The Development of a Permanent Implantable Spacer with the Function of Size Adjustability for Customized Treatment of Regurgitant Heart Valve Disease
Abstract
:1. Introduction
2. Materials and Methods
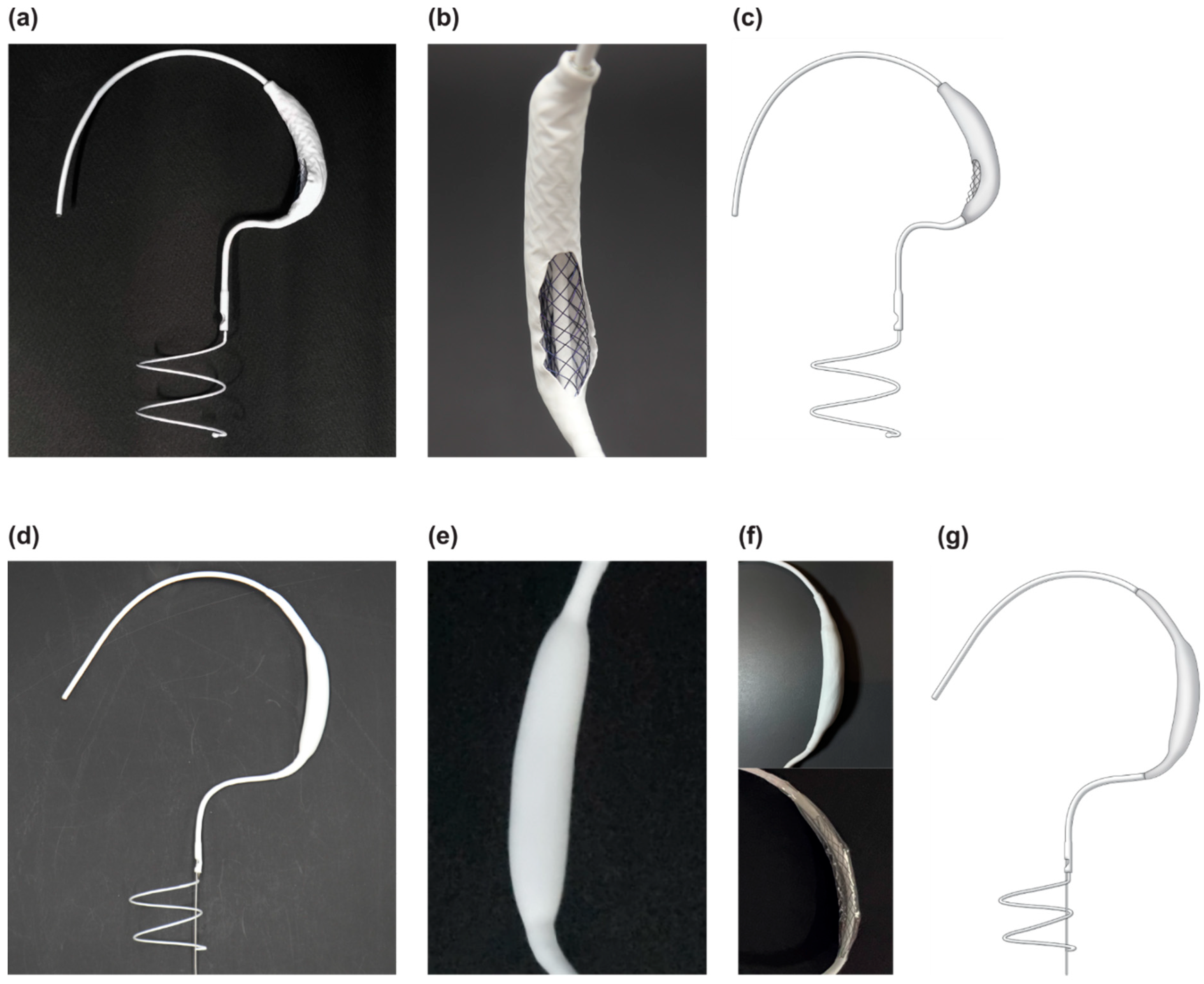
2.1. Innovative 3D Structure of the Pivot Mandu
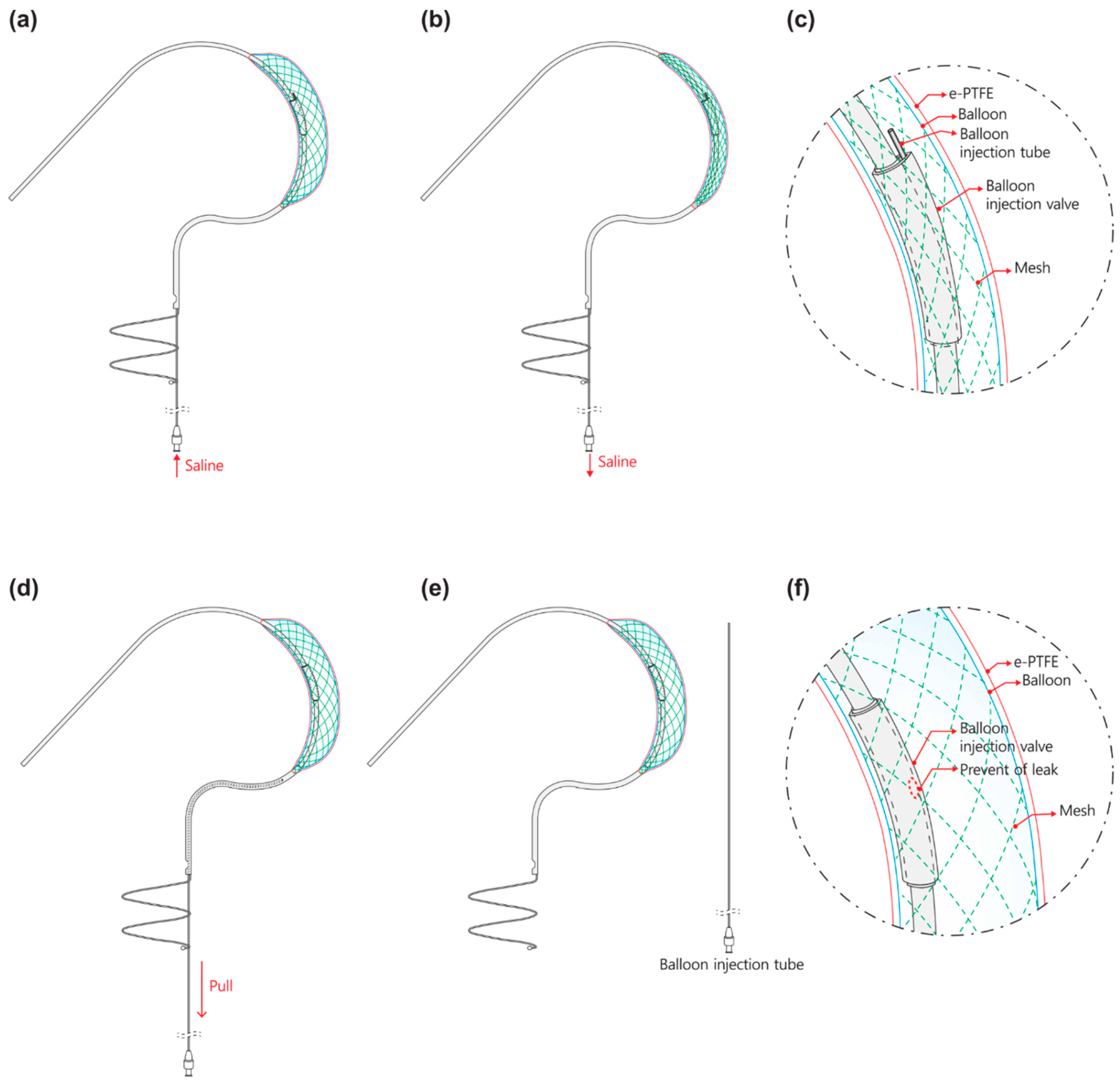
2.1.1. Leak Tight Adjustable 3D Balloon Spacer with Inner Mesh Support
2.1.2. Detachable Fluid Injection System
2.2. Animal
2.3. Functional Efficacy Evaluation
2.3.1. 3D Spacer Efficacy and 3D Balloon Spacer Adjust Bench Test
2.3.2. 3D Spacer Efficacy and 3D Balloon Spacer Patency in Pig Assessment
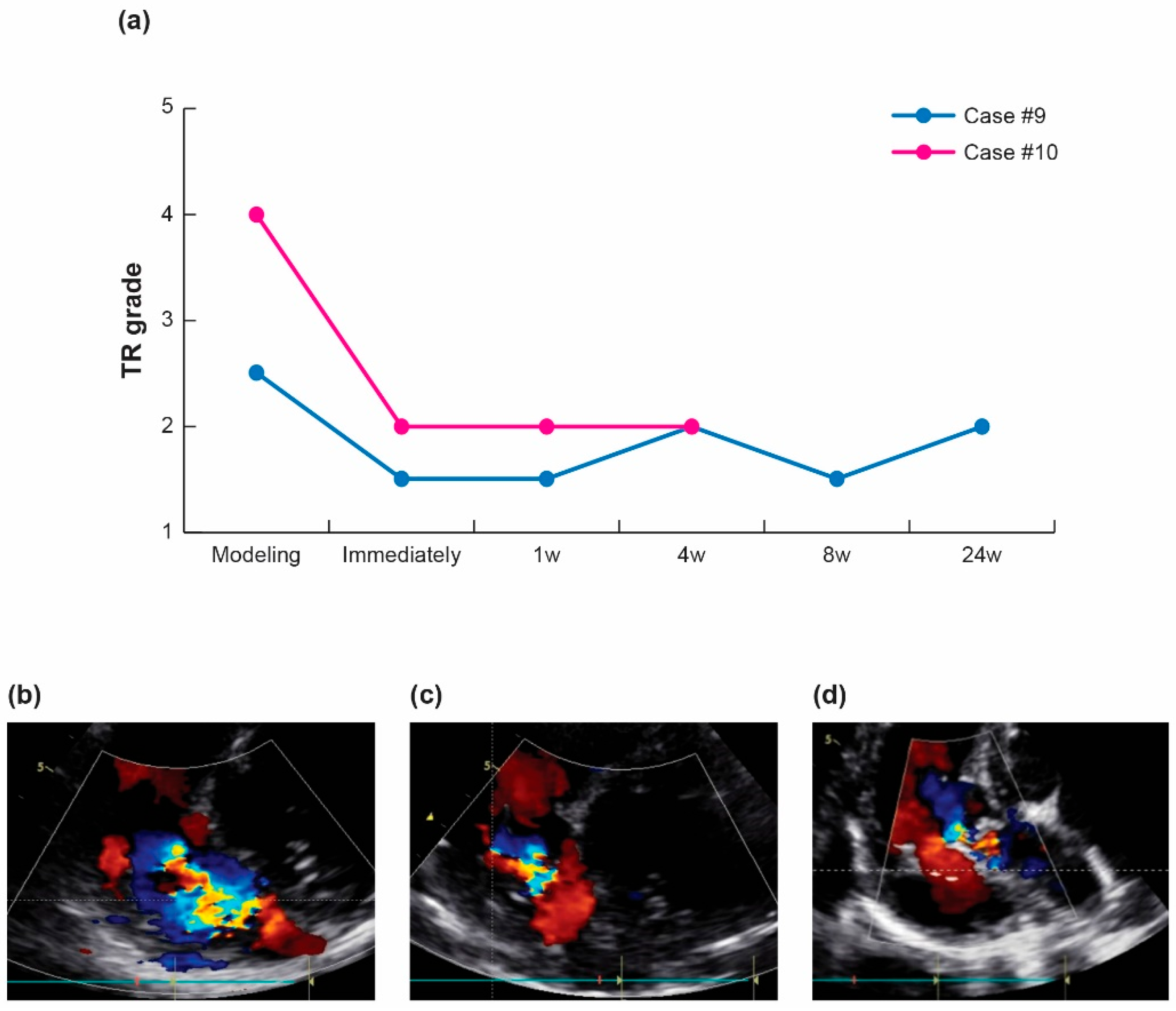
2.3.3. Therapeutic Efficacy and Regurgitation Treatment in Pig Tricuspid Valve
2.4. Long-Term Follow-Up of Animals
2.5. Gross and Pathological Evaluation
2.6. Statistical Analysis
3. Results
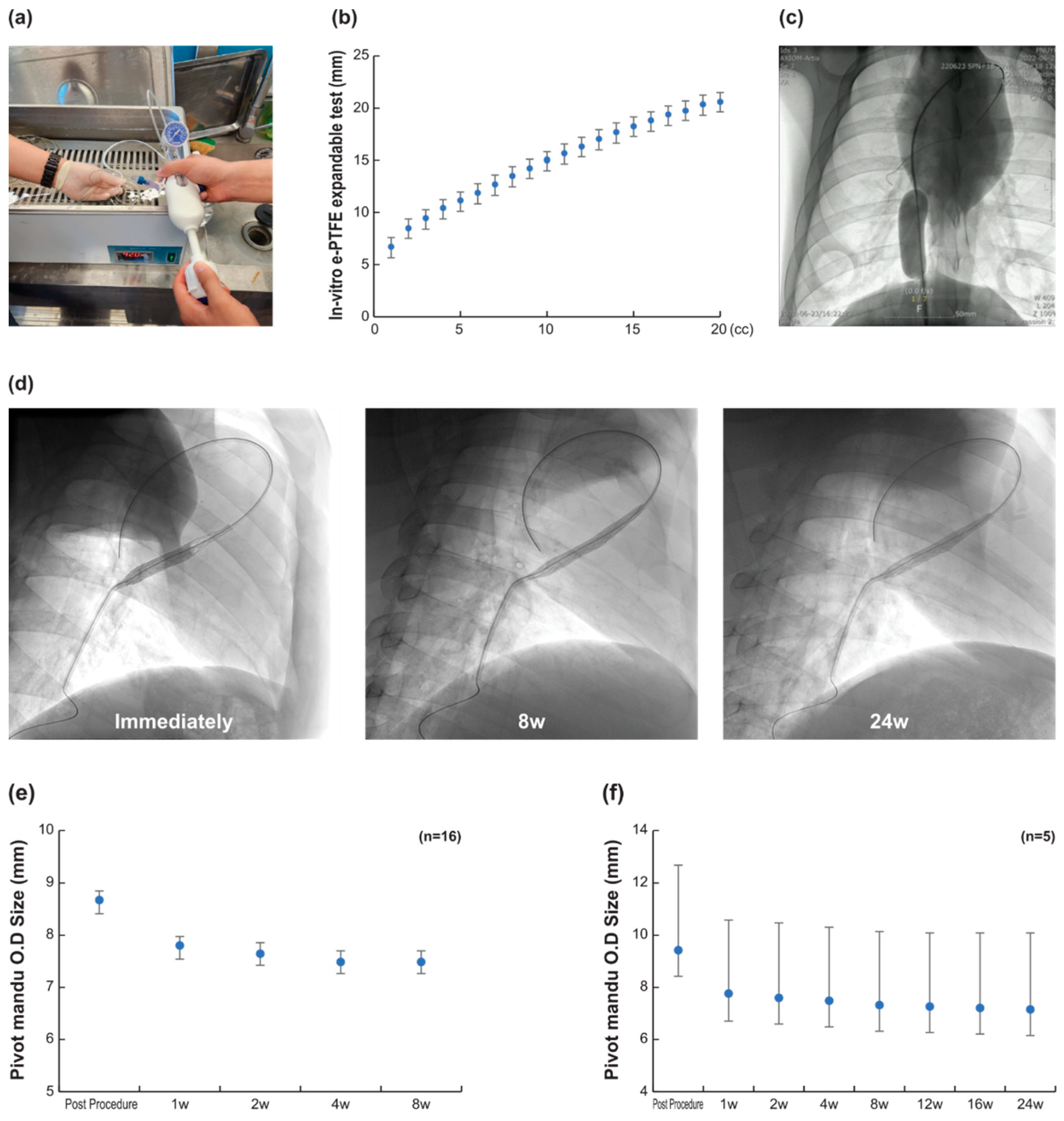
3.1. 3D Balloon Spacer Expansion Bench Test (n = 20)
3.2. In Vivo Efficacy Evaluation (n = 16)
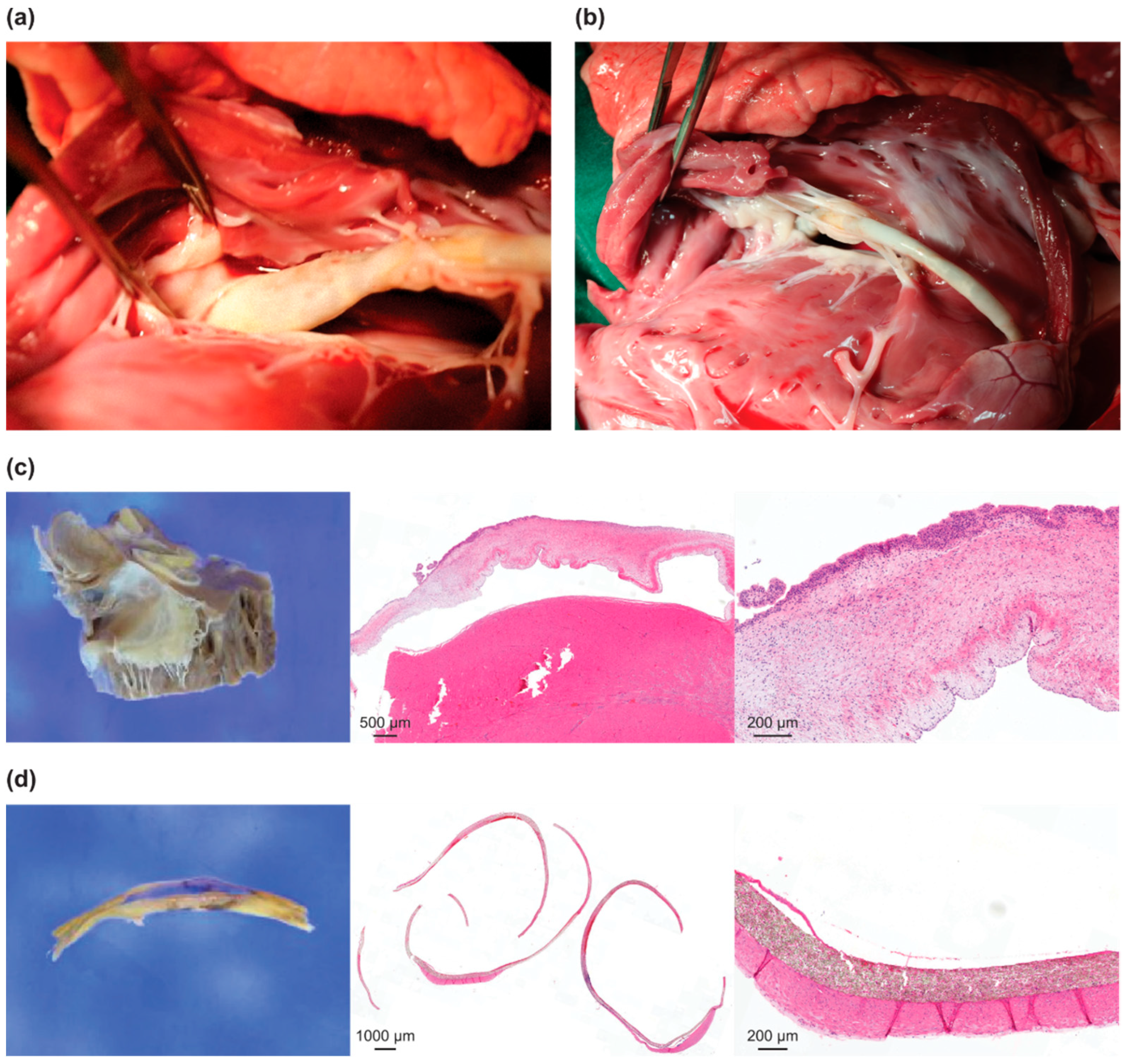
3.3. Safety Assessment and Histological Examination
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thourani, V.H.; Kodali, S.; Makkar, R.R.; Herrmann, H.C.; Williams, M.; Babaliaros, V.; Smalling, R.; Lim, S.; Malaisrie, S.C.; Kapadia, S. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: A propensity score analysis. Lancet 2016, 387, 2218–2225. [Google Scholar] [CrossRef]
- Kim, J.-H.; Sung, S.-C.; Chon, M.-K.; Kim, J.-O.; Lee, S.-H.; Lee, S.-Y.; Je, H.-G.; Choo, K.-S.; Hwang, J.-M.; Kim, J.-S. Mitral loop cerclage as a variant form of mitral cerclage annuloplasty that adds a device (CSTV) for preventing potential complications: A preclinical proof of concept and feasibility study. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2016, 11, e1669–e1679. [Google Scholar] [CrossRef] [PubMed]
- Asmarats, L.; Puri, R.; Latib, A.; Navia, J.L.; Rodés-Cabau, J. Transcatheter tricuspid valve interventions: Landscape, challenges, and future directions. J. Am. Coll. Cardiol. 2018, 71, 2935–2956. [Google Scholar] [CrossRef] [PubMed]
- Asmarats, L.; Perlman, G.; Praz, F.; Hensey, M.; Chrissoheris, M.P.; Philippon, F.; Ofek, H.; Ye, J.; Puri, R.; Pibarot, P. Long-term outcomes of the FORMA transcatheter tricuspid valve repair system for the treatment of severe tricuspid regurgitation: Insights from the first-in-human experience. JACC Cardiovasc. Interv. 2019, 12, 1438–1447. [Google Scholar] [CrossRef]
- Peppas, A.; Furer, A.; Wilson, J.; Yi, G.; Cheng, Y.; Van Wygerden, K.; Seguin, C.; Shibuya, M.; Kaluza, G.L.; Granada, J.F. Preclinical in vivo long-term evaluation of the novel Mitra-Spacer technology: Experimental validation in the ovine model. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 13, 272–279. [Google Scholar] [CrossRef]
- Chon, M.-K.; Lee, S.-W.; Hahn, J.-Y.; Park, Y.-H.; Kim, H.-S.; Lee, S.-H.; Shin, D.-H.; Lee, P.H.; Kim, E.K.; Lee, J.-H. A novel device for tricuspid regurgitation reduction featuring 3-dimensional leaflet and atraumatic anchor: Pivot-TR system. Basic Transl. Sci. 2022, 7, 1249–1261. [Google Scholar] [CrossRef]
- Zanella, A.; Scaravilli, V.; Isgrò, S.; Milan, M.; Cressoni, M.; Patroniti, N.; Fumagalli, R.; Pesenti, A. Fluid leakage across tracheal tube cuff, effect of different cuff material, shape, and positive expiratory pressure: A bench-top study. Intensive Care Med. 2011, 37, 343–347. [Google Scholar] [CrossRef]
- Chon, M.-K.; Shin, D.-H.; Jung, S.-J.; Park, H.-J.; Kim, J.-H. A percutaneous catheter solution as a spacer for regurgitant heart valve disease. Coatings 2021, 11, 926. [Google Scholar] [CrossRef]
- Kibbe, M.R.; Martinez, J.; Popowich, D.A.; Kapadia, M.R.; Ahanchi, S.S.; Aalami, O.O.; Jiang, Q.; Webb, A.R.; Yang, J.; Carroll, T. Citric acid-based elastomers provide a biocompatible interface for vascular grafts. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 314–324. [Google Scholar] [CrossRef]
- Starke, R.M.; Turk, A.; Ding, D.; Crowley, R.W.; Liu, K.C.; Chalouhi, N.; Hasan, D.M.; Dumont, A.S.; Jabbour, P.; Durst, C.R. Technology developments in endovascular treatment of intracranial aneurysms. J. Neurointerv. Surg. 2016, 8, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Pelton, A. Nitinol fatigue: A review of microstructures and mechanisms. J. Mater. Eng. Perform. 2011, 20, 613–617. [Google Scholar] [CrossRef]
- Fiorella, D.; Boulos, A.; Turk, A.S.; Siddiqui, A.H.; Arthur, A.S.; Diaz, O.; Lopes, D.K. The safety and effectiveness of the LVIS stent system for the treatment of wide-necked cerebral aneurysms: Final results of the pivotal US LVIS trial. J. Neurointerv. Surg. 2019, 11, 357–361. [Google Scholar] [CrossRef]
- Kir, D.; Munagala, M. Restructuring the Heart from Failure to Success: Role of Structural Interventions in the Realm of Heart Failure. Front. Cardiovasc. Med. 2022, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Curio, J.; Demir, O.M.; Pagnesi, M.; Mangieri, A.; Giannini, F.; Weisz, G.; Latib, A. Update on the current landscape of transcatheter options for tricuspid regurgitation treatment. Interv. Cardiol. Rev. 2019, 14, 54. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, H.J.; Jung, S.-J.; Kim, J.-H.; Lee, J.S. Optimization of tricuspid membrane mechanism for effectiveness and leaflet longevity through hemodynamic analysis. Eng. Appl. Comput. Fluid Mech. 2022, 16, 1587–1600. [Google Scholar] [CrossRef]
- Ali, M.N.; Busfield, J.J.; Rehman, I.U. Auxetic oesophageal stents: Structure and mechanical properties. J. Mater. Sci. Mater. Med. 2014, 25, 527–553. [Google Scholar] [CrossRef]
- Brooks, A.K.; Chakravarty, S.; Ali, M.; Yadavalli, V.K. Kirigami-inspired biodesign for applications in healthcare. Adv. Mater. 2022, 34, 2109550. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Liu, S. Smart and biostable polyurethanes for long-term implants. ACS Biomater. Sci. Eng. 2018, 4, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- McBane, J.E.; Sharifpoor, S.; Cai, K.; Labow, R.S.; Santerre, J.P. Biodegradation and in vivo biocompatibility of a degradable, polar/hydrophobic/ionic polyurethane for tissue engineering applications. Biomaterials 2011, 32, 6034–6044. [Google Scholar] [CrossRef]
- Soldani, G.; Losi, P.; Bernabei, M.; Burchielli, S.; Chiappino, D.; Kull, S.; Briganti, E.; Spiller, D. Long term performance of small-diameter vascular grafts made of a poly (ether) urethane–polydimethylsiloxane semi-interpenetrating polymeric network. Biomaterials 2010, 31, 2592–2605. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, D.; He, X.; Li, J.; Tan, H.; Li, J.; Fu, Q.; Gu, Q. The degradation and biocompatibility of pH-sensitive biodegradable polyurethanes for intracellular multifunctional antitumor drug delivery. Biomaterials 2012, 33, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, M.J.; Schmitt, B.J.; Coghlan, P.; Finkemeyer, J.P.; Caplash, Y.; Greenwood, J.E. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: A pilot study. Eplasty 2015, 15, e13. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chon, M.-K.; Jung, S.-J.; Seo, J.-Y.; Shin, D.-H.; Park, J.-H.; Kim, H.-S.; Hahn, J.-Y.; Kim, E.-K.; Lee, S.-W.; Park, Y.-H.; et al. The Development of a Permanent Implantable Spacer with the Function of Size Adjustability for Customized Treatment of Regurgitant Heart Valve Disease. Bioengineering 2023, 10, 1016. https://doi.org/10.3390/bioengineering10091016
Chon M-K, Jung S-J, Seo J-Y, Shin D-H, Park J-H, Kim H-S, Hahn J-Y, Kim E-K, Lee S-W, Park Y-H, et al. The Development of a Permanent Implantable Spacer with the Function of Size Adjustability for Customized Treatment of Regurgitant Heart Valve Disease. Bioengineering. 2023; 10(9):1016. https://doi.org/10.3390/bioengineering10091016
Chicago/Turabian StyleChon, Min-Ku, Su-Jin Jung, Jae-Young Seo, Dong-Hoon Shin, Jun-Hui Park, Hyun-Sook Kim, Joo-Yong Hahn, Eun-Kyoung Kim, Seung-Whan Lee, Yong-Hyun Park, and et al. 2023. "The Development of a Permanent Implantable Spacer with the Function of Size Adjustability for Customized Treatment of Regurgitant Heart Valve Disease" Bioengineering 10, no. 9: 1016. https://doi.org/10.3390/bioengineering10091016
APA StyleChon, M.-K., Jung, S.-J., Seo, J.-Y., Shin, D.-H., Park, J.-H., Kim, H.-S., Hahn, J.-Y., Kim, E.-K., Lee, S.-W., Park, Y.-H., Lee, S.-H., & Kim, J.-H. (2023). The Development of a Permanent Implantable Spacer with the Function of Size Adjustability for Customized Treatment of Regurgitant Heart Valve Disease. Bioengineering, 10(9), 1016. https://doi.org/10.3390/bioengineering10091016





