using Shear Wave Elastography. Ultrasound Med. Biol. 2020, 46, 1614–1629. [Google Scholar] [CrossRef]
- Besomi, M.; Salomoni, S.E.; Hug, F.; Tier, L.; Vicenzino, B.; Hodges, P.W. Exploration of shear wave elastography measures of the iliotibial band during different tasks in pain-free runners. Phys. Ther. Sport 2021, 50, 121–129. [Google Scholar] [CrossRef]
- Kozinc, Ž.; Šarabon, N. Shear-wave elastography for assessment of trapezius muscle stiffness: Reliability and association with low-level muscle activity. PLoS ONE 2020, 15, e0234359. [Google Scholar] [CrossRef]
- Heneghan, N.R.; Smith, R.; Tyros, I.; Falla, D.; Rushton, A. Thoracic dysfunction in whiplash associated disorders: A systematic review. PLoS ONE 2018, 13, e0194235. [Google Scholar] [CrossRef]
- Panther, E.J.; Reintgen, C.D.; Cueto, R.J.; Hao, K.A.; Chim, H.; King, J.J. Thoracic outlet syndrome: A review. J. Shoulder Elb. Surg. 2022, 31, e545–e561. [Google Scholar] [CrossRef]
- Kottner, J.; Audigé, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streine, D.L. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J. Clin. Epidemiol. 2011, 64, 96–106. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr. EQUATOR reporting guidelines should also be used by clinicians. J. Clin. Epidemiol. 2020, 117, 149–150. [Google Scholar] [CrossRef]
- Harris, R.J. A Primer of Multivariate Statistics, 2nd ed.; Academic Press: New York, NY, USA, 1985. [Google Scholar]
- VanVoorhis, C.W.; Morgan, B.L. Understanding power and rules of thumb for determining sample sizes. Tutor. Quant. Methods Psychol. 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Larsen, M.N.; Krustrup, P.; Araújo Póvoas, S.C.; Castagna, C. Accuracy and reliability of the InBody 270 multi-frequency body composition analyser in 10–12-year-old children. PLoS ONE 2021, 16, e0247362. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Gómez-Sánchez, S.; Fernández-de-Las-Peñas, C.; Plaza-Manzano, G.; Sánchez-Jorge, S.; Navarro-Santana, M.J. A Procedure for Measuring Anterior Scalene Morphology and Quality with Ultrasound Imaging: An Intra- and Inter-rater Reliability Study. Ultrasound Med. Biol. 2023, 49, 1817–1823. [Google Scholar] [CrossRef]
- Latash, M.L. Muscle coactivation: Definitions, mechanisms, and functions. J. Neurophysiol. 2018, 120, 88–104. [Google Scholar] [CrossRef]
- Groth, M.; Fischer, L.; Herden, U.; Brinkert, F.; Beime, J.; Deindl, P.; Adam, G.; Herrmann, J. Impact of probe-induced abdominal compression on two-dimensional shear wave elastography measurement of split liver transplants in children. Rofo 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Joeng, E.S.; Jeong, Y.C.; Park, B.J.; Kang, S.; Yang, S.N.; Yoon, J.S. Sonoanatomical Change of Phrenic Nerve According to Posture During Ultrasound-Guided Stellate Ganglion Block. Ann. Rehabil. Med. 2016, 40, 244–251. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Bedewi, M.A.; Alhariqi, B.A.; Aldossary, N.M.; Gaballah, A.H.; Sandougah, K.J. Shear wave elastography of the scalene muscles in healthy adults: A preliminary study. Medicine 2021, 100, e26891. [Google Scholar] [CrossRef]
- Varol, U.; Sánchez-Jiménez, E.; Leloup, E.A.A.; Navarro-Santana, M.J.; Fernández-de-Las-Peñas, C.; Sánchez-Jorge, S.; Valera-Calero, J.A. Correlation between Body Composition and Inter-Examiner Errors for Assessing Lumbar Multifidus Muscle Size, Shape and Quality Metrics with Ultrasound Imaging. Bioengineering 2023, 10, 133. [Google Scholar] [CrossRef]
- Varol, U.; Navarro-Santana, M.J.; Gómez-Sánchez, S.; Plaza-Manzano, G.; Sánchez-Jiménez, E.; Valera-Calero, J.A. Inter-Examiner Disagreement for Assessing Cervical Multifidus Ultrasound Metrics Is Associated with Body Composition Features. Sensors 2023, 23, 1213. [Google Scholar] [CrossRef]
- Kuo, W.H.; Jian, D.W.; Wang, T.G.; Wang, Y.C. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med. Biol. 2013, 39, 1356–1361. [Google Scholar] [CrossRef]
- Joshi, S.D.; Joshi, S.S.; Daimi, S.R.; Athavale, S.A. The thyroid gland and its variations: A cadaveric study. Folia Morphol. 2010, 69, 47–50. [Google Scholar]
- Blanpied, P.R.; Gross, A.R.; Elliott, J.M.; Devaney, L.L.; Clewley, C.D.; Walton, D.M.; Sparks, C.; Robertson, E.K. Neck Pain: Revision 2017. J. Orthop. Sports Phys. Ther. 2017, 47, A1–A83. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Al-Buqain-Ortega, A.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Varol, U.; Ortega-Santiago, R. Echo-intensity, fatty infiltration, and morphology ultrasound imaging assessment in healthy and whiplash associated disorders populations: An observational study. Eur. Spine J. 2021, 30, 3059–3067. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Úbeda-D’Ocasar, E.; Caballero-Corella, M.; Fernández-de-Las-Peñas, C.; Sendarrubias, G.M.G.; Arias-Buría, J.L. Cervical Multifidus Morphology and Quality Are Not Associated with Clinical Variables in Women with Fibromyalgia: An Observational Study. Pain Med. 2022, 23, 1138–1143. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Navarro-Santana, M.J.; Plaza-Manzano, G.; Fernández-de-Las-Peñas, C.; Ortega-Santiago, R. Identifying Demographic, Clinical, Muscular and Histological Factors Associated with Ultrasound Cervical Multifidus Measurement Errors in a Chronic Neck Pain Population. Sensors 2022, 22, 8344. [Google Scholar] [CrossRef]
| Variables | Total Sample (n = 34) | Gender | Side | ||||
|---|---|---|---|---|---|---|---|
| Male (n = 24) | Female (n = 10) | Difference | Right (n = 34) | Left (n = 34) | Difference | ||
| Sociodemographic Characteristics | |||||||
| Age (y) | 21.23 ± 4.75 | 21.91 ± 5.43 | 19.60 ± 1.77 | 2.31 (−1.29; 5.92) p = 0.200 | - | - | - |
| Height (m) | 1.72 ± 0.08 | 1.76 ± 0.06 | 1.65 ± 0.05 | 0.11 (0.07; 0.16) p < 0.001 | - | - | - |
| Weight (kg) | 71.95 ± 14.05 | 74.45 ± 13.47 | 65.97 ± 14.26 | 8.48 (−2.02; 18.99) p = 0.110 | - | - | - |
| Body Mass Index (kg/m2) | 24.10 ± 3.95 | 24.02 ± 3.79 | 24.29 ± 4.53 | −0.27 (−3.34; 2.81) p = 0.799 | - | - | - |
| Body Composition Characteristics | |||||||
| Fat Mass (Kg) | 16.90 ± 9.38 | 14.25 ± 8.24 | 23.25 ± 9.25 | 8.99 (2.44;15.54) p = 0.009 | - | - | - |
| Lean Mass (Kg) | 30.93 ± 6.77 | 34.12 ± 5.09 | 23.29 ± 3.17 | 10.83 (7.27; 14.39) p < 0.001 | - | - | - |
| Water Volume (kg) | 40.23 ± 8.11 | 44.00 ± 6.16 | 31.20 ± 3.97 | 12.80 (8.48; 17.11) p < 0.001 | - | - | - |
| Anterior Scalene Muscle Ultrasound Characteristics a | |||||||
| Young’s Modulus (kPa) | 15.69 ± 8.36 | 15.12 ± 7.76 | 17.42 ± 9.83 | 2.29 (−2.23;6.83) p = 0.315 | 16.78 ± 8.99 | 14. 50 ± 7.57 | 2.28 (−1.79; 6.36) p = 0.267 |
| Shear Wave Speed (m/s) | 2.21 ± 0.56 | 2.18 ± 0.53 | 2.33 ± 0.64 | 0.15 (−0.14;0.46) p = 0.312 | 2.29 ± 0.61 | 2.13 ± 0.49 | 0.13 (−0.11; 0.43) p = 0.250 |
| Variables | Young’s Modulus (kPa) | Shear Wave Speed (m/s) |
|---|---|---|
| Mean | 15.69 ± 8.36 | 2.21 ± 0.56 |
| Test | 15.96 ± 8.91 | 15.42 ± 8.52 |
| Re-Test | 2.23 ± 058 | 2.20 ± 0.58 |
| Absolute Difference | 3.30 ± 3.71 | 0.21 ± 0.22 |
| ICC3,2 (95% CI) | 0.912 (0.857–0.946) | 0.923 (0.874–0.952) |
| SEM | 2.47 | 0.15 |
| MDC95 | 3.50 | 0.21 |
| CV (%) | 21.0 | 9.5 |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | ||||||||||
| 2. Height | 0.174 | |||||||||
| 3. Weight | 0.142 | 0.536 ** | ||||||||
| 4. Body Mass Index | 0.075 | 0.039 | 0.854 ** | |||||||
| 5. Water Volume | 0.172 | 0.895 ** | 0.744 ** | 0.347 ** | ||||||
| 6. Lean Mass | 0.171 | 0.890 ** | 0.739 ** | 0.345 ** | 1.000 ** | |||||
| 7. Fat Mass | 0.014 | −0.259 * | 0.614 ** | 0.866 ** | −0.071 | −0.078 | ||||
| 8. Young’s Modulus | −0.085 | −0.125 | −0.102 | −0.020 | −0.126 | −0.125 | −0.004 | |||
| 9. Shear Wave Speed | −0.077 | −0.158 | −0.117 | −0.014 | −0.149 | −0.147 | 0.001 | 0.987 ** | ||
| 10. Young’s Modulus Error | 0.050 | −0.157 | 0.052 | 0.197 | −0.034 | −0.030 | 0.119 | 0.363 ** | 0.390 ** | |
| 11. Shear Wave Speed Error | 0.038 | −0.155 | 0.085 | 0.237 | −0.031 | −0.026 | 0.164 | 0.198 | 0.212 | 0.927 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varol, U.; Valera-Calero, J.A.; Fernández-de-las-Peñas, C.; Buffet-García, J.; Plaza-Manzano, G.; Navarro-Santana, M.J. Body Composition and Demographic Features Do Not Affect the Diagnostic Accuracy of Shear Wave Elastography. Bioengineering 2023, 10, 904. https://doi.org/10.3390/bioengineering10080904
Varol U, Valera-Calero JA, Fernández-de-las-Peñas C, Buffet-García J, Plaza-Manzano G, Navarro-Santana MJ. Body Composition and Demographic Features Do Not Affect the Diagnostic Accuracy of Shear Wave Elastography. Bioengineering. 2023; 10(8):904. https://doi.org/10.3390/bioengineering10080904
Chicago/Turabian StyleVarol, Umut, Juan Antonio Valera-Calero, César Fernández-de-las-Peñas, Jorge Buffet-García, Gustavo Plaza-Manzano, and Marcos José Navarro-Santana. 2023. "Body Composition and Demographic Features Do Not Affect the Diagnostic Accuracy of Shear Wave Elastography" Bioengineering 10, no. 8: 904. https://doi.org/10.3390/bioengineering10080904
APA StyleVarol, U., Valera-Calero, J. A., Fernández-de-las-Peñas, C., Buffet-García, J., Plaza-Manzano, G., & Navarro-Santana, M. J. (2023). Body Composition and Demographic Features Do Not Affect the Diagnostic Accuracy of Shear Wave Elastography. Bioengineering, 10(8), 904. https://doi.org/10.3390/bioengineering10080904







