Abstract
At present, the double-enzyme catalyzed method using maltooligosyltrehalose synthase (MTSase) and maltooligosyltrehalose trehalohydrolase (MTHase) is the mainstream technology for industrial trehalose production. However, MTSase and MTHase are prepared mainly using the heterologous expression in the engineered Escherichia coli strains so far. In this study, we first proved that the addition of 3 U/g neutral pullulanase PulA could enhance the trehalose conversion rate by 2.46 times in the double-enzyme catalyzed system. Then, a CBM68 domain was used to successfully assist the secretory expression of MTSase and MTHase from Arthrobacter ramosus S34 in Bacillus subtilis SCK6. At the basis, an engineered strain B. subtilis PSH02 (amyE::pulA/pHT43-C68-ARS/pMC68-ARH), which co-expressed MTSase, MTHase, and PulA, was constructed. After the 24 h fermentation of B. subtilis PSH02, the optimum ratio of the extracellular multi-enzymes was obtained to make the highest trehalose conversion rate of 80% from 100 g/L maltodextrin. The high passage stability and multi-enzyme preservation stability made B. subtilis PSH02 an excellent industrial production strain. Moreover, trehalose production using these extracellular enzymes produced via the one-step fermentation of B. subtilis PSH02 would greatly simplify the procedure for multi-enzyme preparation and be expected to reduce production costs.
1. Introduction
Trehalose is a stable and non-reducing disaccharide that is composed of two glucose units linked with an α -1, 1-glycosidic bond [,]. This nontoxic carbohydrate is initially considered to primarily act as a stored energy source, similar to glycogen []. However, over the years, it has been revealed that trehalose may also perform specialized biological functions such as protecting proteins and membranes against various stresses [,,]. In recent decades, trehalose has sparked growing interest in a variety of industrial applications, which are mainly attributed to its structural features and its special role in molecular stabilization [,,]. It has been widely used in pharmaceuticals, foodstuffs, cosmetics, and agricultural products [,,]. For example, the ability of trehalose to protect a broad array of biological materials, such as DNA, proteins, cell lines, and tissues, has opened up possibilities in the pharmaceutical and biotechnology industries []. Additionally, studies have proposed that trehalose, as a bioactive nutrient, is a potential tool for regulating blood sugar levels and alleviating diabetes symptoms. It is used as an artificial sweetener and has sweetness equivalent to 40–45% of that of sucrose [,].
So far, there are two main enzymatic processes that can be used for trehalose production. The original enzymatic process is applying maltooligosyltrehalose synthase (MTSase, E.C. 5.4.99.15) and maltooligosyltrehalose trehalohydrolase (MTHase, E.C. 3.2.1.141) for the production of trehalose from maltodextrins or starch, as shown in a schematic diagram Figure S3 [,,,]. Currently, trehalose synthase (TreS, E.C. 5.4.99.16) is used to produce trehalose from maltose [,]. The greatest advantage of this one-step process, through the intermolecular rearrangement mechanism, is simplicity [,]. Most studies on trehalose production via TreS have focused on screening and modifying TreS. Several recombinant TreS genes have been cloned from different bacterial strains and heterologously expressed in different hosts [,,,,]. However, until now, the low expression level of TreS and the relatively high cost of the maltose substrate were the main reasons for making this approach economically unattractive []. The two-enzyme method is still the mainstream technology for industrial trehalose production. Because the main substrate of this process is maltodextrins or starch, the additions of amylase, pullulanase, and glucoamylase are usually used for improving the trehalose conversion rate. Pullulanase, as a debranching enzyme that explicitly cleaves α -1, 6 glycosidic bonds, has been widely recognized in increasing trehalose conversion rate, as shown in Figure S3 []. However, commercial pullulanases are usually acidic enzymes that do not match the optimum reaction pH of the neutral MTSase and MTHase.
Although trehalose production has been industrialized by far, there are still some requirements for producing host strains as the main producing strain is E. coli, which contains endotoxin and is unfavorable for safe and high-quality trehalose production [,,]. Aimed at this, we hired the safe strain B. subtilis approved by the FDA (Food and Drug Administration) as the host and expressed heterologous enzymes MTSase and MTHase in strain B.subtilis SCK6 [,]. This work was thought to establish the basis of high-quality trehalose production with low-endotoxin content. In this present work, a neutral pullulanase PulA, which has been developed with high specific activity, was used to enhance the trehalose conversion rate in the two-enzyme trehalose production method. MTSase and MTHase from different bacterial strains have been heterologously expressed and screened for conversion activity. On this basis, a series of engineered strains, co-expressing the two or three key enzymes with various strategies, have been constructed. Based on the evaluation of the trehalose conversion rate from maltodextrins and strain fermentation characteristics, an optimum production strain that co-expressed three extracellular enzymes in one-step fermentation was screened. In the further trehalose production industry, using this engineered strain to produce extracullular three key enzymes in one-step fermentation would simplify the procedure for multi-enzyme preparation and have the potential in reducing production costs.
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Primers
E. coli BL21 (DE3) TrxB and pET32a were used for gene expressions of MTSase and MTHase to construct recombinant E. coli strains. B. subtilis SCK6 and plasmids pMC68 and pHT43 were used for gene expressions of MTSase and MTHase to construct recombinant B. subtilis strains, respectively. All of the strains and plasmids used in this study are listed in Table S1 of the Supplementary Materials. Moreover, all of the primers designed in this study are listed in Table S2.
2.2. Construction of the Engineered Strains
The encoding genes of MTSase and MTHase from Arthrobacter ramosus S34 (GenBank: AB045141.1), Sulfolobus acidocaldarius ATCC33909 (GenBank: NC_020246.1) and Sulfolobus solfataricus KM1(GenBank: D64128.1) were synthesized by synbio tech Co. Ktd (Suzhou, China) and were inserted into the expression plasmids pET32a, pMC68, and pHT43 using seamless cloning method [], respectively. The obtained recombinant plasmids were then transformed into E. coli BL21 (DE3) TrxB and B. subtilis SCK6, respectively. The recombinant strain, which overexpressed pullulanase PulA, was constructed using a genome integration method according to our previous work []. The encoding gene of PulA has been previously reported [] and deposited in NCBI database with the accession NO. HQ844266.1. The co-expression recombinant strains, which expressed MTSase, MTHase, and PulA, were constructed by transforming both recombinant pMC68 and recombinant pHT43 containing MTSase encoding gene or MTHase encoding gene into the recombinant B. subtilis strain with one copy of PulA encoding gene integrated at amyE position of the B. subtilis SCK6 genome.
2.3. Fermentation Analysis of Recombinant B. subtilis Strains
The fermentation of the recombinant B. subtilis strains for enzyme production was performed according to our previous report []. Single colonies were selected after LB plate (25 µg/mL kanamycin and/or 10 µg/mL chloromycetin) activation and inoculated into fresh LB culture liquid with the same antibiotic addition for 14 h of cultivation. The culture broth was then transferred to 30 mL SR medium (30 g/L tryptone, 50 g/L yeast Extract, and 6 g/L K2HPO4) with 1% inoculum at the related antibiotic addition in a 250 mL shake flask. During 24–72 h cultivation at 37 °C and 220 rpm, the extracellular protein expressions and enzyme activities were detected at fixed intervals. The extracellular supernatant was obtained from the fermentation medium after centrifugation at 12,000 rpm for 10 min. The multiple enzymes solution was prepared and stored via a two-step operation consisting of a filtration with water-based membranes (0.22 μm) and an ultrafiltration concentration by molecular sieving.
2.4. Analysis of Protein Expression and Activity of the Recombinant Enzymes
Extracellular protein expressions were measured using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) method reported []. Pullulanase activity was measured using the modified dinitrosalicylic acid (DNS) method described in our previous work [,]. A mixture composed of 50 μL diluted extracellular pullulanase and 50 μL of pullulan substrates (5% (w/v)) was added into 400 μL phosphate buffer (pH 6.0) and water bathed at 60 °C for 30 min. Then, the reaction mixture was added with 500 μ L DNS solution and boiled for 10 min. After cooling, its absorbance at OD540nm was measured using microplate spectrophotometer Epoch 2TC (BioTeK, Winooski, VT, USA). One standard pullulanase unit was defined as the amount of enzyme that releases 1 μmol reducing sugar per minute.
MTSase activity was measured using the 3, 5-dinitrosalicylic acid (DNS) method [] with minor modifications. A mixture composed of 50 μL diluted extracellular MTSase and 50 μL of maltohexanose (1% (w/v)) was added into 400 μL phosphate buffer (20 mM, pH 6.0) and water bathed at 50 °C for 30 min. Then, 500 μL DNS solution was added to the reaction liquid, and the mixture was boiled for 10 min. After cooling, its absorbance at OD540nm was measured using microplate spectrophotometer Epoch 2TC (BioTeK, Winooski, VT, USA). One unit (U) of MTSase activity was defined as the amount of enzyme needed to convert 1 μmol of maltohexanose into maltotetraosyltrehalose per min.
MTHase activity was measured according to a previous report [] with some modifications. Firstly, maltohexanose was dissolved to a concentration of 1% (w/v) in 20 mM phosphate buffer (pH 6.0). Then, 50 µL ARS solution was mixed with 0.4 mL maltohexanose solution to produce maltotetraosyltrehalose. After 2 h incubation at 50 °C, the reaction was stopped by heating for 10 min in boiling water. After that, the product solution was incubated at 50 °C. Additionally, 50 µL of appropriately diluted MTHase was added, and the mixture was allowed to react for 30 min. Then, 500 μL DNS solution was added to the reaction mixture and boiled for 10 min. After cooling, the absorbance at OD540nm was measured using microplate spectrophotometer Epoch 2TC (BioTeK, Winooski, VT, USA). One unit (U) of MTHase activity was defined as the amount of MTHase needed to produce 1 µmol of trehalose per min under the assay conditions.
2.5. Enzymatic Trehalose Production from Maltodextrin
For the trehalose production with three-enzyme co-catalysis, each enzyme produced by different engineered strains, the dose of MTSase, MTHase, and pullulanase was 90 U, 65 U, and 0–8 U per gram of maltodextrin. The concentration of the substrate maltodextrin (DE 12) was 10% (wt/vol). The reaction was taken at 50 °C and pH 6.0 for 48 h []. For the trehalose production with multi-enzymes produced by the engineered strain in one-step fermentation, 500 μL extracellular enzyme solution was mixed with 500 μL 20% (w/v) maltodextrin (DE 12) solution, and the mixture was incubated at 50 °C for 24–72 h. The reaction was terminated by incubating in boiling water for 10 min. Commercial glucoamylase (LONCT, Shandong, China) was used to hydrolyze the residual maltodextrin at 60 °C for 24 h. The reaction mixture was then reheated in boiling water for 10 min to inactivate the glucoamylase. The quantity of trehalose was determined via HPLC, according to previous reports [,].
2.6. Determination of the Activity of Mixed Enzymes Obtained via One-Step Fermentation
Maltodextrin (DE 12) was dissolved to a concentration of 20% (wt/vol) in 20 mM phosphate buffer (pH 6.0). Then, 500μL maltodextrin substrate solution was mixed with 500 μL extracellular multi-enzymes solution produced via one-step fermentation. The reaction was carried at 50 °C for 48 h. After reaction, the mixture was boiling for 10 min, and then the cooling solution was centrifuged at 14,000× g for 10 min. The supernatant was filtrated with 0.22 μm syringe filters before HPLC analysis. The quantity of product was determined via HPLC using commercial trehalose (Purity ≥ 98%) (Sigma-Aldrich, St. Louis, MO, USA) as standard. The commercial trehalose standard sample was dissolved in double-distilled water with a concentration of 10 mg/mL. The Hypersil NH2-S column was used to determine the concentration of trehalose using a refractive index detector (RID). The mobile phase was acetonitrile/water solution (acetonitrile/water = 75:25) with a flow rate of 1.0 mL/min at 40 °C. The quantitative of trehalose in the sample was determined according to the retention time of the standard. The concentration of the trehalose was calculated based on the peak area of the sample. The calculation formula was as (Cm is concentration of the trehalose, (g/L); Am is peak area of the sample; As is peak area of the standard; Cs is the quality of the standard, g). One unit (U) of the activity of the extracellular multi-enzymes produced via one-step fermentation was defined as the amount of enzymes needed to produce 1 µmol of trehalose during 48 h reaction under the assay conditions. The calculation formula was (Vm is total volume of reaction system).
2.7. Detected Stability of the Strain’s Enzyme Production and Complex Enzymes
The single colony of B. subtilis PSH02 was inoculated into 2 mL of LB liquid medium. After 8 h cultivation, 20 μL bacterial solution was inoculated into 2 mL fresh LB medium and continued to culture for 8 h. After each 8 h interval, the solution was determined as a generation. The 1st, 5th, 10th, 15th, 20th, 25th, and 30th generations were selected for shaking flask fermentation, and then the extracellular multi-enzymes were used to catalyze maltodextrin converting to trehalose. The trehalose conversation rates catalyzed by multi-enzymes from different generation strains were compared to determine the passage stability of B. subtilis PSH02. Meanwhile, the extracellular multi-enzymes solution after 24 fermentation of B. subtilis PSH02 was taken to be filtrated with 0.22 μm syringe filters. The sterile multi-enzyme solutions were stored at room temperature (25–35 °C) and 4 °C for 14 days. The multi-enzyme solutions were selected at the 1st, 3rd, 5th, 7th, and 14th days to detect the trehalose conversation rates from maltodextrin, respectively. The preservation stability of the multi-enzymes solution was determined using the trehalose conversation rate comparisons.
3. Results and Discussion
3.1. Screening of the Malto-Oligosyltrehalose Synthase and Malto-Oligosyltrehalose Trehalohydrolase from Different Sources
Three pairs of malto-oligosyltrehalose synthase (MTSase) and malto-oligosyltrehalose trehalohydrolase (MTHase) from Arthrobacter ramosus S34 and Sulfolobus acidocaldarius ATCC33909 and Sulfolobus solfataricus KM1 were heterologously expressed in E. coli BL21 (DE3) trxB, respectively. MTSase (ARS) and MTHase (ARH) from Arthrobacter ramosus S34; MTHase (SAH) from Sulfolobus acidocaldarius ATCC33909; and MTHase (SSH) from Sulfolobus solfataricus KM1 were solubly expressed with plasmid pET32a in the TrxA-fused version (Figure 1a). However, MTSase (SAS) from Sulfolobus acidocaldarius ATCC33909 and MTSase (SSS) from Sulfolobus solfataricus KM1 were expressed in inclusion body forms (Figure 1a). As only ARS has been expressed in a soluble form showing enzyme activities, we further compared the activities of the recombinant ARH, SAH, and SSH using the reaction products of the recombinant ARS as the reaction substrates. The results showed that the activity of ARH was 7.15 times higher than that of SAH (Figure 1b). Moreover, although SSH showed a similar activity with ARH (Figure 1b), the protein amount of SSH was remarkably higher than that of ARH in the same volume of protein addition (Figure 1). From the results, we could speculate that the key enzymes of the two-step continuous reaction should be from the same source to ensure the best conversion efficiency.
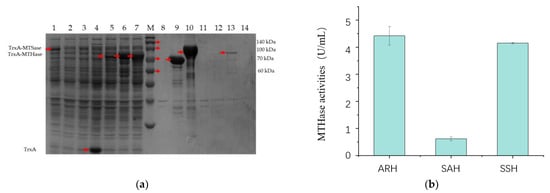
Figure 1.
Heterologous expressions of MTSase and MTHase in E. coli BL21 (DE3) trxB. (a) SDS-PAGE of the intracellular soluble and insoluble proteins of the recombinant E. coli strains. Lane M: protein molecular weight standards. Lanes 1 and 8: the intracellular soluble and insoluble proteins of the recombinant E. coli strains expressing recombinant ARS; lane 2 and 9: the intracellular soluble and insoluble proteins of the recombinant E. coli strains expressing recombinant SAS; lane 3 and 10: the intracellular soluble and insoluble proteins of the recombinant E. coli strains expressing recombinant SSS; lane 4 and 11: the intracellular soluble and insoluble proteins of the control strains with the empty plasmid pET32a in E. coli BL21(DE3) trxB; lane 5 and 12: the intracellular soluble and insoluble proteins of the recombinant E. coli strains expressing recombinant ARH; lane 6 and 13: the intracellular soluble and insoluble proteins of the recombinant E. coli strains expressing recombinant SAH; lane 7 and 14: the intracellular soluble and insoluble proteins of the recombinant E. coli strains expressing recombinant SSH. (b) MTHase activities of ARH, SAH, and SSS using the reaction products of ARS as the reaction substrate. All values in the histogram are expressed in means ± SD (n = 3).
We use the fusion expression tag CBM68, which was developed in our previous study [], to enhance the secretory expressions of ARS and ARH. To our delight, the recombinant ARS and ARH were detected in the medium after the fermentations of the recombinant B. subtilis strains with a high-copy plasmid pMC68 and a low-copy plasmid pHT43-C68, respectively (Figure 2a). Similarly to our previous results [], both the CBM68 fused enzymes and the untagged enzymes were found in the fermentation medium (Figure 2a). The extracellular activities of the recombinant enzymes showed that the CBM68 fused recombinant ARS and ARH with pMC68 plasmid had relatively higher activities by 21.6 U/mL and 14.1 U/mL, which were 5.17 and 3.19 times higher than those of the recombinant ARS and ARH expressed in E.coli, correspondingly (Figure 2b). The relatively lower activities of the CBM68 fused recombinant ARS and ARH in pHT43 plasmids were properly caused by lower protein expression amounts (Figure 2). Meanwhile, these results proved that CBM68 indeed played a significant role in increasing the expression of foreign proteins in B. subtilis once again. Therefore, we selected the CBM68 fused recombinant ARS and ARH for the construction of further expression strains in B. subtilis.
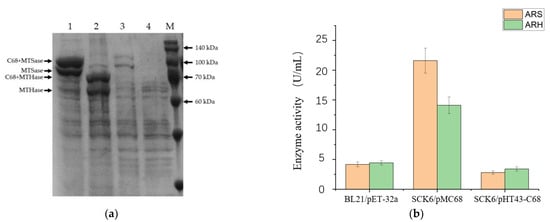
Figure 2.
Heterologous expressions of ARS and ARH in B. subtilis SCK6. (a) SDS-PAGE of the extracellular proteins of the recombinant B. subtilis strains. Lane 1: ARS expressed in B. subtilis SCK6 using the expression plasmid pMC68; lane 2: ARH expressed in B. subtilis SCK6 using the expression plasmid pMC68; lane 3: ARS expressed in B. subtilis SCK6 using the expression plasmid pHT43 (N-terminal fused with CBM68); lane 4: ARH expressed in B. subtilis SCK6 using the expression plasmid pHT43 (N-terminal fused with CBM68); lane M: protein molecular weight standards. (b) The activities of the recombinant enzymes are heterologously expressed by the engineered strains. All values in the histogram are expressed in means ± SD (n = 3).
3.2. Neutral Pullulanase PulA Enhanced Trehalose Conversion Rate
In the vitro conversion system, when the additional amount of ARH was set as 65 U/g maltodextrin substrate, the trehalose conversion rate increased with the increase in the additional amount of ARS (Figure 3a). The conversion rate was 33% at the highest ARS addition of 90 U/g limited by experimental conditions. However, at the fixed ARS additional amount of 90 U/g, the optimum addition amount of ARH was 32 U/g, leading to the highest trehalose conversion rate of 35% (Figure 3b). At the basis, a neutral pullulanase PulA was added with different additional amounts of 0 U/g, 1 U/g, 3 U/g, 6 U/g, and 8 U/g, respectively. As shown in Figure 3c, when the additional amount of PulA was 3 U/g, the trehalose conversion rate was up to 86%, which was 2.46 times higher than that without the pullulanase addition. It indicated that PulA was very useful for the enzymatic synthesis of trehalose from maltodextrin. Therefore, we decided to make PulA, ARS, and ARH the target enzymes to be co-overexpressed in B. subtulis. Moreover, based on the results of vitro multi-enzymatic conversion, pullulanase supplementation was very important to improve the trehalose conversion rate, but the requirement for pullulanase activity is relatively lower compared with the main enzymes ARS and ARH. Thus, to keep a high trehalose conversion rate, the construction of an engineered strain for one-step fermentation-produced complex enzymes still should ensure the expressions of ARS and ARH as high as possible in the first place.
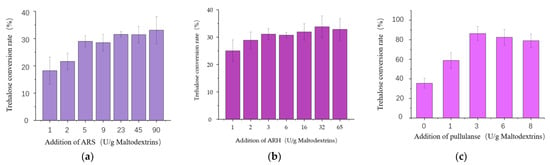
Figure 3.
Effect of different enzyme additions on trehalose conversion rate. (a) The optimum additional amount of ARS for trehalose production. (b) The optimum additional amount of ARH for trehalose production. (c) The optimum additional amount of PulA for trehalose production. All values are expressed as the means ± SDs (n = 3).
3.3. Construction of the Engineered Strains for Co-Production of the Three Enzymes
For the co-expression of the three enzymes, the PulA encoding gene was inserted at the amyE site of the genome of B. subtilis SCK6, while the ARS and ARH encoding genes were co-expressed using plasmids pMC68 or pHT43-C68, respectively. Four recombinant strains were successfully constructed to determine the extracellular expression of each target protein and the trehalose conversion rates catalyzed by the extracellular multi-enzymes produced by different strains: B. subtilis PSH01 (amyE::pulA/pMC68-ARS/pHT43-C68-ARH), B. subtilis SH01 (pMC68-ARS/pHT43-C68-ARH), B. subtilis PSH02 (amyE::pulA/pHT43-C68-ARS/pMC68-ARH), and B. subtilis SH02 (pHT43-C68- ARS/pMC68-ARH). The results were shown in Figure 4 and Figure S2. Although the strains B. subtilis PSH01 and B. subtilis SH01 showed relatively higher expression amounts of ARS after 48 h of fermentation using the high copy number plasmid pMC68 (Figure 4a), the strains B. subtilis PSH02 and B. subtilis SH02 showed relatively higher trehalose conversion rates at different fermentation times (Figure 4b). However, because ARH was expressed using pMC68 in the recombinant strains B. subtilis PSH02 and B. subtilis SH02, the ARH expression levels of B. subtilis PSH02 and B. subtilis SH02 showed relatively higher rates than those of B. subtilis PSH01 and B. subtilis SH01 (Figure 4a). It indicated that the over-expression of ARH in the system, when a single strain was used to produce multiple enzymes, was relatively important for the improvement of the trehalose conversion rate. Moreover, when compared with B. subtilis SH02, the trehalose conversion rate in B. subtilis PSH02 was 1.45 times higher catalyzed by its extracellular multi-enzymes after 24 h fermentation (Figure 4b and Figure S2). It was concluded that the co-expression of PulA in the multi-enzyme expression system played a significant role in improving the trehalose conversion rate. As shown in Figure 4b, the effects of different fermentation times of the four recombinant strains on the trehalose conversion rates were evaluated. According to the results, B. subtilis PSH02 could reach the highest trehalose conversion rate after 24 h fermentation, while B. subtilis SH02 needed 48 h of fermentation to reach its highest trehalose conversion rate (Figure 4b). Moreover, the highest trehalose conversion rate by B. subtilis PSH02 was up to 80%, which was 1.29 times higher than that of B. subtilis SH02 (Figure 4b). However, with the extension of fermentation time, the trehalose conversion rates in B. subtilis PSH02 declined gradually, which was contrary to the increases in the extracellular expressions of target proteins (Figure 4b,c). As the cells grew and plasmids replications, the ARH was expressed and accumulated into a high but unmatched amount to the ARS and substrate, which led to the hydrolysis of the substrate and the reduced trehalose conversion rate accordingly (Figure S3). This unconventional hydrolysis was also proved by the partial increase in trehalose yielding during the determinations of the PulA lacking strain SH02, as shown in Figure 4b. ARH caused the hydrolysis of some α-1,4-glycosidic bonds of maltodextrin, and more reducing ends were revealed to be new substrates, increasing the trehalose production temporarily. With the prolongation of the fermentation time, this enhancement was limited due to the unmatched expression level of ARS and ARH, which caused the decreasing trend of trehalose production ultimately. It indicated that compared with increasing the expression level of each target protein, keeping the appropriate ratios of the three enzymes was more important for enhancing the trehalose conversion rate catalyzed via this multi-enzymatic system produced using one-step fermentation. As shown in Figure 4, at the fermentation time of 24 h, B. subtilis PSH02, producing the optimum ratios of ARS, ARH, and PulA in the extracellular medium made the trehalose conversion rate up to 80% from 10 g/L maltodextrin during 48 h of reaction at 50 °C.
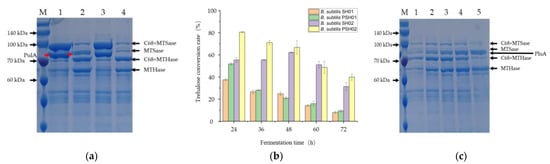
Figure 4.
Co-expression of ARS, ARH and PulA in B. subtilis SCK6. (a) SDS-PAGE of the extracellular proteins of the co-expression strains. Lane M: protein molecular weight standards; lane 1: extracellular proteins of the recombinant strain B. subtilis PSH01 (amyE::pulA/pMC68-ARS/pHT43-C68-ARH) after 48 h fermentation; lane 2: extracellular proteins of the recombinant strain B. subtilis PSH02 (amyE::pulA/pHT43-C68-ARS/pMC68-ARH) after 48 h fermentation; lane 3: extracellular proteins of the recombinant strain B. subtilis SH01 (pMC68-ARS/pHT43-C68-ARH) after 48 h fermentation; lane 4: extracellular proteins of the recombinant strain B. subtilis SH02 (pHT43-C68-ARS/pMC68-ARH) after 48 h fermentation. (b) Trehalose conversion rates using the extracellular enzymes produced by the co-expression strains after different fermentation times. (c) SDS-PAGE of the extracellular proteins of B. subtilis PSH02 with different fermentation times. lane 1: 24 h; lane 2: 36 h; lane 3: 48 h; lane 4: 60 h; lane 5: 72 h. All values in the histogram are expressed in means ± SD (n = 3).
The engineered strains, which were inserted with ARS and/or ARH into the genome of B. subtilis SCK6, were also constructed in this work. Firstly, ARS and ARH were separately inserted into the amyE, nprB, or ytxE position of the genome of B. subtilis SCK6 to test the enzyme activity. The results are shown in Figure S1A, the same enzyme which showed different activities at different inserted positions. Similarly, ARS and ARH showed remarkably different enzyme activity values when they were inserted into the same positions. However, all recombinant ARS and ARH, which were expressed by genome integration, showed activities of no more than 5 U/mL (Figure S1A). Moreover, the co-expressions of ARS and ARH in different combination sites also showed different trehalose conversion rates, and the highest trehalose conversion rate was only 26% (Figure S1B). Therefore, compared with the engineered strains which over-expressed ARS and ARH by plasmids, the genome integration strains showed relatively lower enzyme activities and were not suitable to be multi-enzyme-producing strains for the production of trehalose.
3.4. Properties of the Engineered Strain B. subtilis PSH02 for Trehalose Enzymatic Synthesis
Because various enzyme production ratios of ARS, ARH, and PulA would lead to different trehalose conversion rates, in order to evaluate the transformation ability of the multi-enzymes produced by the engineered strains for trehalose production, the multi-enzyme activity was defined as the number of enzymes that produce 1 μM trehalose during 48 h of reaction at 50 °C. The results showed that the multi-enzymes activities produced by B. subtilis SH01, B. subtilis PSH01, B. subtilis SH02, and B. subtilis PSH02 were 23.2 U/mL, 36.5 U/mL, 38.4 U/mL, and 51.7 U/mL after 24 h fermentation, correspondingly. The multi-enzymes activity of each strain corresponded to its trehalose conversion rate very well (Figure 4b). On this basis, we used the extracellular multi-enzymes produced by B. subtilis PSH02 after 24 h of fermentation to further optimize the substrate concentration and enzyme dosage. As shown in Figure 5a, 15 U/mL of the multi-enzymes could catalyze more than 80% of 10 g/L maltodextrin substrate to trehalose. When the concentration of maltodextrin substrate was 100 g/L, the additional dosage of the multi-enzymes needed to be 150 U/mL to reach the eligible trehalose conversion rate, which was more than 80% (Figure 5b). It illustrated that the three times concentration of the extracellular multi-enzymes produced by B. subtilis PSH02 after 24 h if fermentation could obtain more than 80% of trehalose conversion rate with the high substrate concentration of 100 g/L. Compared with the work carried out by Liu, who utilized the recombinant Bacillus subtilis to secrete TreS and converted maltose to trehalose with a conversion rate of 75.5% [], a higher conversion rate was obtained during our work as the engineered strain B. subtilis PSH02 showed a good potential for industrial application of trehalose enzymatic synthesis.
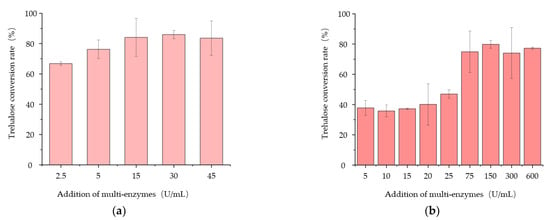
Figure 5.
Effects of different addition dosages of multi-enzymes on the trehalose conversion rate at the maltodextrin substrate concentration of 10 g/L (a) and 100 g/L (b). All values are expressed as the means ± SDs (n = 3).
In order to further evaluate the production characteristics of B. subtilis PSH02, the passage stability of B. subtilis PSH02 and the stability of its extracellular multi-enzyme activity were determined in this work. As shown in Figure 6a, the relative activity of the extracellular multi-enzymes of B. subtilis PSH02 was higher than 88% after a successive passage to 30 generations. It indicated that B. subtilis PSH02 had a high production stability of the extracellular multi-enzymes. Moreover, both at 4 °C and room temperature (25–35 °C), the extracellular multi-enzymes could keep high relative activities during 7 days of preservation (Figure 6b). After 14 days of preservation at 4 °C and room temperature (25–35 °C), the relative activities of the extracellular multi-enzymes were 82% and 83%, respectively. Thus, the stabilities, both in B. subtilis PSH02 generations and its extracellular multi-enzymes activities, made B. subtilis PSH02 a potential industrial production strain.
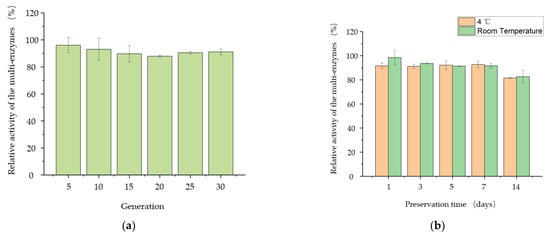
Figure 6.
Evaluation of the production characteristics from B. subtilis PSH02. (a) Passage stability of B. subtilis PSH02. The activity of extracellular multi-enzymes produced by the original generation B. subtilis PSH02 after 24 h fermentation was set as 100%. (b) Stability of its extracellular multi-enzymes activity. The activity of the fresh extracellular multi-enzymes produced by B. subtilis PSH02 after 24 h fermentation was set as 100%. All values are expressed as the means ± SDs (n = 3).
4. Conclusions
In this study, four engineered strains were constructed to produce the key enzymes via one-step fermentation for trehalose enzymatic synthesis from maltodextrin: B. subtilis PSH01 (amyE::pulA/pMC68-ARS/pHT43-C68-ARH), B. subtilis SH01 (pMC68-ARS/pHT43-C68-ARH), B. subtilis PSH02 (amyE::pulA/pHT43-C68-ARS/pMC68-ARH) and B. subtilis SH02 (pHT43-C68- ARS/pMC68-ARH). The optimum strain B. subtilis PSH02, which co-expressed ARS, ARH, and PulA at an appropriate ratio after 24 h fermentation, could make the highest trehalose conversion rate of 80% from 100 g/L maltodextrin substrate. Moreover, B. subtilis PSH02 showed high generational stability within 30 generations. Its extracellular multi-enzymes could keep high preservation stability within 7 days at room temperature without adding any protective agent. Thus, the engineered strain B. subtilis PSH02 showed a high prospect for industrial application in trehalose enzymatic synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering10080977/s1, Figure S1: Expression of ARS and/or ARH via genome integration in B. subtilis SCK6; Figure S2: The HPLC chromatograms of extracellular enzymes solutions produced constructed strains; Figure S3: Converting pathway from maltodextrin to trehalose via double enzymes system. Table S1: Strains and plasmids used in this study; Table S2: Primers used in this study.
Author Contributions
Conceptualization, H.Z. and X.S.; methodology, J.Y. and X.F.; software, J.Y.; validation, J.Y., X.Z. and J.Z.; formal analysis, J.Y.; investigation, J.Y.; resources, H.S. and J.X.; data curation, J.Y. and X.F.; writing—original draft preparation, H.Z. and J.Y.; writing—review and editing, H.Z. and J.Y.; visualization, J.Y.; supervision, H.Z. and W.B.; project administration, H.Z. and W.B.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Key Research and Development Program of China, grant number 2021YFC2100403; Tianjin Research Innovation Project for Postgraduate Students, grant number 2021XY008; and Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project, grant number TSBICIP-CXRC-037, TSBICIP-PTJJ-007-13, and TSBICIP-KJGG-009-0202.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the text of the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Qiao, Y.; Wang, W.; Lu, X. Engineering cyanobacteria as cell factories for direct trehalose production from CO2. Metab. Eng. 2020, 62, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chi, Z.; Liu, G.L.; Xue, S.J.; Wang, Z.P.; Hu, Z.; Chi, Z.M. Improved pullulan production by a mutant of Aureobasidium melanogenum TN3-1 from a natural honey and capsule shell preparation. Int. J. Biol. Macromol. 2019, 141, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Vanaporn, M.; Titball, R.W. Trehalose and bacterial virulence. Virulence 2020, 11, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Tapia, H.; Goddard, J.; Gibney, P. Trehalose and its applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5004–5037. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, X.; Liu, F.; Xie, J.; Zhu, Q.; Tan, S. Trehalose in Biomedical Cryopreservation-Properties, Mechanisms, Delivery Methods, Applications, Benefits, and Problems. ACS Biomater. Sci. Eng. 2023, 9, 1190–1204. [Google Scholar] [CrossRef]
- MacIntyre, A.M.; Barth, J.X.; Pellitteri Hahn, M.C.; Scarlett, C.O.; Genin, S.; Allen, C. Trehalose Synthesis Contributes to Osmotic Stress Tolerance and Virulence of the Bacterial Wilt Pathogen Ralstonia solanacearum. Mol. Plant-Microbe Interact. 2020, 33, 462–473. [Google Scholar] [CrossRef]
- Chen, A.; Gibney, P. Dietary Trehalose as a Bioactive Nutrient. Nutrients 2023, 15, 1393. [Google Scholar] [CrossRef]
- Sharma, E.; Shruti, P.S.; Singh, S.; Singh, T.; Kaur, P.; Jodha, B.; Srivastava, Y.; Munshi, A.; Singh, S. Trehalose and its diverse biological potential. Curr. Protein Pept. Sci. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Trakarnpaiboon, S.; Champreda, V. Integrated Whole-Cell Biocatalysis for Trehalose Production from Maltose Using Permeabilized Pseudomonas monteilii Cells and Bioremoval of Byproduct. J. Microbiol. Biotechnol. 2022, 32, 1054–1063. [Google Scholar] [CrossRef]
- Su, L.; Yao, K.; Wu, J. Improved Activity of Sulfolobus acidocaldarius Maltooligosyltrehalose Synthase through Directed Evolution. J. Agric. Food Chem. 2020, 68, 4456–4463. [Google Scholar] [CrossRef]
- Su, L.; Wu, S.; Feng, J. High-efficiency expression of Sulfolobus acidocaldarius maltooligosyl trehalose trehalohydrolase in Escherichia coli through host strain and induction strategy optimization. Bioprocess Biosyst. Eng. 2019, 42, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Su, L.; Xu, F.; Xia, Y. Improved Thermostability of Maltooligosyltrehalose Synthase from Arthrobacter ramosus by Directed Evolution and Site-Directed Mutagenesis. J. Agric. Food Chem. 2019, 67, 5587–5595. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Su, P.C.; Chen, P.T. Production and characterization of a recombinant thermophilic trehalose synthase from Thermus antranikianii. J. Biosci. Bioeng. 2020, 129, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Singh, S. A Novel Trehalose Synthase for the Production of Trehalose and Trehalulose. Microbiol. Spectr. 2021, 9, e0133321. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, B.; Song, Z.; Jiang, L. Permeabilized TreS-Expressing Bacillus subtilis Cells Decorated with Glucose Isomerase and a Shell of ZIF-8 as a Reusable Biocatalyst for the Coproduction of Trehalose and Fructose. J. Agric. Food Chem. 2020, 68, 4464–4472. [Google Scholar] [CrossRef]
- Dong, L.; Lin, X.; Yu, D.; Huang, L.; Wang, B.; Pan, L. High-level expression of highly active and thermostable trehalase from Myceliophthora thermophila in Aspergillus niger by using the CRISPR/Cas9 tool and its application in ethanol fermentation. J. Ind. Microbiol. Biotechnol. 2019, 47, 133–144. [Google Scholar] [CrossRef]
- Liu, H.; Yang, S.; Wang, X.; Wang, T. Production of trehalose with trehalose synthase expressed and displayed on the surface of Bacillus subtilis spores. Microb. Cell Factories 2013, 18, 100. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Yang, S.; Wang, R.; Wang, T. Saturation mutagenesis and self-inducible expression of trehalose synthase in Bacillus subtilis. Biotechnol. Prog. 2019, 35, e2826. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Yang, S.; Wang, R.; Wang, T. Improved Expression and Optimization of Trehalose Synthase by Regulation of Pglv in Bacillus subtilis. Sci. Rep. 2019, 9, 6585. [Google Scholar] [CrossRef]
- Kobayashi, K.; Komeda, T.; Miura, Y.; Kettoku, M.; Kato, M. Production of Trehalose from Starch by Novel Trehalose-Producing Enzymes from Sulfolobus solfataricus KM1. J. Ferment. Bioeng. 1997, 83, 296–298. [Google Scholar] [CrossRef]
- Jiang, X.R.; Lin, Y.F.; Chen, P.T. Trehalose production via merged secretion, purification, and immobilization of trehalose synthase in Bacillus subtilis. Taiwan Inst. Chem. Eng. 2018, 82, 23–27. [Google Scholar] [CrossRef]
- Shu, W.; Zheng, H.; Fu, X.; Zhen, J.; Tan, M.; Xu, J.; Zhao, X.; Yang, S.; Song, H.; Ma, Y. Enhanced Heterologous Production of Glycosyltransferase UGT76G1 by Co-Expression of Endogenous prpD and malK in Escherichia coli and Its Transglycosylation Application in Production of Rebaudioside. Int. J. Mol. Sci. 2020, 21, 5752. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, X.; Zhao, X.; Xu, J.; Liu, Y.; Zheng, H.; Bai, W. Overexpression of a Thermostable α-Amylase through Genome Integration in Bacillus subtilis. Fermentation 2023, 9, 139. [Google Scholar] [CrossRef]
- Xu, J.; Ren, F.; Huang, C.H.; Zheng, Y.; Zhen, J.; Sun, H.; Ko, T.P.; He, M.; Chen, C.C.; Chan, H.C.; et al. Functional and structural studies of pullulanase from Anoxybacillus sp. LM18-11. Proteins 2014, 82, 1685–1693. [Google Scholar] [CrossRef]
- Zhen, J.; Zheng, H.; Zhao, X.; Fu, X.; Yang, S.; Xu, J.; Song, H.; Ma, Y. Regulate the hydrophobic motif to enhance the non-classical secretory expression of Pullulanase PulA in Bacillus subtilis. Int. J. Biol. Macromol. 2021, 193, 238–246. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, J.; Fu, X.; Tan, M.; Liu, F.; Zheng, H.; Song, H. Effects of different carbohydrate-binding modules on the enzymatic properties of pullulanase. Int. J. Biol. Macromol. 2019, 137, 973–981. [Google Scholar] [CrossRef]
- Han, C.; Su, L.; Hong, R.; Wu, S. A comparative study of maltooligosyltrehalose synthase from Sulfolobus acidocaldarius expressed in Pichia pastoris and Escherichia coli. Process Biochem. 2017, 60, 35–41. [Google Scholar] [CrossRef]
- Yamamoto, T.; Maruta, K.; Watanabe, H.; Yamashita, H.; Kubota, M.; Fukuda, S.; Kurimoto, M. Trehalose-producing Operon treYZ from Arthrobacter ramosus S34. Biosci. Biotechnol. Biochem. 2001, 65, 1419–1423. [Google Scholar] [CrossRef]
- Fang, T.; Tseng, W.C.; Pan, C.; Chun, Y.; Wang, M. Protein Engineering of Sulfolobus solfataricus Maltooligosyltrehalose Synthase To Alter Its Selectivity. J. Agric. Food Chem. 2007, 55, 5588–5594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).