1. Introduction
The human circulatory system serves as the source of energy and nutrients for our bodies. This system facilitates the transfer of oxygen, carbon dioxide, and nutrients between cells, while eliminating metabolic waste. The circulatory system primarily comprises the lungs, heart, arteries, veins, and microcirculation. Microcirculation refers to the network of the tiniest blood vessels, with blood vessels having a diameter smaller than 100 μm [
1]. This microvasculature is responsible for substance exchange between the blood and cells, including oxygen, carbon dioxide, and nutrients. The functioning of microcirculation is mainly regulated by endothelial cells, which control aspects such as blood vessel dilation and constriction, vascular permeability, local blood flow control, and anticoagulation. When the functions of endothelial cells are disrupted, it can lead to cardiovascular diseases.
Research using optical technology has shown the capability of observing vascular images on the skin’s surface, and categorized abnormalities in microcirculation into five groups [
2].
Class I (all capillaries stagnant): This phenomenon was observed in certain septic shock patients following the excessive use of vasopressors to normalize blood pressure, potentially hindering microcirculatory flow. The absence of capillary flow in this class would result in tissue hypoxia.
Class II (capillaries with flowing red blood cells next to capillaries with no flow): Predominantly observed during cardiopulmonary bypass surgery, this involves disruptions in normal blood flow in micro-veins. This leads to variations in blood flow between two micro-vessels, where one may experience a lack of blood flow, resulting in localized tissue hypoxia [
3]. Despite the presence of higher blood hemoglobin saturation, the second vessel exhibits a reduction in the gas exchange surface area [
4], a characteristic often observed in patients utilizing an extracorporeal membrane oxygenation (ECMO) device. These circumstances pose a potential threat to tissue perfusion in affected patients.
Class III (stagnant capillaries next to flowing capillaries): This is noted in conditions such as sepsis, reperfusion injury, sickle cell crisis, and malaria. Regions with stagnant capillaries are likely to be hypoxic due to insufficient circulation.
Class IV (hyperdynamic and stagnant capillaries): This is evident in resuscitated hyperdynamic septic patients. The presence of stagnant capillaries contributes to regional tissue hypoxia.
Class V (hyperdynamic flow in all vessels): This is identified in resuscitated sepsis and extreme exercise. It remains unclear whether this leads to hypoxia or signifies shunting from other organs.
In summary, Classes I, II, III, and IV are characterized by areas of stagnant capillary flow, rendering them incapable of delivering oxygen and resulting in regional tissue hypoxia. The impact of Class V is less distinct based on the provided information. It is noteworthy, however, that our research focuses specifically on the investigation of Class II in this paper. Monitoring these microcirculatory changes could prove valuable in guiding more targeted shock resuscitation.
ECMO is a life-support system used in clinical settings. ECMO plays a pivotal role in saving lives during critical situations and typically involves establishing peripheral vascular access, which can be achieved through various routes such as the femoral artery, femoral vein, or internal jugular vein. ECMO primarily operates in two modes: veno-venous (VV-ECMO) and veno-arterial (VA-ECMO). In VV-ECMO mode, healthcare professionals withdraw venous blood, oxygenate it, and then return it to the patient’s venous system. This mode is appropriate for patients with normal heart function but impaired lung function, such as adults with acute respiratory distress syndrome (ARDS). VV-ECMO effectively provides respiratory support while minimizing the complications associated with arterial cannulation [
5,
6]. Conversely, in the VA-ECMO mode, venous blood is withdrawn from the patient and oxygenated through an oxygenator before being returned to the patient’s arterial system. This device provides life support for patients with heart and lung failure, sepsis, or cardiogenic shock using ECMO technology.
During the process of using the ECMO treatment, clinical physicians adjust parameters such as the temperature, blood pump speed, and oxygen concentration based on various physiological indicators such as body temperature, blood pressure, heart rate, blood oxygen saturation, coagulation time, mean arterial pressure, and cardiac output to assist patients in achieving optimal circulatory conditions. In recent years, ECMO-assisted therapy has significantly improved patient survival rates. However, as the duration of ECMO use increases, the risk of complications also rises. These complications may include hemolysis due to rapid adjustments in the blood pump speed and the formation of blood clots in the ECMO circuit due to the increased contact between blood and the gas exchange membrane, leading to the activation of coagulation factors and inflammatory mediators. This can increase the risk of peripheral tissue ischemia and the need for amputation. Therefore, patients need continuous administration of anticoagulants during ECMO use to prevent clot formation [
6,
7]. The administration of anticoagulants during ECMO placement may reduce the platelet count and increase the bleeding risk, particularly in surgical sites, the digestive tract, and intracranial regions [
8]. Despite the limitations and possible complications of using physiological indicators as monitoring standards in clinical practice, these parameters still have limitations, and complications cannot be entirely avoided. For instance, neurological problems, stroke, renal replacement therapy, and infections occur at relatively high rates [
9,
10]. The occurrence of complications increases the mortality rate. Therefore, an increasing number of research teams are dedicated to studying how to prevent complications and exploring various factors contributing to complications and mortality [
11,
12,
13] to enhance the survival rate of ECMO patients. In the past, many research teams have developed scoring systems like SAVE, PRESET, and ENCOURAGE, which utilize logistic regression to predict the survival and mortality rates of ECMO patients based on various clinical physiological parameters, enabling clinical physicians to have better access to assess the patient’s condition [
14,
15,
16]. Currently, when patients are undergoing ECMO, clinical physicians can only adjust the ECMO mode based on fundamental physiological signs and the relevant dosage of medications. Nevertheless, using this method to detect changes in the hemodynamics of peripheral tissues in patients is relatively sensitive.
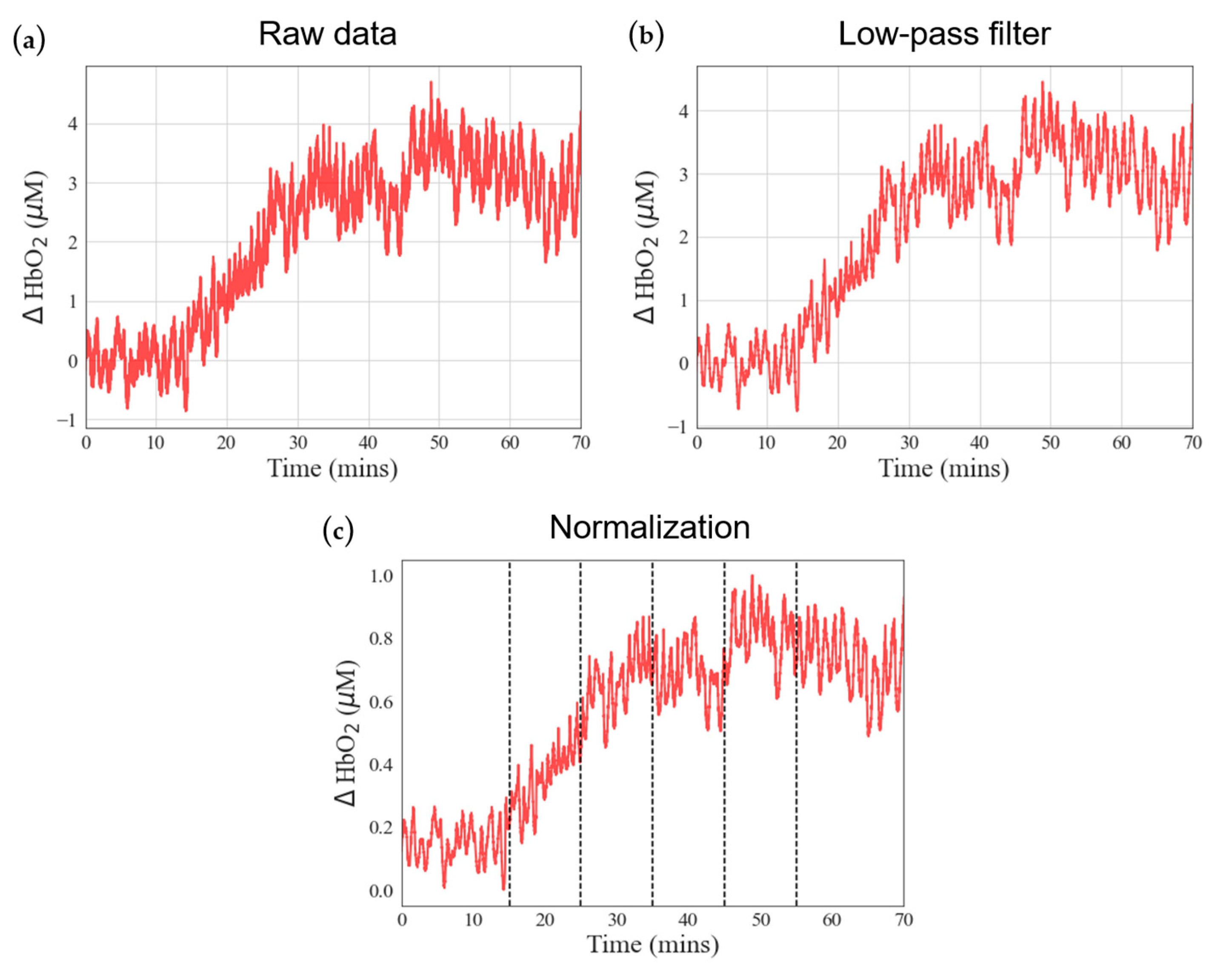
Near-infrared spectroscopy (NIRS) is a non-radiating, non-invasive, and real-time monitoring technique. The objective of this study is to utilize NIRS to monitor blood perfusion in the lower limb of ECMO patients. The goal is to establish the correlation between the optical observation of blood oxygen dynamics during ECMO pump speed adjustments and the corresponding physiological indicators. Researchers have recently applied this technology to identify postoperative complications arising from insufficient local blood flow. In 2012, Wong et al. first used NIRS devices to measure cerebral and limb blood perfusion in ECMO patients [
17]. They integrated this technology into treatment protocols, indicating that immediate interventions are necessary when the regional oxygen saturation (rSO
2) drops below 40% of the baseline or rises above 25% [
18,
19]. Lamb et al. confirmed that continuous monitoring using NIRS can detect ischemia in the limbs of ECMO patients [
20]. In addition to the interest in complication detection, research on the application of NIRS for monitoring systemic circulation has been growing. In 2020, our laboratory conducted a study utilizing NIRS technology to examine the adjustment of the blood pump speed in ECMO patients as part of clinical physiological parameter monitoring. During the research, we also observed perfusion in peripheral tissues. Our preliminary findings have been published in the
Journal of Biophotonics [
21], highlighting the potential of NIRS in providing real-time insights into patients’ physiological responses during ECMO treatment.
At Taipei Veterans General Hospital, where the experiments were conducted, there are an average of 200–250 ECMO patients annually. Drawing from our long-term experience in caring for a large number of ECMO patients, we have observed that the natural physiological response of the human body is to prioritize blood circulation to central organs in cases of severe injury or shock (with the exception of the kidneys, which need to retain sufficient fluid in the body). Peripheral tissue circulation is often reduced or sacrificed first, and the reduction in blood perfusion is most evident in the distal regions, particularly the feet. In ECMO patients, good blood circulation in the distal legs suggests a higher chance of survival, whereas poor circulation is associated with a higher likelihood of mortality. Due to challenges in measuring SPO2 or NIRS changes in the foot and toe regions, such as insufficient muscle mass, low temperature, and intense microvascular constriction, the calf was chosen as the measurement site for NIRS in our serial studies.
Beyond the technical applications, we believe that this study holds significant implications for enhancing medical procedures in clinical practice. Through the implementation of non-invasive optical technology, we can observe real-time changes in patients’ blood oxygen levels, gaining further insights into the severity of the disease.
For patients in the high-severity group, our study provides a valuable indicator for physicians when considering adjustments in ECMO support or medication dosages. Conversely, for patients with a lower disease severity, physicians may contemplate gradually reducing the ECMO rotation speed, allowing patients to rely more on their circulatory system, potentially leading to the complete cessation of ECMO treatment.
In essence, this research aims to equip clinical physicians with precise predictions of critical conditions in severe patients, thereby reducing the risk of complications and minimizing the use of healthcare resources. The technical advancements in NIRS not only contribute to our understanding of ECMO dynamics but also offer tangible benefits in improving patient outcomes and optimizing clinical decision making.
4. Discussions
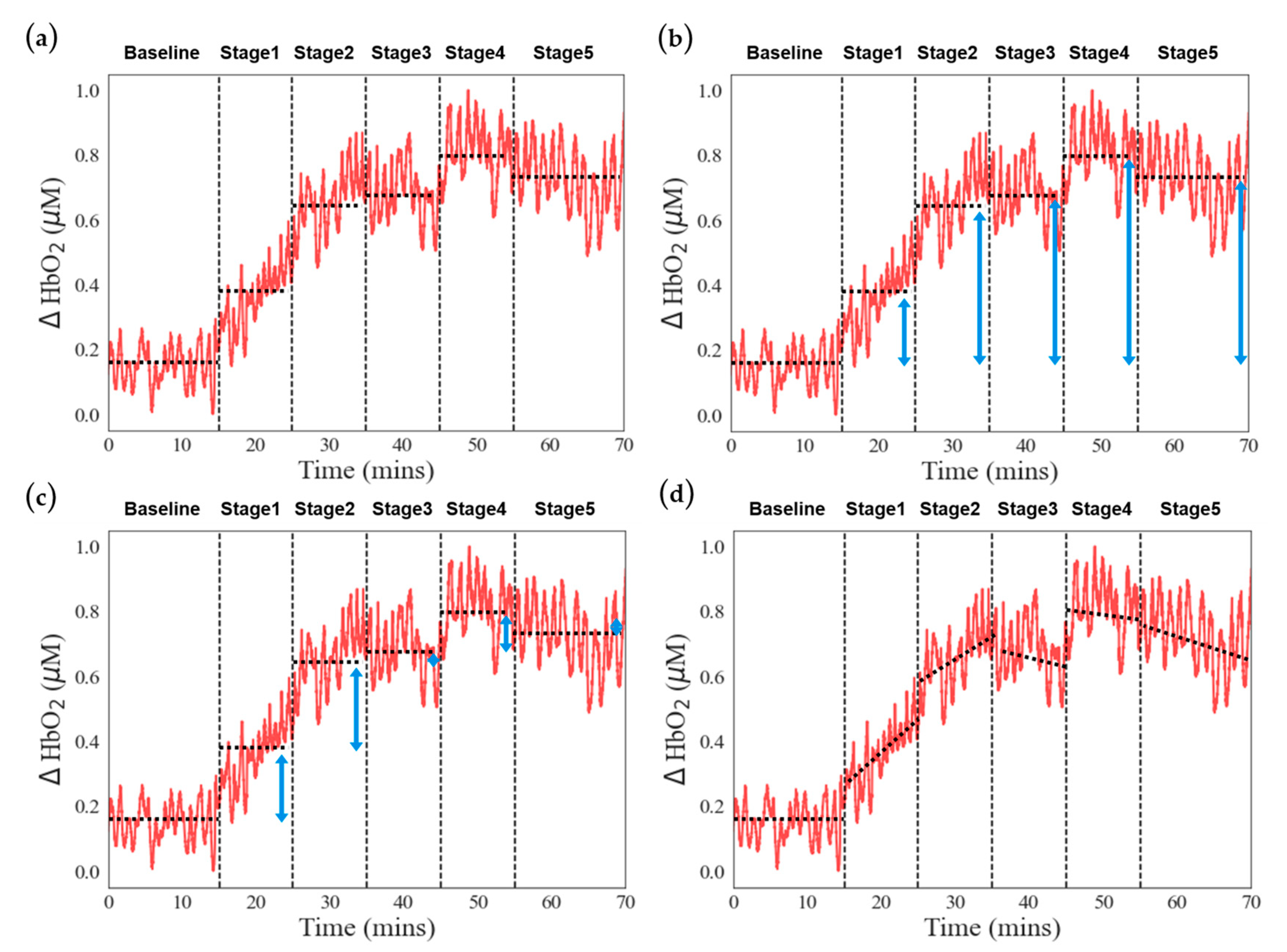
This study aims to differentiate between low- and high-scoring groups in the VV-ECMO and VA-ECMO populations by combining near-infrared spectroscopy (NIRS) with the APACHE II severity-of-disease scoring system. In the classification results for the VV-ECMO population, we constructed an SVM model using NIRS features that exhibited significant differences based on statistical tests. The top-performing classification model consisted of two feature values, each associated with stages where rotational speed adjustments were minimal.
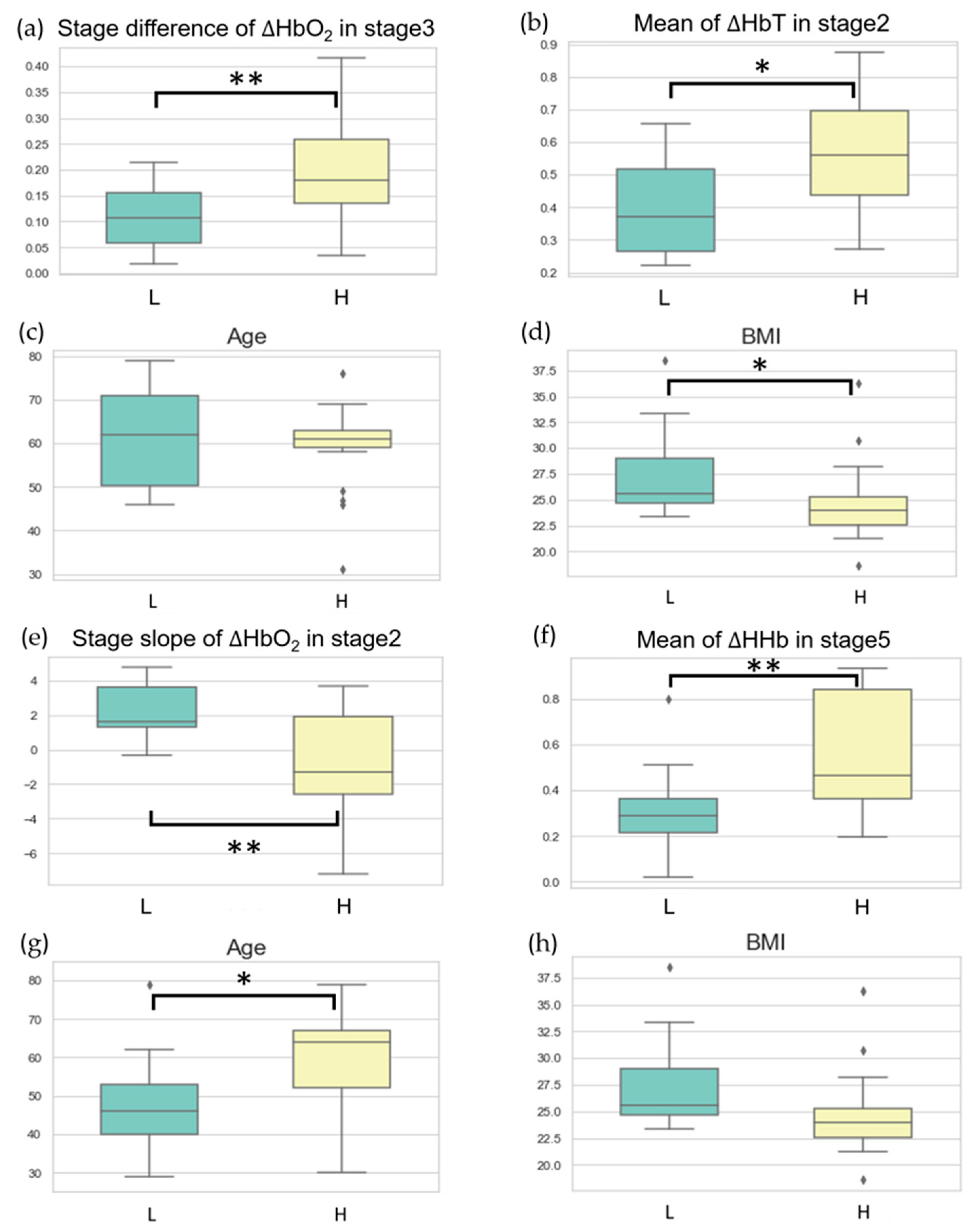
Figure 5a,b illustrate the data distribution of these two features in the high-scoring and low-scoring populations within the VV-ECMO group. On average, in the high-scoring group, there was a significant increase in the average change in oxygenated hemoglobin concentration during Task Stage 3 when the pump speed was increased by one unit compared to the average change in oxygenated hemoglobin concentration during Task Stage 2. This observation suggests that the high-severity group requires higher pump speeds to achieve better oxygenation at Stage 3.
Additionally, in the high-scoring group, the average increase in total hemoglobin concentration during Task Stage 2 exceeded that in the low-scoring group. This finding implies that the high-severity group has relatively poorer lung circulation. When the pump speed was increased by one unit from the minimum level at Stage 1, peripheral blood vessels exhibited a more pronounced expansion.
Incorporating clinical parameters into the VV-ECMO population can enhance the model’s sensitivity, especially for accurately diagnosing highly severe patients.
Figure 5c,d depict the distribution of age and BMI data in the high-score and low-score groups within the VV-ECMO cohort. There were no significant differences in age between these two groups. While the APACHE II scale does not explicitly include BMI as an indicator, BMI, nevertheless, reflects APACHE II scores. We observed that patients in the low-score group had higher overall BMI. Recent studies have indicated that a patient’s BMI can influence ICU survival rates. Research findings have suggested that underweight patients have a higher risk of in-hospital mortality, whereas overweight patients have a lower mortality risk [
30]. Patients with a slightly higher BMI tend to store more calories, making them more resilient when faced with severe illnesses and providing them with more energy to combat diseases, resulting in higher survival rates. In the future, combining BMI indicators with the NIRS system may aid in assessing the ultimate outcomes of ECMO patients, whether they survive or succumb.
Regarding the classification outcomes in the VA-ECMO population, the better-performing classification models correlate with stages where the patient’s internal microcirculation is significantly affected by adjustments in the blood pump speed.
Figure 5e,f illustrates the data distribution of two classification features within the VA-ECMO cohort among the high-score and low-score groups. In the low-score group, there is a more pronounced trend in the change of oxygenated blood concentration during Task Stage 2 compared to the high-score group. This finding is because patients in the low-score group have relatively better circulatory function, resulting in a higher increase in oxygenated blood concentration when increasing the blood pump speed during Task Stage 2. Conversely, in the high-score group, when reducing the pump speed by two units during Task Stage 5, a substantial reduction in ECMO support occurs. During this time, peripheral tissues experience extreme hypoxia, leading to a higher change in hypoxic blood concentration during Task Stage 5 compared to the low-score group. We incorporate clinical parameters into the VA-ECMO population with the expectation of achieving improved performance in training the model and enhancing its generalization capability.
Figure 5g,h depicts the distribution of age and BMI data among high-score and low-score groups within the VA-ECMO cohort. In contrast to the VV-ECMO population, BMI did not show significant differences between the high-score and low-score groups. However, in the low-score group, patients tended to be younger. This outcome finds its explanation in the classification labels’ foundation on APACHE II scores, incorporating patient age as one of the scoring criteria. Therefore, older patients may receive higher APACHE II scores, which is also why patients in the low-score group generally have a younger age.
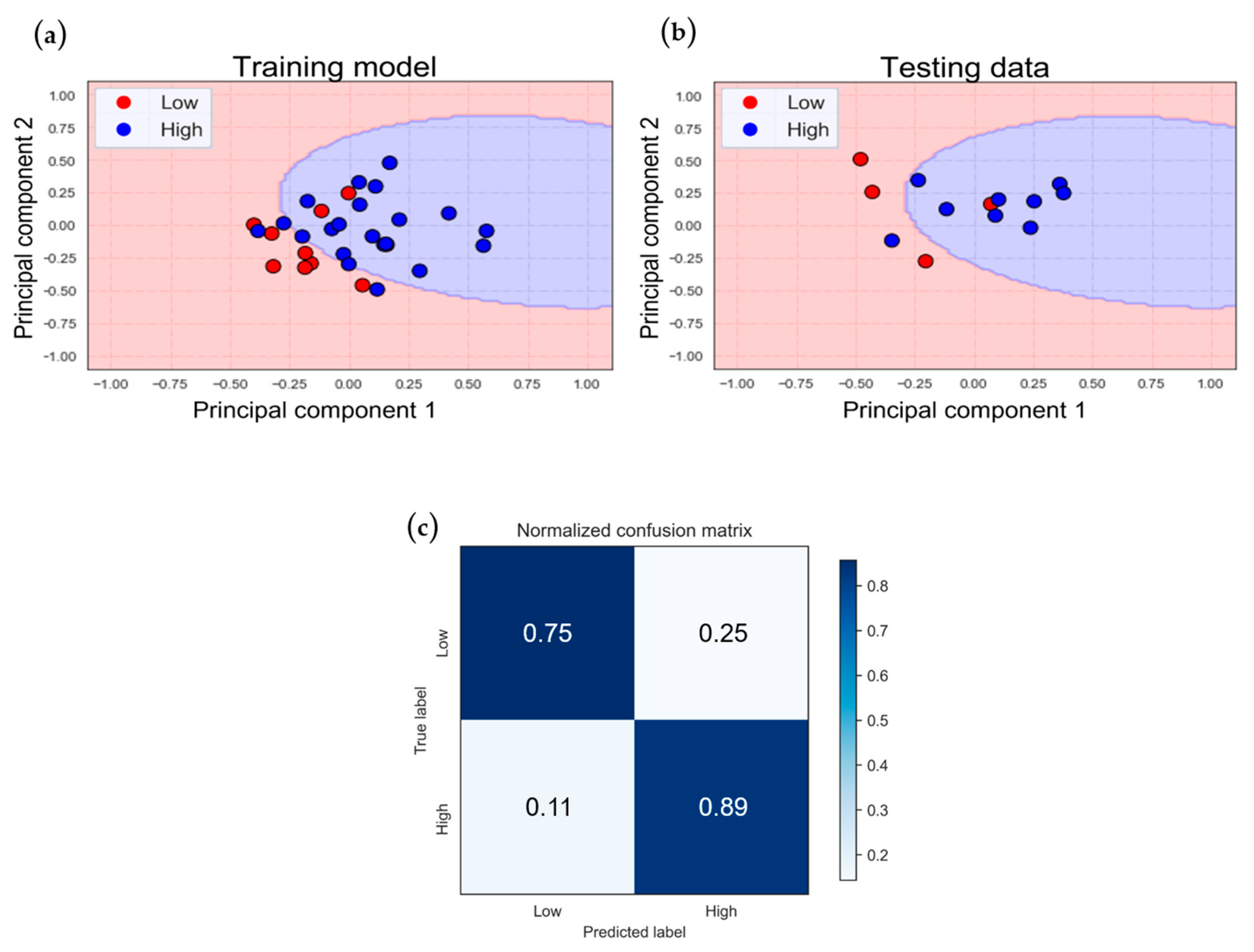
From
Figure 6 to
Figure 9, we can observe certain phenomena. In terms of model accuracy, whether using NIRS oxygenation features for classification or optimizing the model by incorporating clinical parameters, the classification model for the VA-ECMO population outperforms that of the VV-ECMO population. Overall, the VA-ECMO population’s model demonstrates superior generalization. The two data categories exhibit a clear separation in the two-dimensional feature distribution plot, indicating that they do not overlap. Consequently, the decision boundary effectively distinguishes the majority of data points between these two categories. Both sensitivity and specificity highlight the VA-ECMO population’s model as having superior discriminative abilities compared to the VV-ECMO population’s model.
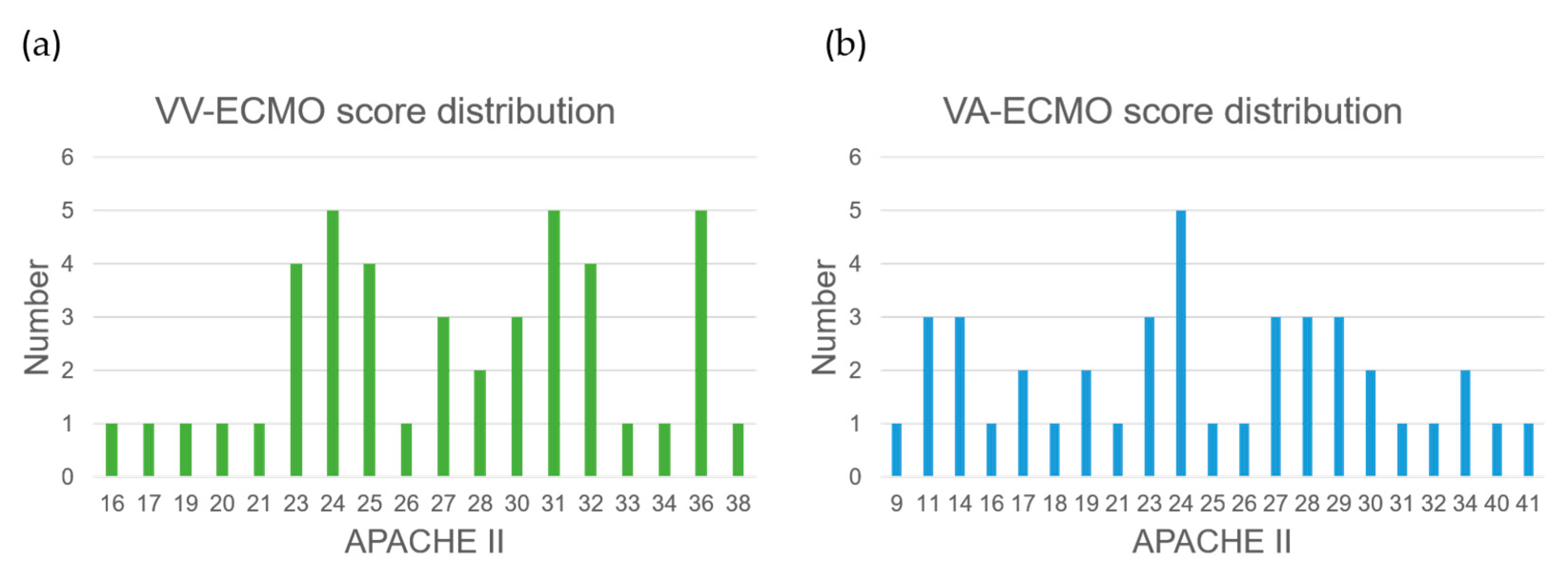
The VV-ECMO population’s model exhibits relatively poorer performance due to several factors. Firstly, there was an imbalance in the number of samples between the two VV-ECMO subgroups, leading to an overemphasis on the majority category during model training, resulting in a reduced performance for the minority category samples. Secondly, based on the statistics of APACHE II scores (
Figure 10a,b), a significant number of data points near the APACHE II score of 24 were observed within the VV-ECMO population. Within this range, the severity of illness is quite similar, and, as a result, any misclassification of data points in this interval significantly impacts the overall classification accuracy. In contrast, VA-ECMO is less affected by such scenarios.
In future research directions, we anticipate that, with an increase in patient sample size, traditional analytical methods such as multivariate models incorporating classical biomarkers can be employed. These can be compared with established prognostic scores like APACHE II and SOFA to provide alternative predictive techniques beyond traditional analysis. Due to the preliminary nature of this series of studies and the uncertainty surrounding the future value of big data, coupled with the limited number of patients, this article does not present statistical analyses for the small patient subset.
This near-infrared spectroscopy (NIRS) study demonstrates significant differences in patient prognostic scoring compared to traditional methods. In addition to offering alternative models for predicting patient outcomes, it also aids ICU physicians in timely adjustments of ECMO settings, such as early adjustments of ECMO support when patients show improvement, thereby enhancing the quality of ECMO care.
Despite the early literature on ECMO mentioning risk factors for predicting patient outcomes, the predictive capability for individual patients remains insufficient. Based on the analysis results presented in this paper, we hypothesize that NIRS may have the potential to surpass previous multivariate risk factor analysis models in terms of the predictive capability for individual patients.