Development and Utility of an Imaging System for Internal Dosimetry of Astatine-211 in Mice
Abstract
:1. Introduction
2. Results
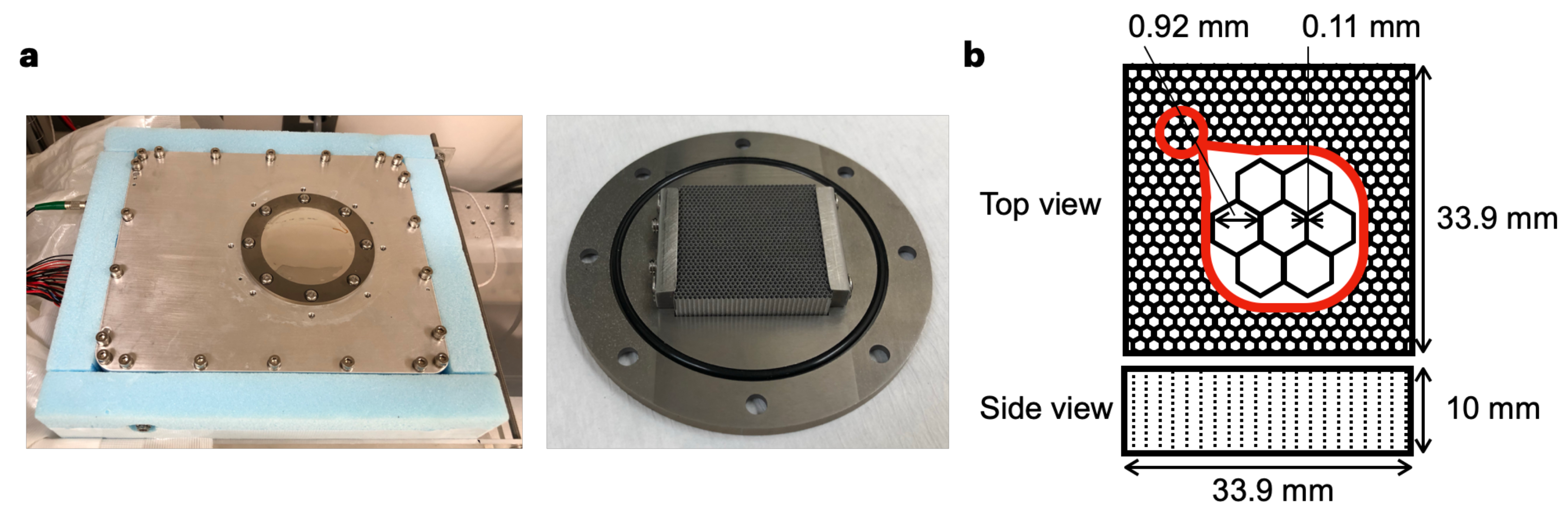
2.1. Specification of the Imager
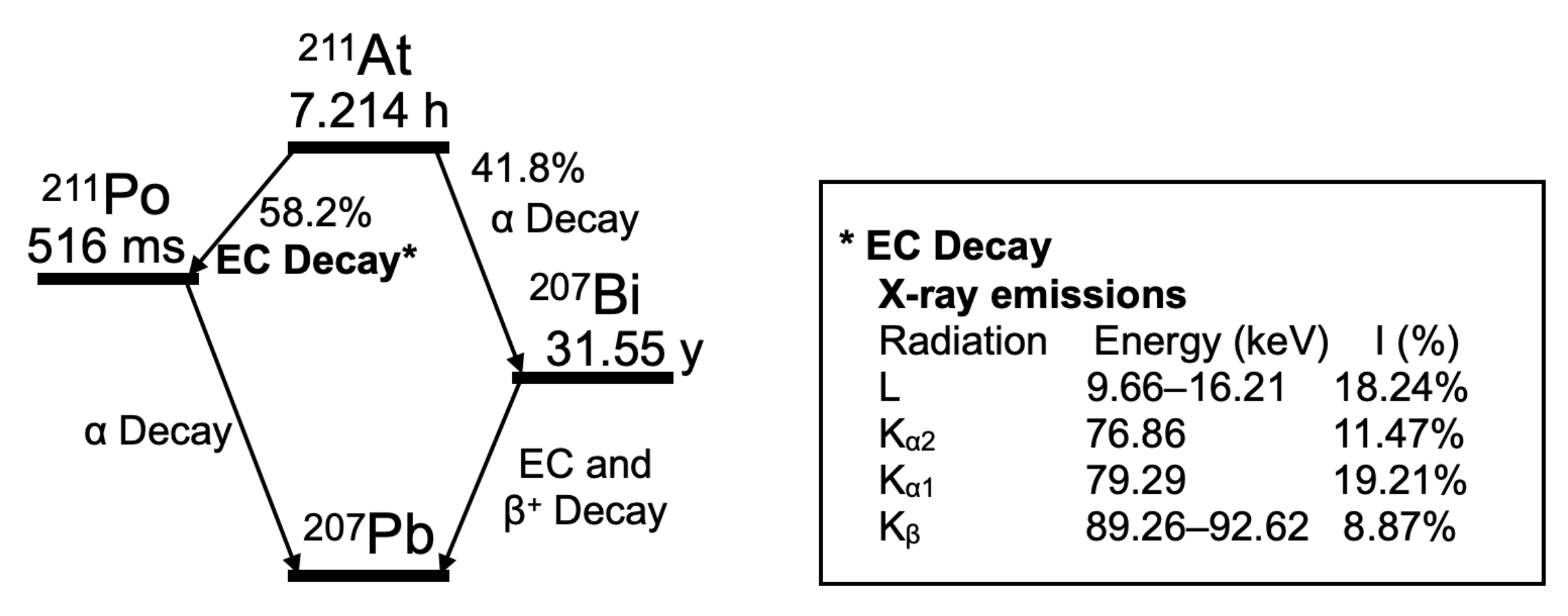
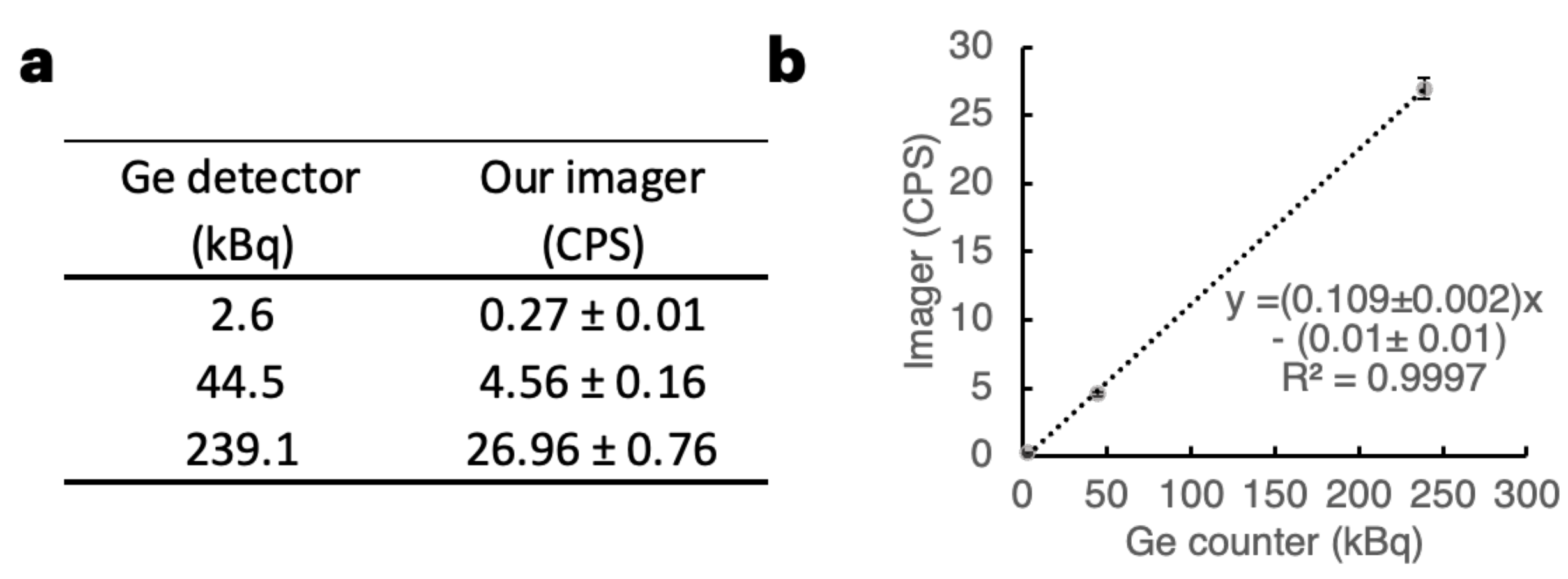
2.2. Performance of the Imager with Respect to 211At X-rays
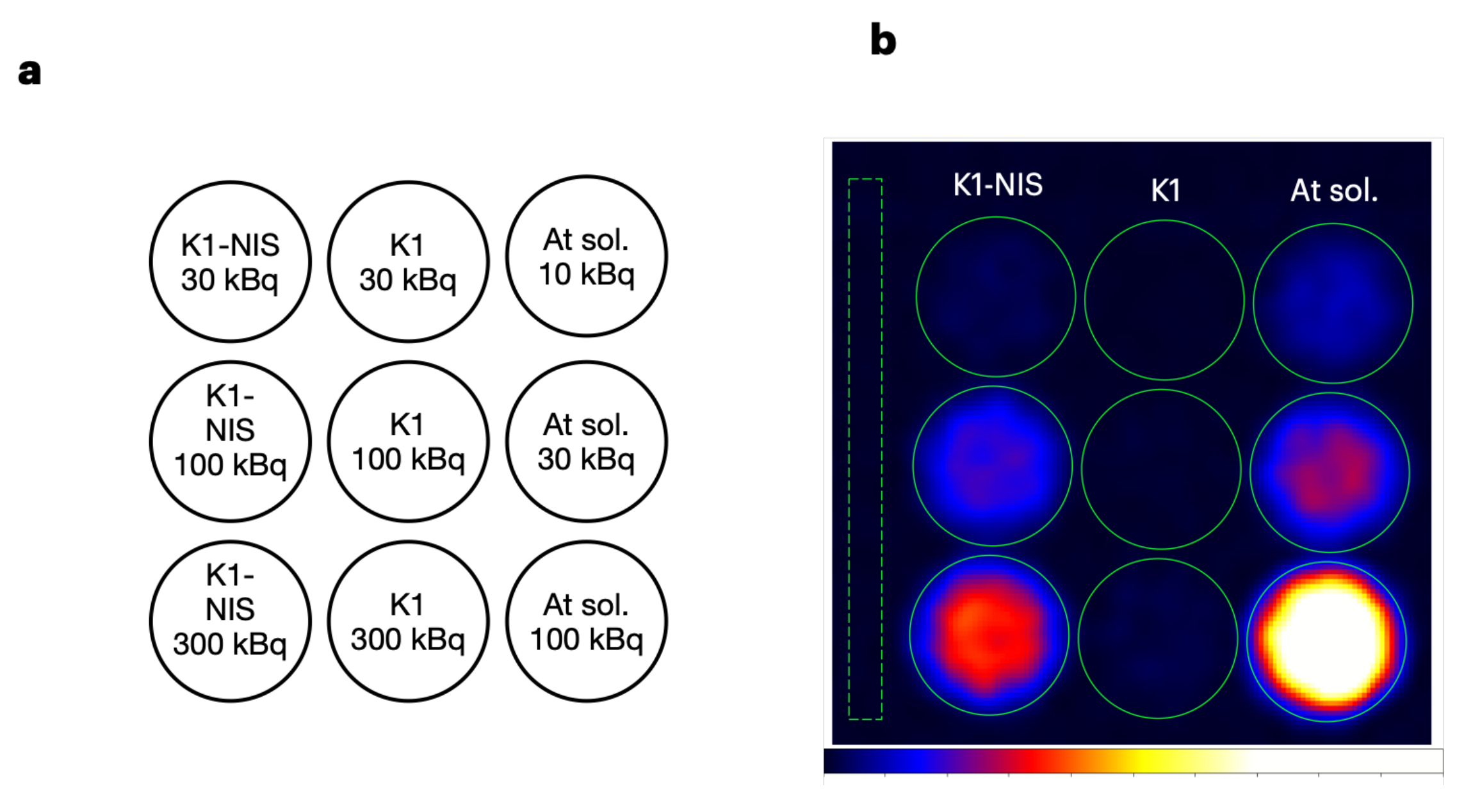
2.3. Cell Imaging Using 211At-NaAt Solution
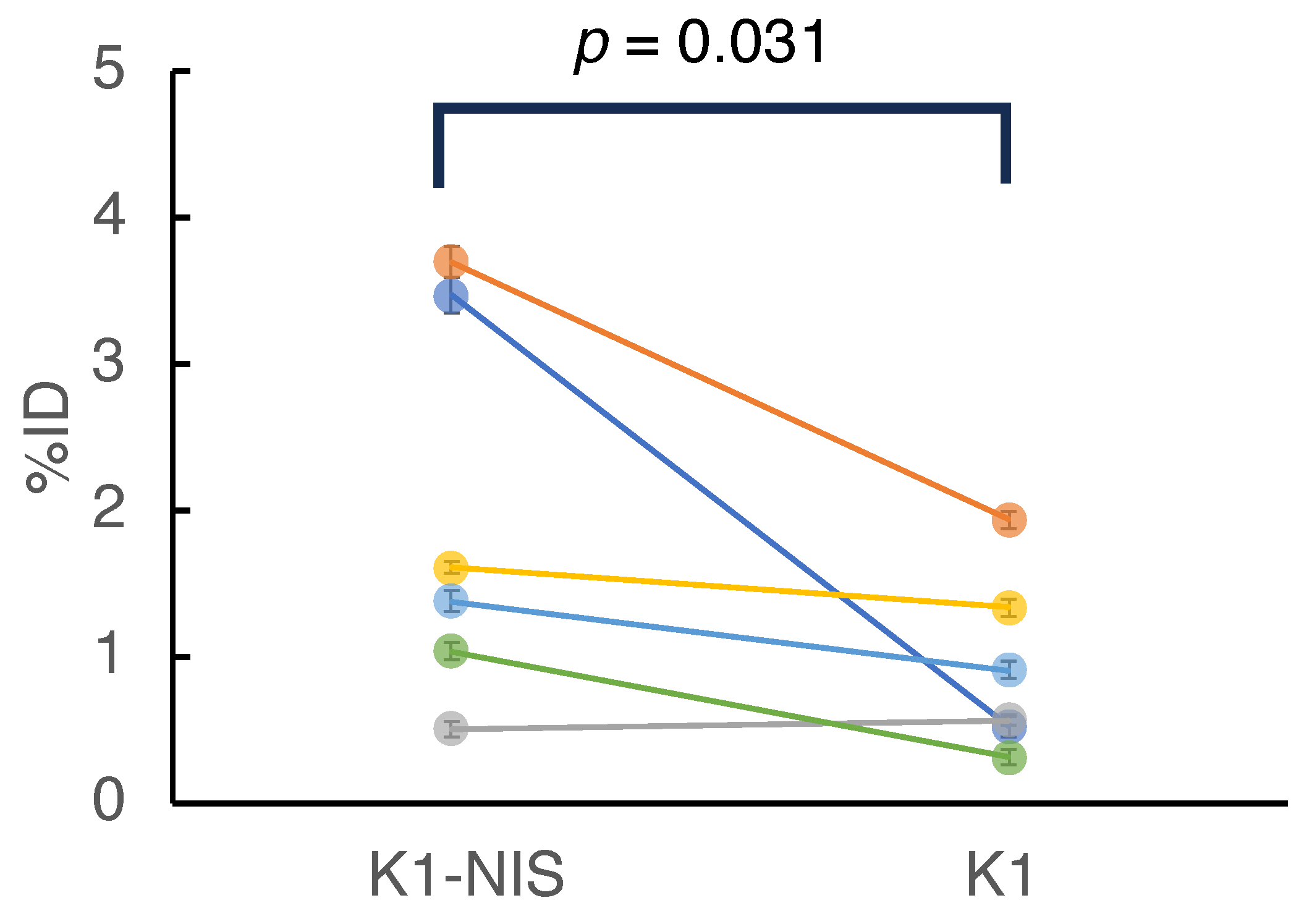
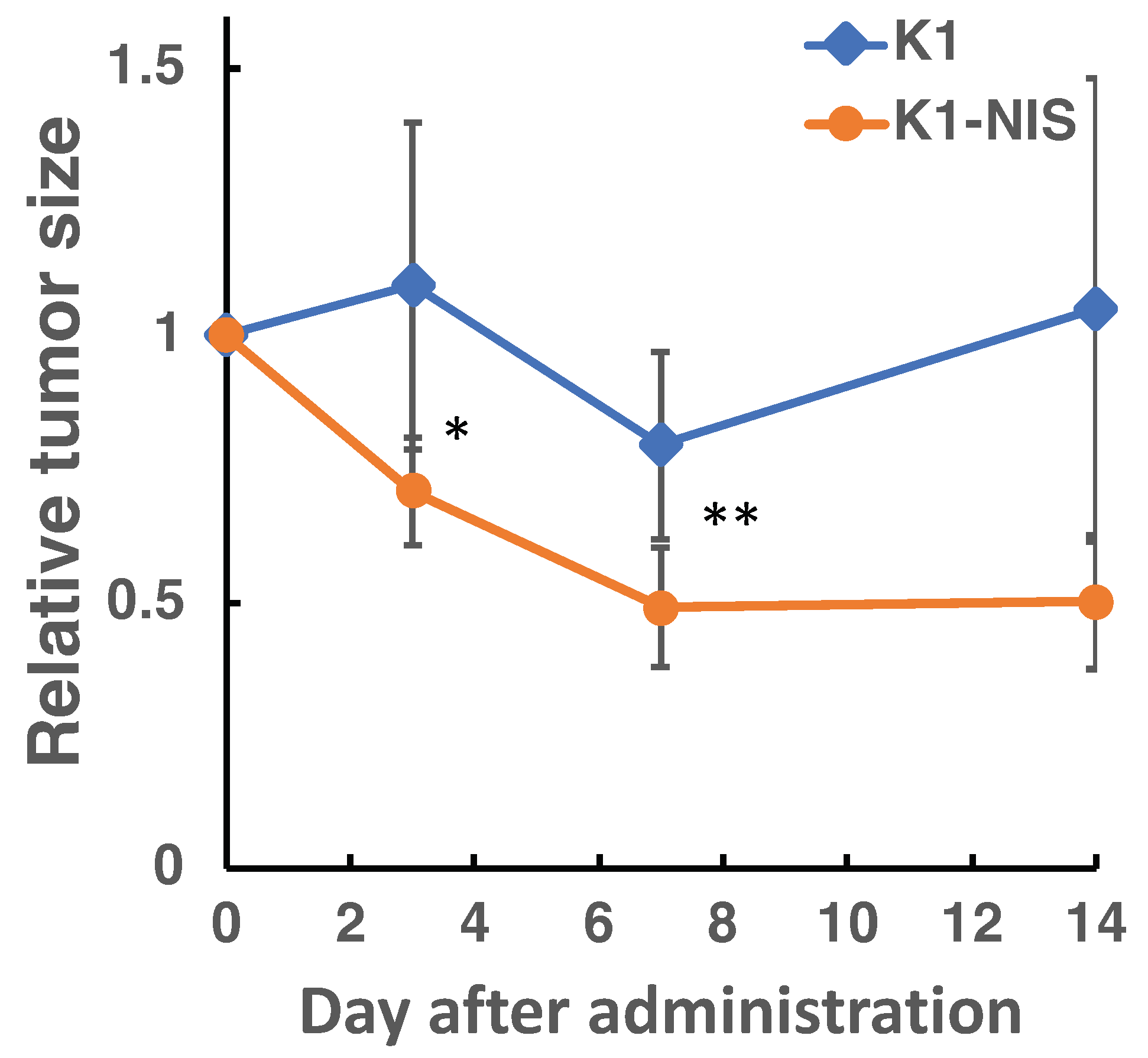
2.4. In Vivo Imaging in 211At Radionuclide Therapy
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Imager Setup and Its Performance
5.2. Preparation of 211At Solutions
5.3. Cell Culture
5.4. Preparation of Animals
5.5. Imaging Conditions for Cellular and Animal Imaging
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPECT | single-photon emission computed tomography |
| NIS | sodium iodide symporter |
| SN-ratio | signal-to-noise ratio |
| CdTe | cadmium telluride |
| CPS | count per second |
| ROI | region of interest |
| %ID | percentage of injected dose |
| FOV | field of view |
References
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A.M.; Lewis, J.S. Radiotheranostics in oncology: Current challenges and emerging opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef] [PubMed]
- Pomykala, K.L.; Hadaschik, B.A.; Sartor, O.; Gillessen, S.; Sweeney, C.J.; Maughan, T.; Hofman, M.S.; Herrmann, K. Next generation radiotheranostics promoting precision medicine. Ann. Oncol. 2023, 34, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Kendi, A.T.; Johnson, G.B.; Halfdanarson, T.R.; Sartor, O. Targeted Alpha-Particle Therapy: A Review of Current Trials. Int. J. Mol. Sci. 2023, 24, 11626. [Google Scholar] [CrossRef] [PubMed]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I.; Adelstein, S.J. Radiobiologic Principles in Radionuclide Therapy. J. Nucl. Med. 2005, 46 (Suppl. 1), 4S–12S. [Google Scholar]
- Flux, G.D.; Haq, M.; Chittenden, S.J.; Buckley, S.; Hindorf, C.; Newbold, K.; Harmer, C.L. A dose-effect correlation for radioiodine ablation in differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 270–275. [Google Scholar] [CrossRef]
- Dewaraja, Y.K.; Schipper, M.J.; Shen, J.; Smith, L.B.; Murgic, J.; Savas, H.; Youssef, E.; Regan, D.; Wilderman, S.J.; Roberson, P.L.; et al. Tumor-Absorbed Dose Predicts Progression-Free Survival Following 131I-Tositumomab Radioimmunotherapy. J. Nucl. Med. 2014, 55, 1047–1053. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Cremonesi, M.; Ferrari, M.E.; Bodei, L.; Chiesa, C.; Sarnelli, A.; Garibaldi, C.; Pacilio, M.; Strigari, L.; Summers, P.E.; Orecchia, R.; et al. Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2426–2441. [Google Scholar] [CrossRef]
- Strigari, L.; Konijnenberg, M.; Chiesa, C.; Bardies, M.; Du, Y.; Gleisner, K.S.; Lassmann, M.; Flux, G. The evidence base for the use of internal dosimetry in the clinical practice of molecular radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1976–1988. [Google Scholar] [CrossRef] [PubMed]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Decay Characteristics of Some Medically Important Radionuclides. In Physics in Nuclear Medicine, 4th ed.; Elsevier: Philadelphia, PA, USA, 2012; pp. 449–475. [Google Scholar]
- Chu, S.Y.F.; Ekström, L.P.; Firestone, R.B. The Lund/LBNL Nuclear Data Search, Version 2.0. 1999. Available online: http://nucleardata.nuclear.lu.se/toi/ (accessed on 1 May 2023).
- Takeda, S.; Orita, T.; Yagishita, A.; Katsuragawa, M.; Yabu, G.; Tomaru, R.; Moriyama, F.; Sugawara, H.; Watanabe, S.; Mizuma, H.; et al. CdTe-DSD SPECT-I: An Ultra-High-Resolution Multi-Isotope Tomographic Imager for Mice. IEEE Trans. Radiat. Plasma Med. Sci. 2023, 7, 860–870. [Google Scholar] [CrossRef]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; Lindegren, S.; Timperanza, C.; Smerud, K.; Palm, S. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front. Med. 2022, 9, 1076210. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Ohnuki, K.; Takeda, S.; Katsuragawa, M.; Yagishita, A.; Yabu, G.; Watanabe, S.; Takahashi, T. A Pure Tungsten Collimator Manufactured Using 3D Printing Technology for the Evaluation of 211At Radionuclide Therapy. Radioisotopes 2022, 71, 141–151. [Google Scholar] [CrossRef]
- Spetz, J.; Rudqvist, N.; Forssell-Aronsson, E. Biodistribution and dosimetry of free 211At, 125I− and 131I− in rats. Cancer Biother. Radio. 2013, 28, 657–664. [Google Scholar] [CrossRef]
- Maheshwari, Y.K.; Hill, C.S., Jr.; Haynie, T.P., III; Hickey, R.C.; Samaan, N.A. 131I therapy in differentiated thyroid carcinoma: MD Anderson Hospital experience. Cancer 1981, 47, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Maxon, H., 3rd; Englaro, E.E.; Thomas, S.R.; Hertzberg, V.S.; Hinnefeld, J.D.; Chen, L.S.; Smith, H.; Cummings, D.; Aden, M.D. Radioiodine-131 therapy for well-differentiated thyroid cancer—A quantitative radiation dosimetric approach: Outcome and validation in 85 patients. J. Nucl. Med. 1992, 33, 1132–1136. [Google Scholar] [PubMed]
- Ciarallo, A.; Rivera, J. Radioactive iodine therapy in differentiated thyroid cancer: 2020 update. Am. J. Roentgenol. 2020, 215, 285–1291. [Google Scholar] [CrossRef]
- Watabe, T.; Kaneda-Nakashima, K.; Liu, Y.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Fukuda, M.; Shinohara, A.; Hatazawa, J. Enhancement of 211At Uptake via the Sodium Iodide Symporter by the Addition of Ascorbic Acid in Targeted α-Therapy of Thyroid Cancer. J. Nucl. Med. 2019, 60, 1301–1307. [Google Scholar] [CrossRef]
- Kaneda-Nakashima, K.; Shirakami, Y.; Watabe, T.; Ooe, K.; Yoshimura, T.; Toyoshima, A.; Wang, Y.; Haba, H.; Fukase, K. Effect to Therapy of Sodium-Iodine Symporter Expression by Alpha-Ray Therapeutic Agent via Sodium/Iodine Symporter. Int. J. Mol. Sci. 2022, 23, 15509. [Google Scholar] [CrossRef]
- Takashima, H.; Ohnuki, K.; Manabe, S.; Koga, Y.; Tsumura, R.; Anzai, T.; Wang, Y.; Yin, X.; Sato, N.; Shigekawa, Y.; et al. Tumor Targeting of 211At-Labeled Antibody under Sodium Ascorbate Protection against Radiolysis. Mol. Pharmaceut. 2023, 20, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, Y.; Sudo, H.; Watanabe, S.; Nagatsu, K.; Tsuji, A.B.; Sakashita, T.; Ito, Y.M.; Yoshinaga, K.; Higashi, T.; Ishioka, N.S. Antitumor effects of radionuclide treatment using α-emitting meta-211At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Liu, Y.; Ooe, K.; Teramoto, T.; Toyoshima, A.; Shimosegawa, E.; Nakano, T.; Kanai, Y.; et al. Targeted alpha therapy using astatine (211At)-labeled phenylalanine: A preclinical study in glioma bearing mice. Oncotarget 2020, 11, 1138–1398. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Kadonaga, Y.; Ooe, K.; Wang, Y.; Haba, H.; Toyoshima, A.; Cardinale, J.; Giesel, F.L.; et al. Targeted α-therapy using astatine (211At)-labeled PSMA1, 5, and 6: A preclinical evaluation as a novel compound. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Yang, Y.; Zhang, J.; Yin, Y.; Liu, W.; Zhang, H.; Fan, X.; Yang, M.; Yu, F. Effective Treatment of SSTR2-Positive Small Cell Lung Cancer Using 211At-Containing Targeted α-Particle Therapy Agent Which Promotes Endogenous Antitumor Immune Response. Mol. Pharmaceut. 2023, 20, 5543–5553. [Google Scholar] [CrossRef]
- Kaneda-Nakashima, K.; Zhang, Z.; Manabe, Y.; Shimoyama, A.; Kabayama, K.; Watabe, T.; Kanai, Y.; Ooe, K.; Toyoshima, A.; Shirakami, Y.; et al. α-Emitting cancer therapy using 211At-AAMT targeting LAT1. Cancer Sci. 2021, 112, 1132–1140. [Google Scholar] [CrossRef]
- Lindencrona, U.; Nilsson, M.; Forssell-Aronsson, E. Similarities and differences between free 211At and 125I transport in porcine thyroid epithelial cells cultured in bicameral chambers. Nucl. Med. Biol. 2001, 28, 41–50. [Google Scholar] [CrossRef]
- Yagishita, A.; Takeda, S.i.; Katsuragawa, M.; Kawamura, T.; Matsumura, H.; Orita, T.; Umeda, I.O.; Yabu, G.; Caradonna, P.; Takahashi, T.; et al. Simultaneous visualization of multiple radionuclides in vivo. Nat. Biomed. Eng. 2022, 6, 640–647. [Google Scholar] [CrossRef]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. The Gamma camera, performance characteristics. In Physics in Nuclear Medicine, 4th ed.; Elsevier: Philadelphia, PA, USA, 2012; pp. 209–231. [Google Scholar]


| Accuracy (Statistical Error) | Acquisition Time (min) | |||
|---|---|---|---|---|
| 10 | 20 | 30 | 60 | |
| 5% | 6.12 | 3.06 | 2.04 | 1.02 |
| 10% | 1.53 | 0.76 | 0.51 | 0.25 |
| 15% | 0.68 | 0.34 | 0.23 | 0.11 |
| Well | Loaded Activity (kBq) | CPS | Activity (kBq) | Uptake Rate (%) |
|---|---|---|---|---|
| K1-NIS cell | 300 | 4.77 | 44.07 | 14.69 |
| 100 | 2.58 | 23.94 | 23.94 | |
| 30 | 0.35 | 3.34 | 11.13 | |
| K1 cell | 300 | 0.28 | 2.7 | 0.9 |
| 100 | 0.08 | 0.83 | 0.83 | |
| 30 | n.d. | n.a. | n.a. | |
| Reference (no cell) | 100 | 11.58 | 106.84 | - |
| 30 | 3.3 | 30.55 | - | |
| 10 | 1.11 | 10.36 | - |
| Mouse No. | Dose (MBq) | K1-NIS | K1 | ||
|---|---|---|---|---|---|
| Activity (kBq) | %ID (%) | Activity (kBq) | %ID (%) | ||
| 1 | 1.16 | 40.34 ± 1.31 | 3.47 ± 0.11 | 6.13 ± 0.69 | 0.53 ± 0.06 |
| 2 | 1.07 | 39.68 ± 1.29 | 3.7 ± 0.12 | 20.81 ± 0.9 | 1.94 ± 0.08 |
| 3 | 1.07 | 5.49 ± 0.44 | 0.51 ± 0.04 | 6.14 ± 0.59 | 0.57 ± 0.06 |
| 4 | 1.05 | 16.91 ± 0.7 | 1.61 ± 0.07 | 14.07 ± 0.65 | 1.34 ± 0.06 |
| 5 | 1.04 | 14.32 ± 0.62 | 1.38 ± 0.06 | 9.48 ± 0.52 | 0.91 ± 0.05 |
| 6 | 1.07 | 11.08 ± 0.56 | 1.04 ± 0.05 | 3.46 ± 0.36 | 0.32 ± 0.03 |
| Requirements | Achieved Results | |
|---|---|---|
| Sensitivity | Is quantitative imaging of many therapeutics possible concerning dose and tumor accumulation rate? | The system is capable of handling many cases as detailed in Table 1 and the results. |
| Spatial resolution | Is spatial resolution sufficient to distinguish mouse organs? | The spatial resolution is better than 1.6 mm, which is sufficient to identify small organs such as the thyroid gland. |
| Energy resolution | Is the energy resolution sufficient to distinguish it from noise signals? | Its high energy resolution makes it possible to distinguish between X-rays from 211At and tungsten fluorescent X-rays. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagishita, A.; Katsuragawa, M.; Takeda, S.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Takahashi, T.; Watabe, T. Development and Utility of an Imaging System for Internal Dosimetry of Astatine-211 in Mice. Bioengineering 2024, 11, 25. https://doi.org/10.3390/bioengineering11010025
Yagishita A, Katsuragawa M, Takeda S, Shirakami Y, Ooe K, Toyoshima A, Takahashi T, Watabe T. Development and Utility of an Imaging System for Internal Dosimetry of Astatine-211 in Mice. Bioengineering. 2024; 11(1):25. https://doi.org/10.3390/bioengineering11010025
Chicago/Turabian StyleYagishita, Atsushi, Miho Katsuragawa, Shin’ichiro Takeda, Yoshifumi Shirakami, Kazuhiro Ooe, Atsushi Toyoshima, Tadayuki Takahashi, and Tadashi Watabe. 2024. "Development and Utility of an Imaging System for Internal Dosimetry of Astatine-211 in Mice" Bioengineering 11, no. 1: 25. https://doi.org/10.3390/bioengineering11010025
APA StyleYagishita, A., Katsuragawa, M., Takeda, S., Shirakami, Y., Ooe, K., Toyoshima, A., Takahashi, T., & Watabe, T. (2024). Development and Utility of an Imaging System for Internal Dosimetry of Astatine-211 in Mice. Bioengineering, 11(1), 25. https://doi.org/10.3390/bioengineering11010025







