Automated Segmentation and Measurements of Pulmonary Cysts in Lymphangioleiomyomatosis across Multiple CT Scanner Platforms over a Period of Two Decades
Abstract
:1. Introduction
1.1. Variability of the Scanner Platform and Imaging Conditions in Longitudinal Studies
1.2. Variability of the Radiodensity of Air Inside and Outside the Chest
1.3. Variability of the Radiodensity of the Pulmonary Parenchyma
2. Materials and Methods
2.1. Study Population
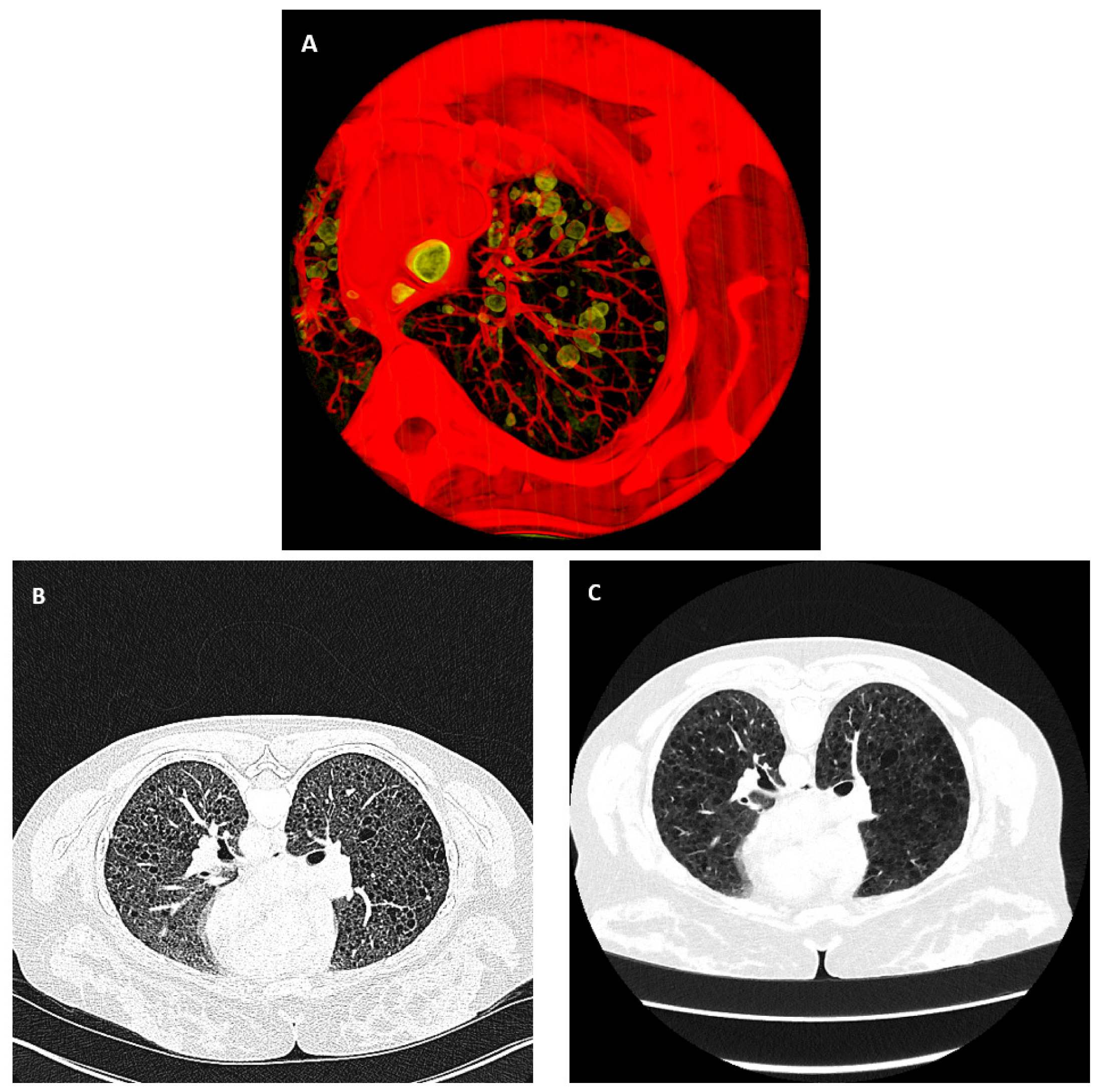
2.2. Metrics for Assessing the Quality of the Cyst Segmentation
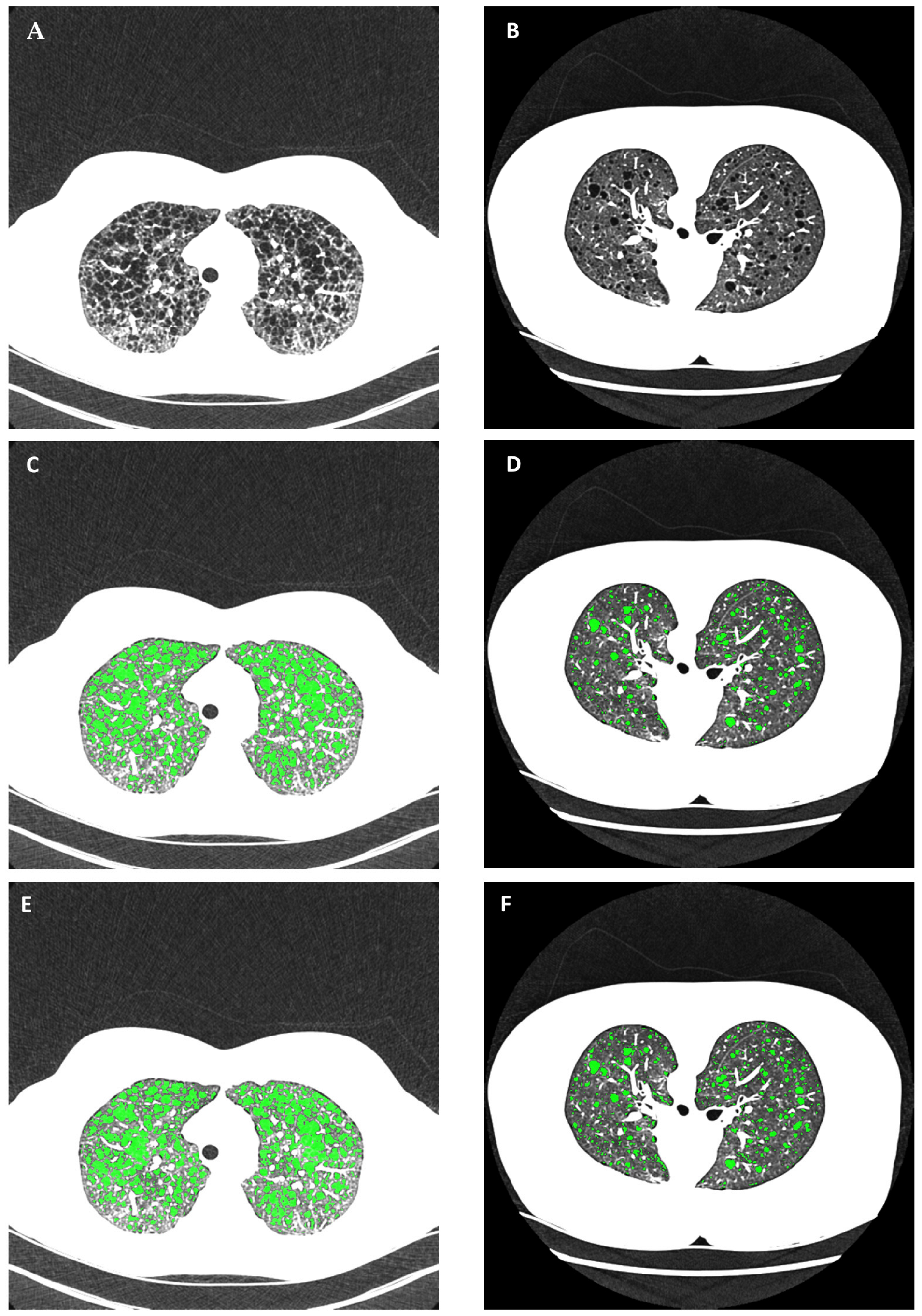
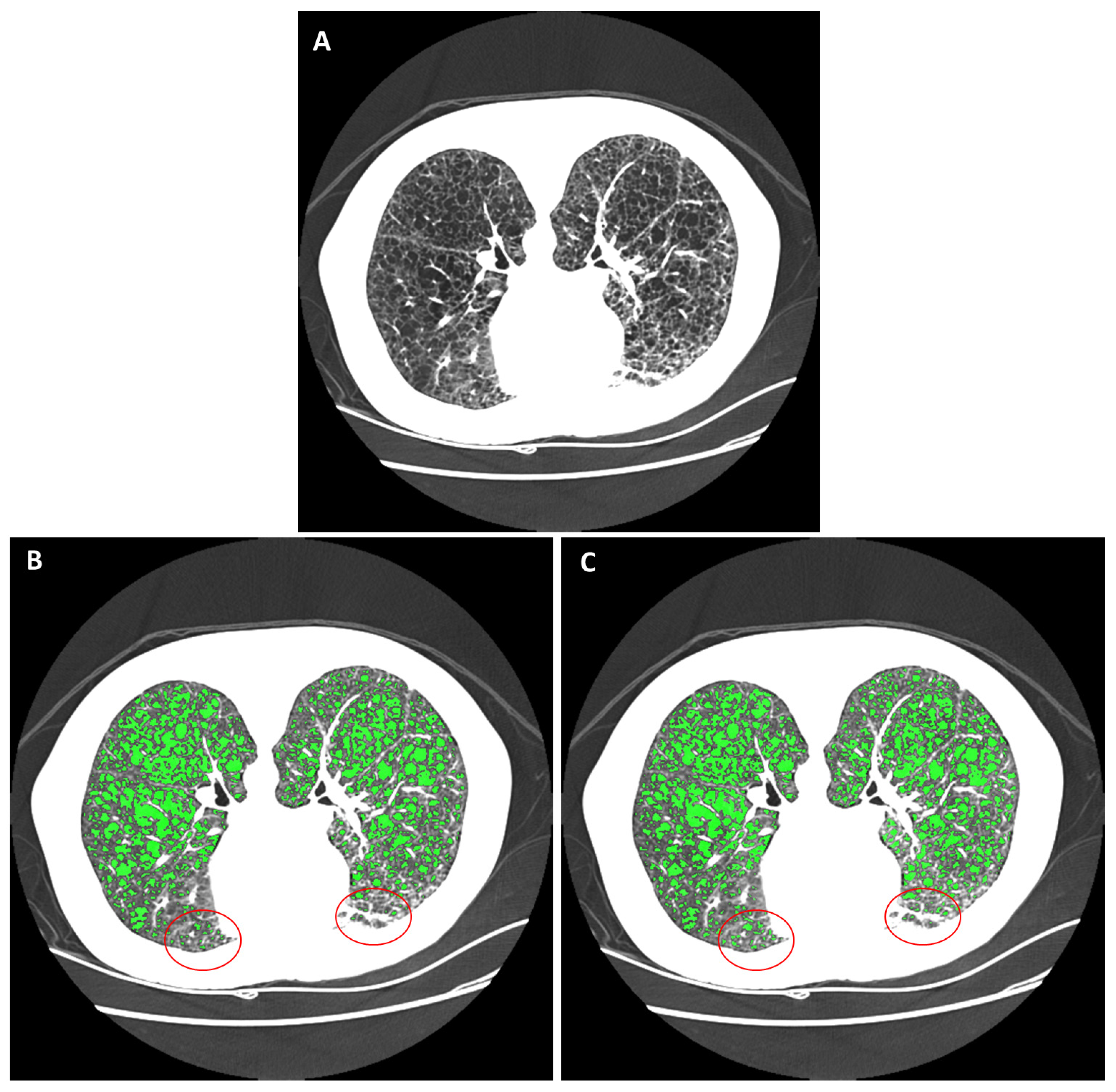
2.3. Reference Gold-Standard Semi-Automated Cyst Segmentation
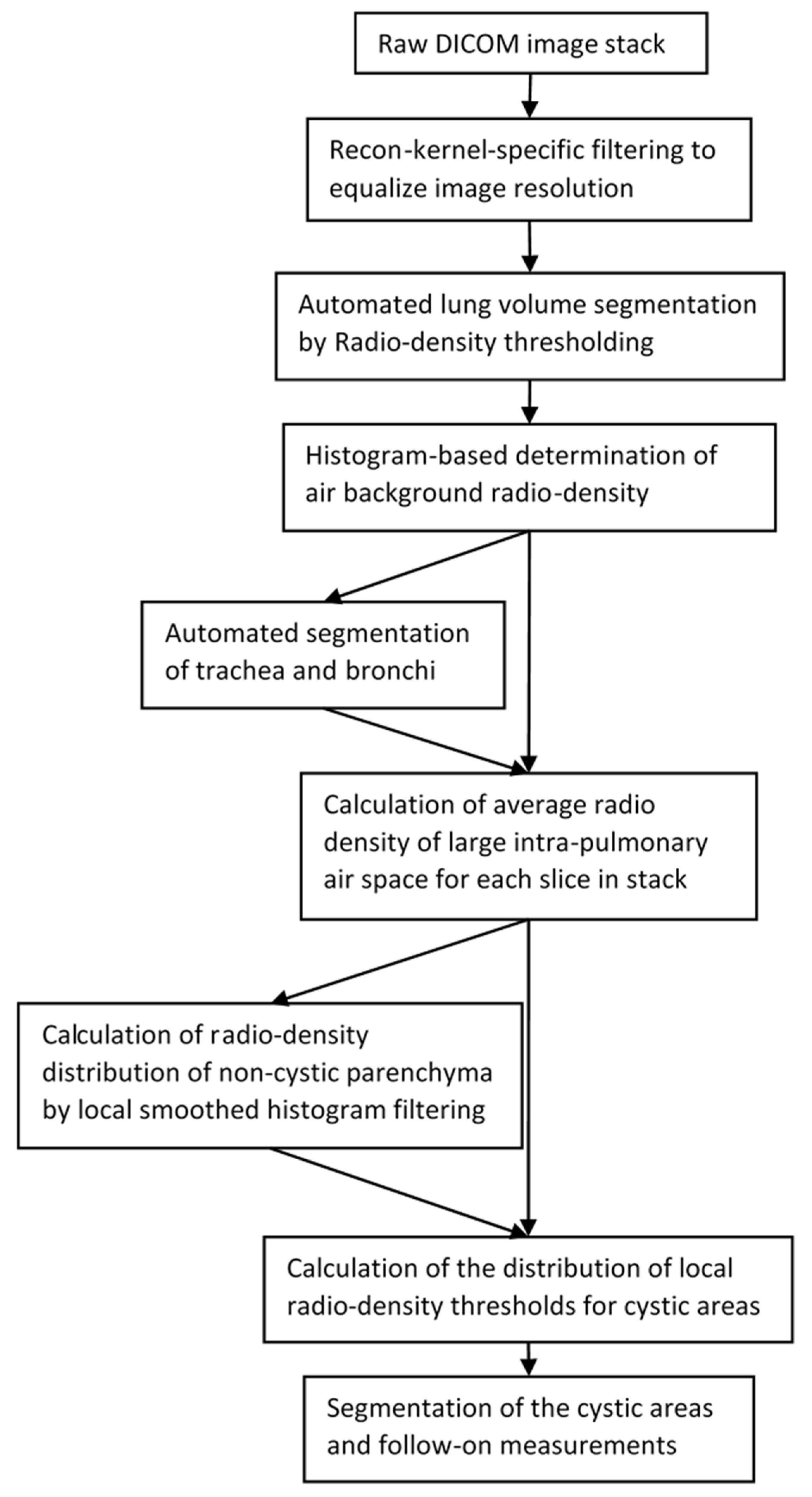
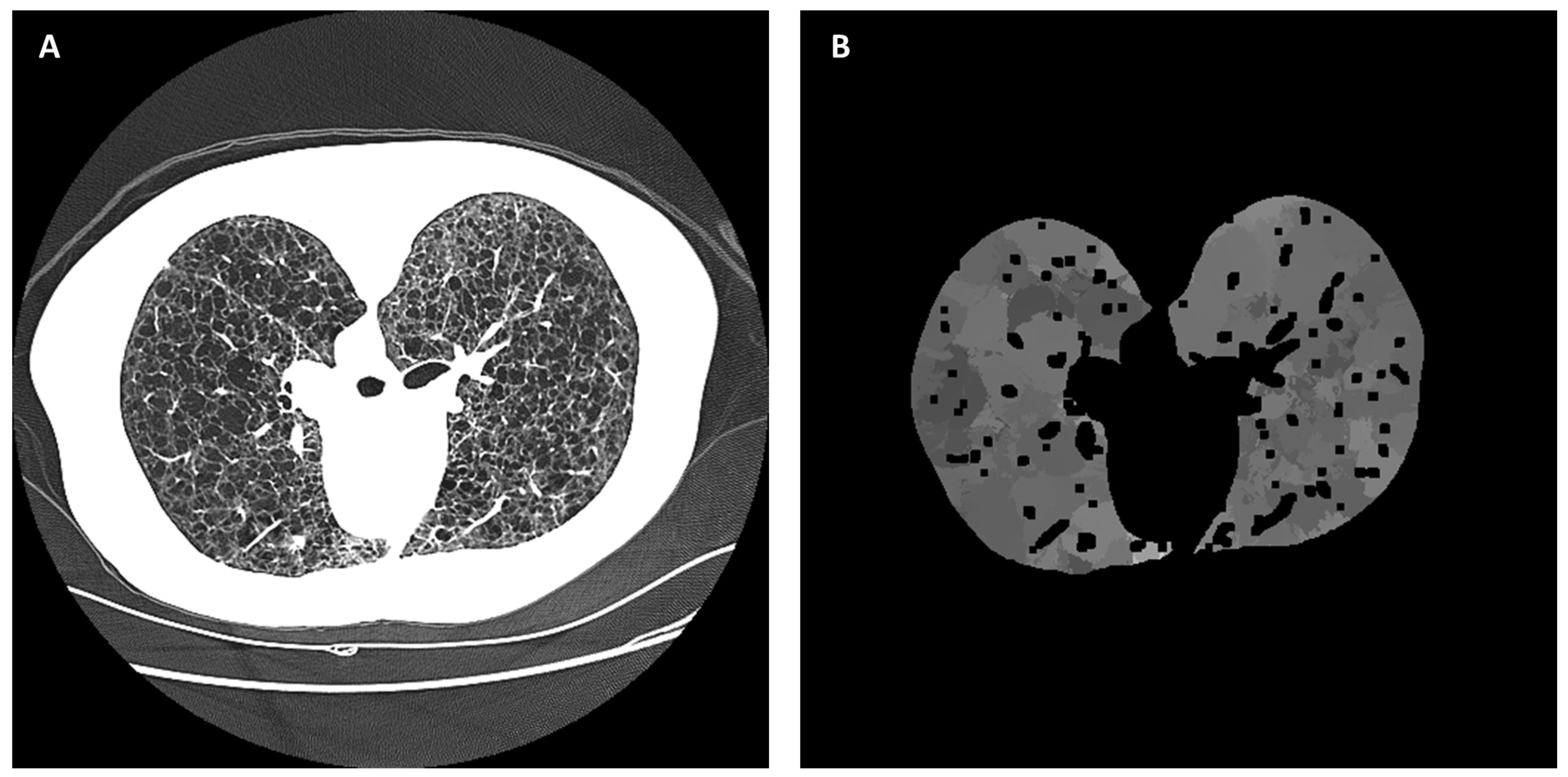
2.4. Automated Cyst Segmentation
3. Results
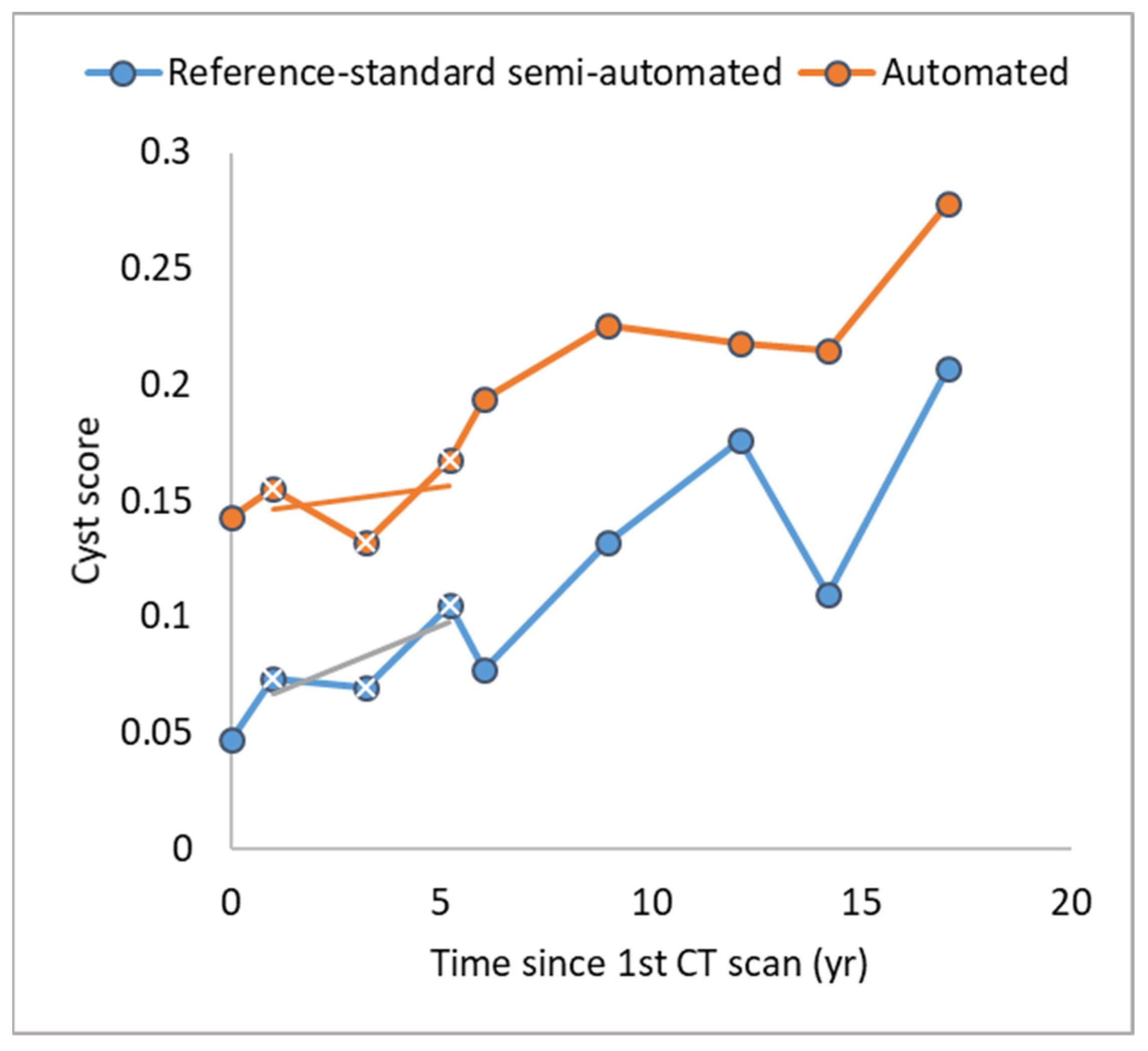
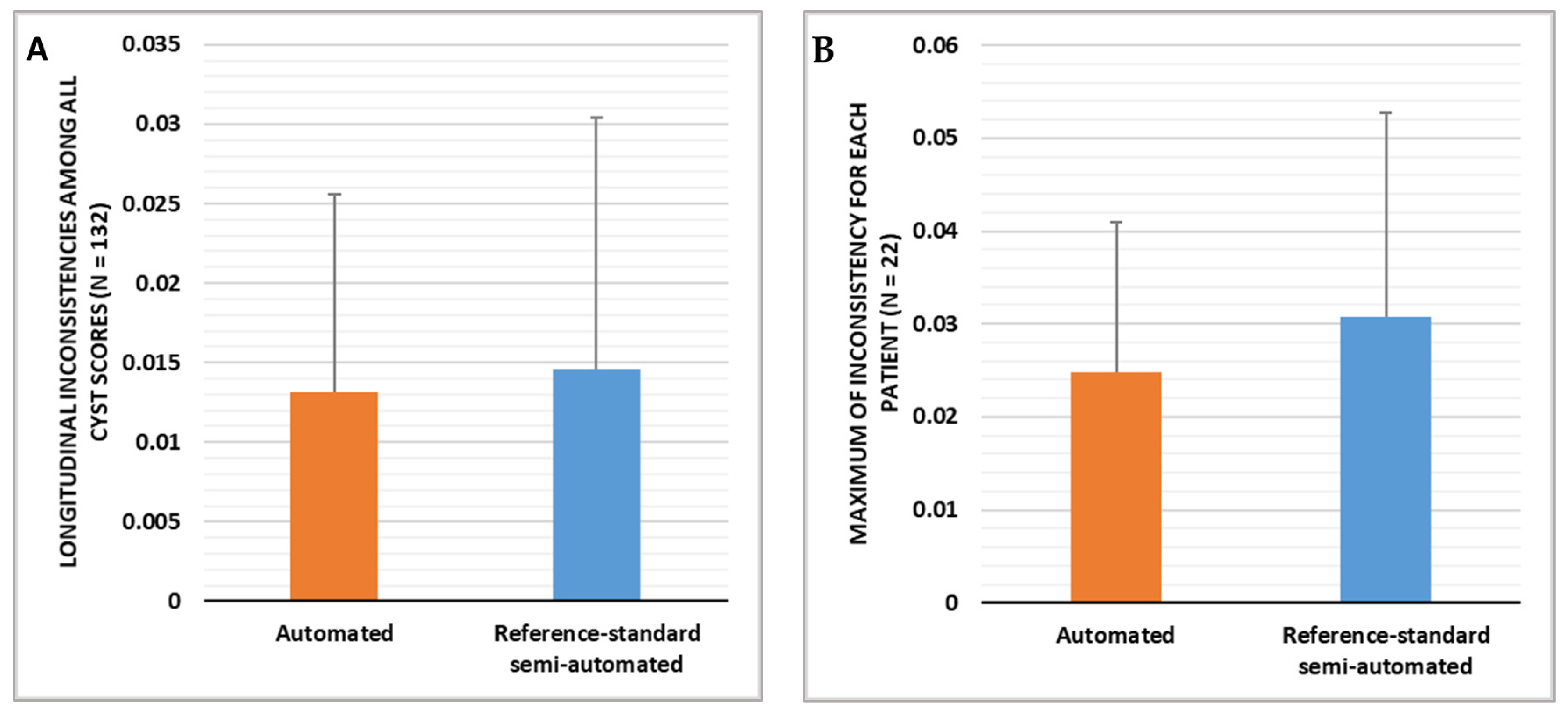
3.1. Comparison of the Longitudinal Consistency of Cyst Scores over Time
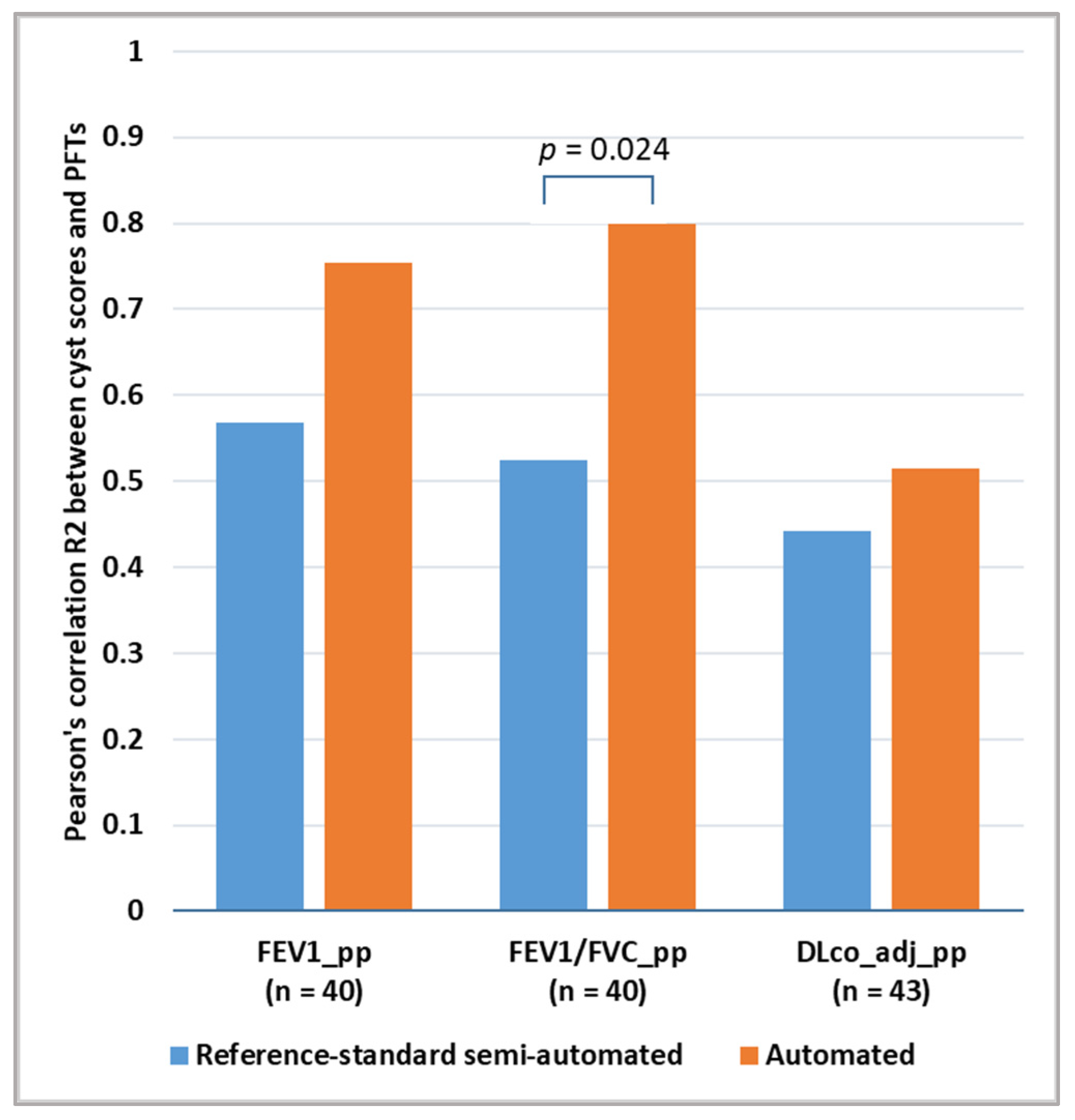
3.2. Comparison of the Correlation between Cyst Scores and Pulmonary Function Test Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hohman, D.W.; Noghrehkar, D.; Ratnayake, S. Lymphangioleiomyomatosis: A Review. Eur. J. Intern. Med. 2008, 19, 319–324. [Google Scholar] [CrossRef]
- Gupta, N.; Vassallo, R.; Wikenheiser-Brokamp, K.A.; McCormack, F.X. Diffuse Cystic Lung Disease. Part I Am. J. Respir. Crit. Care Med. 2015, 191, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Tobino, K.; Johkoh, T.; Fujimoto, K.; Sakai, F.; Arakawa, H.; Kurihara, M.; Kumasaka, T.; Koike, K.; Takahashi, K.; Seyama, K. Computed Tomographic Features of Lymphangioleiomyomatosis: Evaluation in 138 Patients. Eur. J. Radiol. 2015, 84, 534–541. [Google Scholar] [CrossRef]
- Moir, L.M. Lymphangioleiomyomatosis: Current Understanding and Potential Treatments. Pharmacol. Ther. 2016, 158, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Seibert, D.; Hong, C.-H.; Takeuchi, F.; Olsen, C.; Hathaway, O.; Moss, J.; Darling, T.N. Recognition of Tuberous Sclerosis in Adult Women: Delayed Presentation with Life-Threatening Consequences. Ann. Intern. Med. 2011, 154, 806. [Google Scholar] [CrossRef] [PubMed]
- Taveira-DaSilva, A.M.; Moss, J. Clinical Features, Epidemiology, and Therapy of Lymphangioleiomyomatosis. Clin. Epidemiol. 2015, 7, 249–257. [Google Scholar] [CrossRef]
- Xu, K.-F.; Lo, B.H. Lymphangioleiomyomatosis: Differential Diagnosis and Optimal Management. Ther. Clin. Risk Manag. 2014, 10, 691–700. [Google Scholar] [CrossRef]
- Cohen, M.M.; Pollock-BarZiv, S.; Johnson, S.R. Emerging Clinical Picture of Lymphangioleiomyomatosis. Thorax 2005, 60, 875–879. [Google Scholar] [CrossRef]
- Oprescu, N.; McCormack, F.X.; Byrnes, S.; Kinder, B.W. Clinical Predictors of Mortality and Cause of Death in Lymphangioleiomyomatosis: A Population-Based Registry. Lung 2013, 191, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Crausman, R.S.; Lynch, D.A.; Mortenson, R.L.; King, T.E.; Irvin, C.G.; Hale, V.A.E.; Newell, J.D. Quantitative CT Predicts the Severity of Physiologic Dysfunction in Patients with Lymphangioleiomyomatosis. Chest 1996, 109, 131–137. [Google Scholar] [CrossRef]
- Taveira-DaSilva, A.M.; Stylianou, M.P.; Hedin, C.J.; Hathaway, O.; Moss, J. Decline in Lung Function in Patients with Lymphangioleiomyomatosis Treated with or without Progesterone. Chest 2004, 126, 1867–1874. [Google Scholar] [CrossRef]
- Johnson, S.R.; Taveira-DaSilva, A.M.; Moss, J. Lymphangioleiomyomatosis. Clin. Chest Med. 2016, 37, 389–403. [Google Scholar] [CrossRef]
- Tobino, K.; Hirai, T.; Johkoh, T.; Fujimoto, K.; Kawaguchi, A.; Tomiyama, N.; Takahashi, K.; Seyama, K. Difference of the Progression of Pulmonary Cysts Assessed by Computed Tomography among COPD, Lymphangioleiomyomatosis, and Birt-Hogg-Dubé Syndrome. PLoS ONE 2017, 12, e0188771. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Yao, J.; Steagall, W.K.; Avila, N.A.; Taveira-DaSilva, A.M.; Stylianou, M.; Chen, M.Y.; Moss, J. Use of CT Imaging to Quantify Progression and Response to Treatment in Lymphangioleiomyomatosis. Chest 2019, 155, 962–971. [Google Scholar] [CrossRef]
- Matthew, B.P.; Hasani, A.M.; Chen, Y.-C.; Pirooznia, M.; Stylianou, M.; Rollison, S.F.; Machado, T.R.; Quade, N.M.; Jones, A.M.; Julien-Williams, P.; et al. Ultra-Small Lung Cysts Impair Diffusion without Obstructing Air Flow in Lymphangioleiomyomatosis. Chest 2021, 160, 199–208. [Google Scholar] [CrossRef]
- Matthew, B.P.; Lebron, A.; Chen, Y.-C.; Lohr, W.H.; Rollison, S.F.; Worthy, T.A.; Jones, A.M.; Julien-Williams, P.; Pirooznia, M.; Chen, M.Y.; et al. Novel Pulmonary Cyst Characteristics Associated with the Diffusing Capacity of the Lung in Lymphangioleiomyomatosis: A Cross-Sectional Clinical Trial. Ann. Am. Thorac. Soc. 2023, 20, 1073–1076. [Google Scholar] [CrossRef]
- Argula, R.G.; Kokosi, M.; Lo, P.; Kim, H.J.; Ravenel, J.G.; Meyer, C.; Goldin, J.; Lee, H.-S.; Strange, C.; McCormack, F.X. A Novel Quantitative Computed Tomographic Analysis Suggests How Sirolimus Stabilizes Progressive Air Trapping in Lymphangioleiomyomatosis. Ann. Am. Thorac. Soc. 2016, 13, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Schmithorst, V.J.; Altes, T.A.; Young, L.R.; Franz, D.N.; Bissler, J.J.; McCormack, F.X.; Dardzinski, B.J.; Brody, A.S. Automated Algorithm for Quantifying the Extent of Cystic Change on Volumetric Chest CT: Initial Results in Lymphangioleiomyomatosis. Am. J. Roentgenol. 2009, 192, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Hansell, D.M.; Brown, K.; Tashkin, D.P. Lymphangiomyomatosis–CT, Chest Radiographic, and Functional Correlations. Radiology 1990, 176, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Avila, N.A.; Kelly, J.A.; Dwyer, A.J.; Johnson, D.L.; Jones, E.C.; Moss, J. Lymphangioleiomyomatosis: Correlation of Qualitative and Quantitative Thin-Section CT with Pulmonary Function Tests and Assessment of Dependence on Pleurodesis. Radiology 2002, 223, 189–197. [Google Scholar] [CrossRef]
- Hu-Wang, E.; Schuzer, J.L.; Rollison, S.; Leifer, E.S.; Steveson, C.; Gopalakrishnan, V.; Yao, J.H.; Machado, T.; Jones, A.M.; Julien-Williams, P.; et al. Chest CT Scan at Radiation Dose of a Posteroanterior and Lateral Chest Radiograph Series A Proof of Principle in Lymphangioleiomyomatosis. Chest 2019, 155, 528–533. [Google Scholar] [CrossRef]
- Yao, J.; Taveira-DaSilva, A.M.; Colby, T.V.; Moss, J. CT Grading of Lung Disease in Lymphangioleiomyomatosis. Am. J. Roentgenol. 2012, 199, 787–793. [Google Scholar] [CrossRef]
- Hasani, A.M.; Matthew, B.; Goljamali, M.; Chen, Y.-C.; Stylianou, M.; Pirooznia, M.; Taveira-DaSilva, A.; Chen, M.Y.; Wen, H.; Moss, J. Effect of Non-Uniform Cyst Distribution in Lymphangioleiomyomatosis on Pulmonary Function: A Cross-Sectional Study. Eur. Respir. J. 2021, 57, 2003769. [Google Scholar] [CrossRef]
- Tanabe, N.; Muro, S.; Sato, S.; Oguma, T.; Sato, A.; Hirai, T. Fractal Analysis of Low Attenuation Clusters on Computed Tomography in Chronic Obstructive Pulmonary Disease. BMC Pulm. Med. 2018, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.; Solomon, J. Smoothed Local Histogram Filters. ACM Trans. Graph. 2010, 29, 1–10. [Google Scholar] [CrossRef]
- Wen, H.; Huapaya, J.A.; Kanth, S.M.; Sun, J.; Matthew, B.P.; Lee, S.C.; Do, M.; Chen, M.Y.; Malayeri, A.A.; Suffredini, A.F. Quantitative CT Metrics Associated with Variability in the Diffusion Capacity of the Lung of Post-COVID-19 Patients with Minimal Residual Lung Lesions. J. Imaging 2023, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Taveira-DaSilva, A.M.; Moss, J. Lymphangioleiomyomatosis. In Encyclopedia of Respiratory Medicine, 2nd ed.; Janes, S.M., Ed.; Academic Press: Oxford, UK, 2022; pp. 447–465. ISBN 978-0-08-102724-0. [Google Scholar]
- McCormack, F.X.; Gupta, N.; Finlay, G.R.; Young, L.R.; Taveira-DaSilva, A.M.; Glasgow, C.G.; Steagall, W.K.; Johnson, S.R.; Sahn, S.A.; Ryu, J.H.; et al. Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2016, 194, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Official ERS Technical Standards: Global Lung Function Initiative Reference Values for the Carbon Monoxide Transfer Factor for Caucasians|European Respiratory Society. Available online: https://erj.ersjournals.com/content/50/3/1700010 (accessed on 10 August 2022).
- Coxson, H.O.; Mayo, J.R.; Behzad, H.; Moore, B.J.; Verburgt, L.M.; Staples, C.A.; Pare, P.D.; Hogg, J.C. Measurement of Lung Expansion with Computed Tomography and Comparison with Quantitative Histology. J. Appl. Physiol. 1995, 79, 1525–1530. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lebron, A.; Matthew, B.; Moss, J.; Wen, H. Automated Segmentation and Measurements of Pulmonary Cysts in Lymphangioleiomyomatosis across Multiple CT Scanner Platforms over a Period of Two Decades. Bioengineering 2023, 10, 1255. https://doi.org/10.3390/bioengineering10111255
Lee S, Lebron A, Matthew B, Moss J, Wen H. Automated Segmentation and Measurements of Pulmonary Cysts in Lymphangioleiomyomatosis across Multiple CT Scanner Platforms over a Period of Two Decades. Bioengineering. 2023; 10(11):1255. https://doi.org/10.3390/bioengineering10111255
Chicago/Turabian StyleLee, Simone, Alfredo Lebron, Brianna Matthew, Joel Moss, and Han Wen. 2023. "Automated Segmentation and Measurements of Pulmonary Cysts in Lymphangioleiomyomatosis across Multiple CT Scanner Platforms over a Period of Two Decades" Bioengineering 10, no. 11: 1255. https://doi.org/10.3390/bioengineering10111255
APA StyleLee, S., Lebron, A., Matthew, B., Moss, J., & Wen, H. (2023). Automated Segmentation and Measurements of Pulmonary Cysts in Lymphangioleiomyomatosis across Multiple CT Scanner Platforms over a Period of Two Decades. Bioengineering, 10(11), 1255. https://doi.org/10.3390/bioengineering10111255






