The Translation of Mobile-Exoneuromusculoskeleton-Assisted Wrist–Hand Poststroke Telerehabilitation from Laboratory to Clinical Service
Abstract
:1. Introduction
2. Materials and Methods
2.1. WH-ENMS and Preparation of Translation
2.2. Home-Based Self-Help Telerehabilitation Program
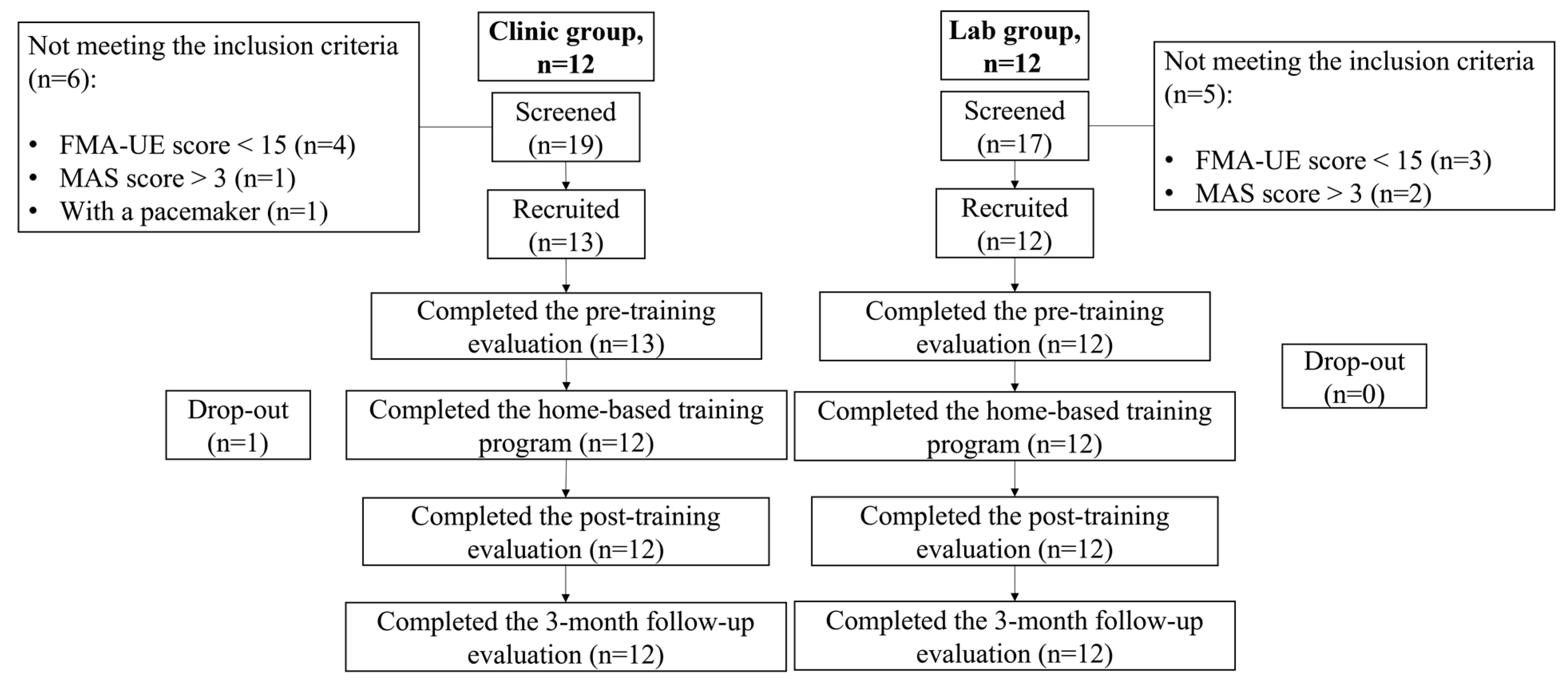
2.2.1. Participant Recruitment
2.2.2. Intervention Protocol
Pre-Training Tutorial
Training Protocol in Sessions
Logistics Management of Self-Help Training at Home
2.2.3. Clinic Group versus Lab Group—Variations
2.3. Evaluation of Training Outcomes
2.3.1. Clinical Assessments
2.3.2. EMG Evaluation
2.3.3. Kinematic Evaluation
2.3.4. Developed Questionnaire
2.3.5. Statistical Analysis
3. Results
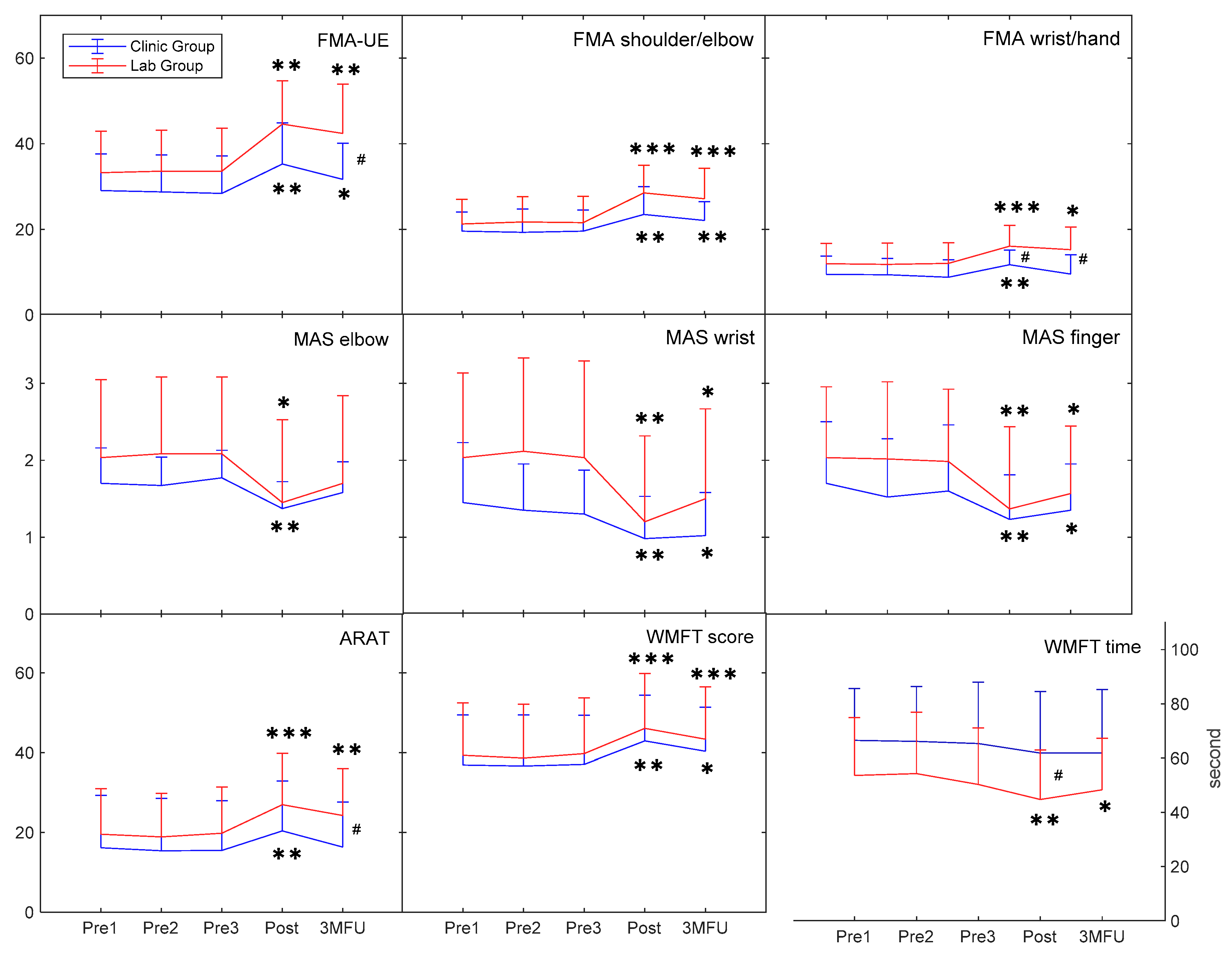
3.1. Behavioral Improvements in Clinical Assessments
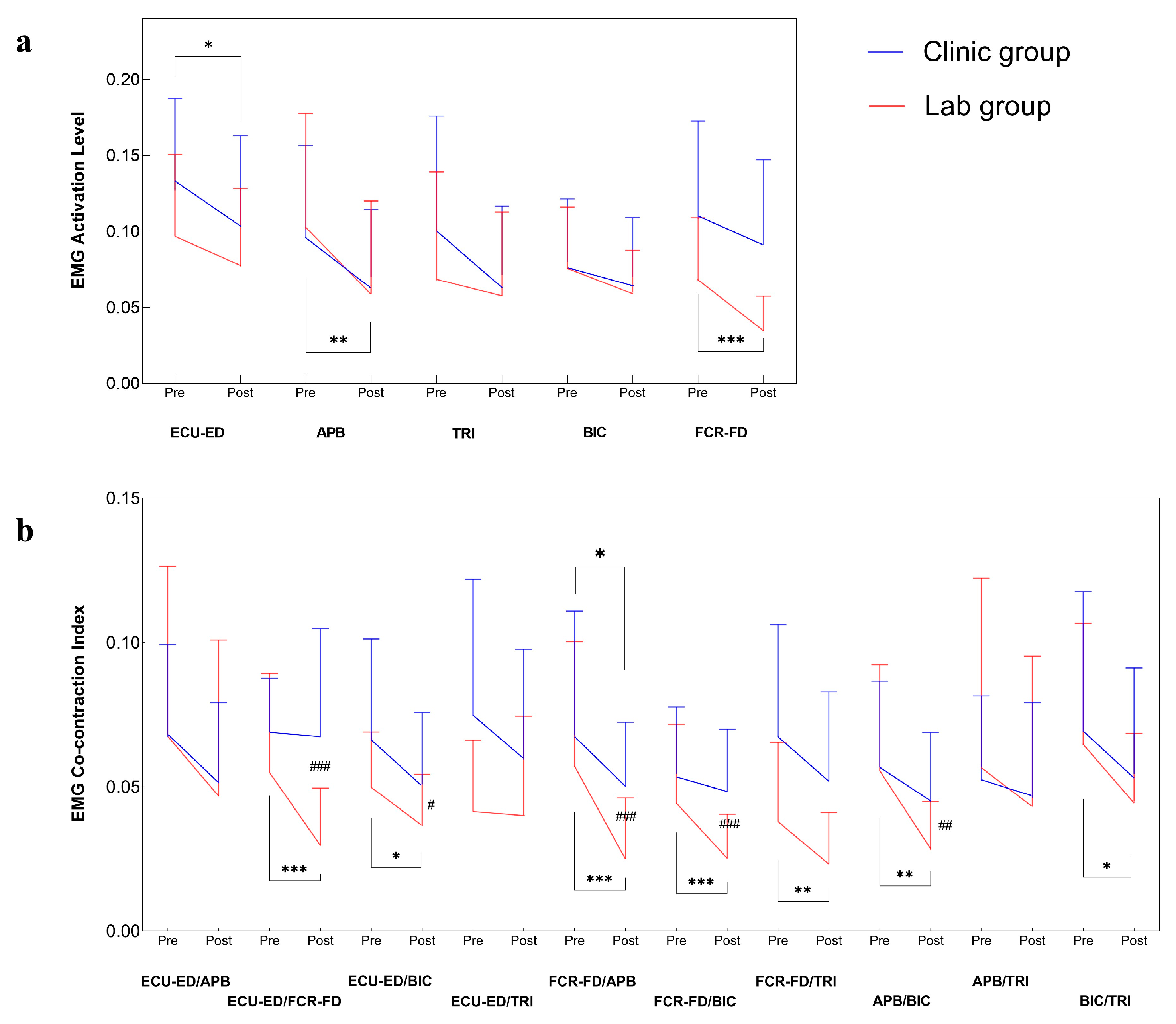
3.2. Improvements in Muscular Coordination by EMG
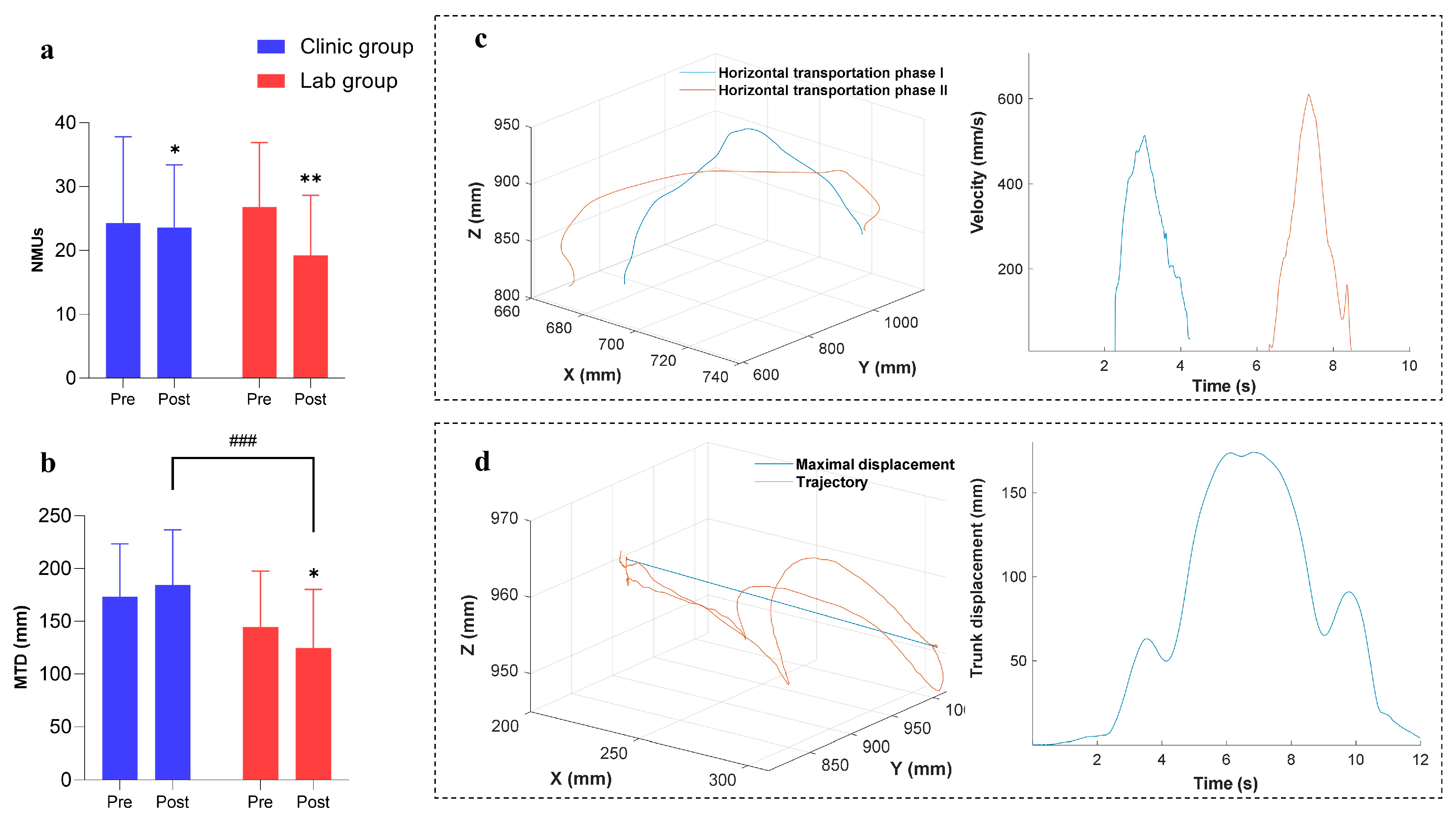
3.3. Improvements in Kinematic Performance
3.4. Remote Monitoring of Training Progresses
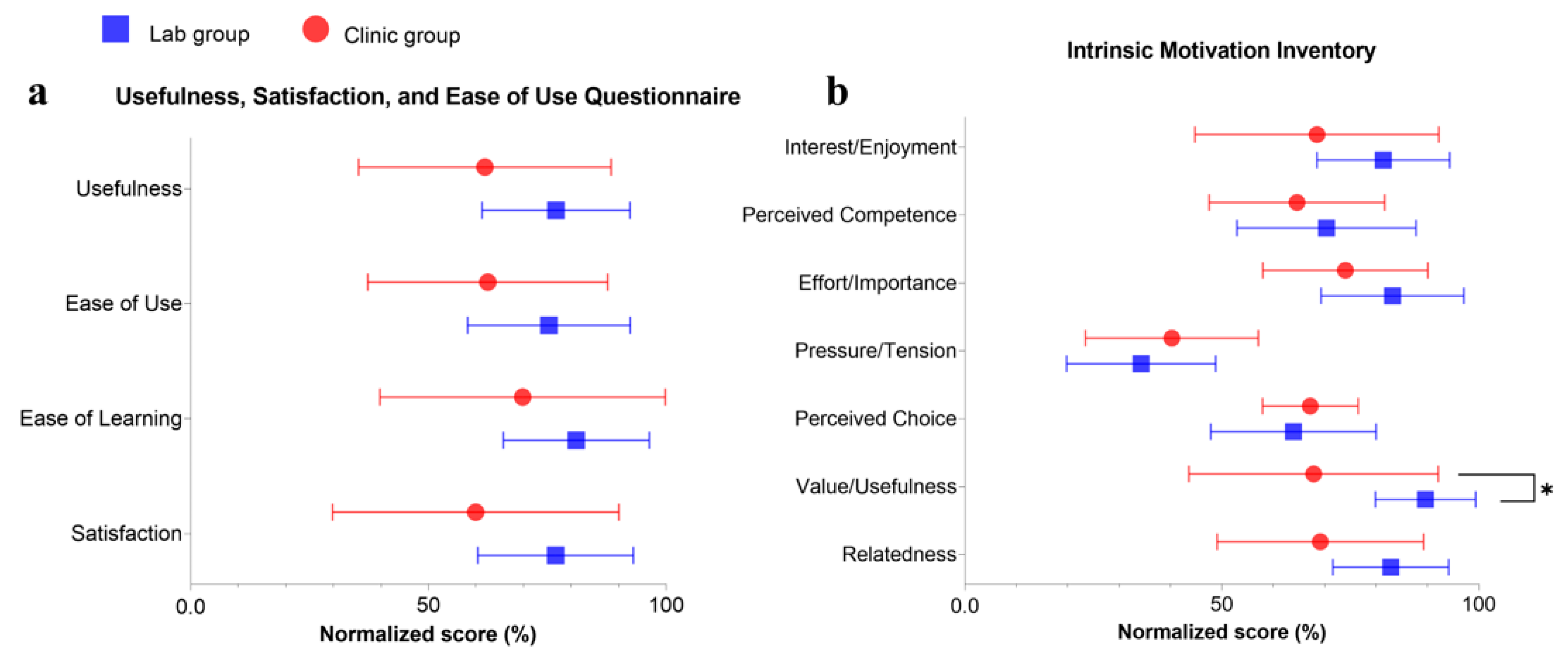
3.5. Questionnaire Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Evaluation Data by Group
| Clinical Assessments | Clinic Group (n = 12) | Lab Group (n = 12) | p (Cohen’s d) | p (r) |
|---|---|---|---|---|
| FMA-UE | 28.75 ± 8.56 | 33.47 ± 9.71 | 0.094 (0.342) | |
| FMA shoulder/elbow | 19.50 ± 4.75 | 21.53 ± 5.91 | 0.385 (0.177) | |
| FMA wrist/hand | 9.25 ± 4.02 | 11.97 ± 4.76 | 0.140 (0.301) | |
| ARAT | 15.69 ± 12.88 | 19.44 ± 11.29 | 0.326 (0.200) | |
| FIM | 62.75 ± 3.93 | 65.58 ± 1.78 | 0.038 * (0.927) | |
| WMFT score | 36.89 ± 12.48 | 39.22 ± 13.48 | 0.402 (0.171) | |
| WMFT time | 65.96 ± 20.54 | 52.77 ± 21.09 | 0.135 (0.634) | |
| MAS elbow | 1.71 ± 0.35 | 2.07 ± 1.00 | 0.173 (0.278) | |
| MAS wrist | 1.37 ± 0.58 | 2.06 ± 1.17 | 0.130 (0.309) | |
| MAS finger | 1.61 ± 0.77 | 2.01 ± 0.94 | 0.258 (0.474) |
| Clinical Assessments | Group | Pre1 | Pre2 | Pre3 | Post | 3MFU | 1-Way Repeated Measures ANOVA | Friedman Test | Quade’s ANCOVA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Value (95% Confidence Interval) | p (Partial η2) | p (Kendall’s W) | p Post (Partial η2) | p 3MFU (Partial η2) | ||||||
| FMA-UE | Clinic | 29.08(23.64~34.53) | 28.75(23.24~34.26) | 28.42(22.87~33.96) | 35.25(29.15~41.35) | 31.67(26.29~37.05) | <0.001 *** (0.771) | 0.056(0.156) | 0.019 # (0.226) | |
| Lab | 33.25(27.11~39.39) | 33.58(27.48~39.69) | 33.58(27.22~39.95) | 44.58(38.14~51.03) | 42.42(35.12~49.71) | <0.001 *** (0.771) | ||||
| FMA shoulder/elbow | Clinic | 19.58(16.72~22.44) | 19.33(15.90~22.76) | 19.58(16.47~22.70) | 23.50(19.38~27.62) | 22.08(19.30~24.87) | <0.001 *** (0.702) | 0.060(0.152) | 0.100(0.118) | |
| Lab | 21.25(17.59~24.91) | 21.75(18.01~25.49) | 21.58(17.68~25.49) | 28.50(24.40~32.60) | 27.17(22.65~31.68) | <0.001 *** (0.527) | ||||
| FMA wrist/hand | Clinic | 9.50(6.77~12.23) | 9.42(6.99~11.85) | 8.83(6.22~11.44) | 11.75(9.56~13.94) | 9.58(6.72~12.44) | 0.005 ** (0.442) | 0.047 # (0.167) | 0.016 # (0.237) | |
| Lab | 12.00(9.00~15.00) | 11.83(8.68~14.99) | 12.08(9.01~15.15) | 16.08(13.02~19.14) | 15.25(11.91~18.59) | <0.001 *** (0.471) | ||||
| ARAT | Clinic | 16.17(7.80~24.53) | 15.42(7.05~23.79) | 15.50(7.59~23.41) | 20.42(12.46~28.37) | 16.33(9.16~23.51) | 0.001 *** (0.608) | 0.118(0.107) | 0.014 # (0.246) | |
| Lab | 19.58(12.32~26.85) | 18.92(11.96~25.87) | 19.83(12.46~27.20) | 27.00(18.88~35.12) | 24.25(16.79~31.71) | <0.001 *** (0.554) | ||||
| FIM | Clinic | 62.75(60.25~65.25) | 62.75(60.25~65.25) | 62.75(60.25~65.25) | 63.25(61.10~65.40) | 63.50(61.34~65.66) | 0.061(0.233) | 0.284(0.052) | 0.671(0.008) | |
| Lab | 65.58(64.45~66.72) | 65.58(64.45~66.72) | 65.58(64.45~66.72) | 65.75(64.60~66.90) | 65.75(64.60~66.90) | 0.166(0.167) | ||||
| WMFT score | Clinic | 36.92(28.95~44.89) | 36.67(28.54~44.80) | 37.08(29.27~44.90) | 42.92(35.64~50.19) | 40.33(33.30~47.36) | <0.001 *** (0.780) | 0.549(0.017) | 0.352(0.040) | |
| Lab | 39.33(30.99~47.68) | 38.58(29.99~47.18) | 39.75(30.90~48.60) | 46.08(37.34~54.83) | 43.33(34.97~51.69) | <0.001 *** (0.543) | ||||
| WMFT time | Clinic | 66.48(54.34~78.61) | 66.09(53.22~78.97) | 65.32(50.92~79.71) | 61.77(47.33~76.20) | 61.89(47.07~76.71) | 0.338(0.090) | 0.017 # (0.232) | 0.496(0.021) | |
| Lab | 53.63(40.13~67.14) | 54.35(40.01~68.69) | 50.34(37.15~63.54) | 44.80(33.26~56.34) | 48.35(36.36~60.34) | <0.001 *** (0.293) | ||||
| MAS elbow | Clinic | 1.70(1.41~1.99) | 1.67(1.44~1.90) | 1.77(1.54~2.00) | 1.37(1.14~1.59) | 1.58(1.33~1.83) | 0.005 ** (0.438) | 0.247(0.060) | 0.126(0.103) | |
| Lab | 2.03(1.39~2.68) | 2.08(1.45~2.72) | 2.08(1.45~2.72) | 1.45(0.77~2.13) | 1.70(0.98~2.42) | <0.001 *** (0.406) | ||||
| MAS wrist | Clinic | 1.45(0.95~1.95) | 1.35(0.97~1.73) | 1.30(0.94~1.66) | 0.98(0.63~1.33) | 1.02(0.66~1.37) | 0.007 ** (0.419) | 0.257(0.058) | 0.997(<0.001) | |
| Lab | 2.03(1.33~2.73) | 2.12(1.35~2.89) | 2.03(1.24~2.83) | 1.20(0.49~1.91) | 1.50(0.76~2.24) | <0.001 *** (0.477) | ||||
| MAS finger | Clinic | 1.70(1.19~2.21) | 1.52(1.04~2.00) | 1.60(1.05~2.15) | 1.23(0.87~1.60) | 1.35(0.97~1.73) | 0.014 * (0.354) | 0.134(0.099) | 0.219(0.068) | |
| Lab | 2.03(1.45~2.62) | 2.02(1.38~2.65) | 1.98(1.39~2.58) | 1.37(0.69~2.05) | 1.57(1.01~2.12) | <0.001 *** (0.658) | ||||
| EMG Parameters | Group | Pre | Post | Paired t-Test | Wilcoxon Signed Rank Test | Independent t-Test | Mann–Whitney U Test |
|---|---|---|---|---|---|---|---|
| Mean Value (95% Confidence Interval) | p (Cohen’s d) | p (r) | p (Cohen’s d) | p (r) | |||
| Normalized EMG activation level | |||||||
| ECU-ED | Clinic | 0.13(0.10~0.15) | 0.10(0.08~0.13) | 0.032 * (0.31) | 0.143(0.21) | ||
| Lab | 0.10(0.07~0.12) | 0.08(0.06~0.10) | 0.228(0.37) | ||||
| APB | Clinic | 0.10(0.07~0.12) | 0.07(0.05~0.09) | 0.123(0.22) | 0.108(0.23) | ||
| Lab | 0.10(0.07~0.13) | 0.06(0.03~0.08) | 0.003 ** (0.43) | ||||
| TRI | Clinic | 0.10(0.07~0.13) | 0.07(0.05~0.09) | 0.209(0.18) | / | ||
| Lab | 0.07(0.04~0.10) | 0.06(0.03~0.08) | 0.525(0.16) | ||||
| BIC | Clinic | 0.08(0.06~0.10) | 0.07(0.05~0.09) | 0.306(0.25) | 0.314(0.29) | ||
| Lab | 0.08(0.06~0.09) | 0.06(0.05~0.07) | 0.086(0.44) | ||||
| FCR-FD | Clinic | 0.11(0.08~0.14) | 0.09(0.07~0.11) | 0.123(0.22) | / | ||
| Lab | 0.07(0.05~0.09) | 0.03(0.02~0.04) | <0.001 *** (0.99) | ||||
| Normalized co-contraction index | |||||||
| ECU-ED/APB | Clinic | 0.07(0.05~0.08) | 0.05(0.04~0.06) | 0.067(0.26) | 0.083(0.25) | ||
| Lab | 0.07(0.04~0.09) | 0.05(0.02~0.07) | 0.169(0.37) | ||||
| ECU-ED/FCR-FD | Clinic | 0.07(0.06~0.08) | 0.07(0.05~0.08) | 0.376(0.13) | <0.001 ### (0.55) | ||
| Lab | 0.05(0.04~0.07) | 0.03(0.02~0.04) | 0.001 *** (0.90) | ||||
| ECU-ED/BIC | Clinic | 0.07(0.05~0.08) | 0.05(0.04~0.06) | 0.059(0.27) | 0.026 # (0.67) | ||
| Lab | 0.05(0.04~0.06) | 0.04(0.03~0.04) | 0.019 * (0.71) | ||||
| ECU-ED/TRI | Clinic | 0.07(0.05~0.09) | 0.06(0.04~0.08) | 0.290(0.15) | / | ||
| Lab | 0.04(0.03~0.05) | 0.04(0.03~0.05) | 0.843(0.04) | ||||
| FCR-FD/APB | Clinic | 0.07(0.05~0.09) | 0.05(0.04~0.06) | 0.050 * (0.52) | <0.001 ### (0.53) | ||
| Lab | 0.06(0.04~0.08) | 0.03(0.02~0.03) | 0.001 *** (0.47) | ||||
| FCR-FD/BIC | Clinic | 0.05(0.04~0.06) | 0.05(0.04~0.06) | 0.248(0.28) | <0.001 ### (1.23) | ||
| Lab | 0.04(0.03~0.06) | 0.03(0.02~0.03) | 0.001 *** (0.85) | ||||
| FCR-FD/TRI | Clinic | 0.07(0.05~0.08) | 0.05(0.04~0.07) | 0.179(0.19) | / | ||
| Lab | 0.04(0.03~0.05) | 0.02(0.02~0.03) | 0.006 ** (0.64) | ||||
| APB/BIC | Clinic | 0.06(0.04~0.07) | 0.04(0.03~0.05) | 0.106(0.44) | 0.008 ## (0.80) | ||
| Lab | 0.06(0.04~0.07) | 0.03(0.02~0.04) | 0.003 ** (0.95) | ||||
| APB/TRI | Clinic | 0.05(0.04~0.06) | 0.05(0.03~0.06) | 0.440(0.11) | 0.099(0.24) | ||
| Lab | 0.06(0.03~0.08) | 0.04(0.02~0.07) | 0.351(0.23) | ||||
| BIC/TRI | Clinic | 0.07(0.05~0.09) | 0.05(0.04~0.07) | 0.219(0.18) | 0.536(0.09) | ||
| Lab | 0.06(0.05~0.08) | 0.04(0.03~0.05) | 0.031 * (0.58) | ||||
| Kinematic Parameters | Group | Pre | Post | Paired t-Test | Wilcoxon Signed Rank Test | Independent t-Test | Mann–Whitney U Test |
|---|---|---|---|---|---|---|---|
| Mean Value (95% Confidence Interval) | p (Cohen’s d) | p (r) | p (Cohen’s d) | p (r) | |||
| NMUs | Clinic | 30.02(24.33~35.71) | 23.63(19.50~27.77) | 0.040 * (0.30) | 0.054(0.28) | ||
| Lab | 26.83(22.57~31.09) | 19.25(15.28~23.22) | 0.003 ** (0.78) | ||||
| MTD | Clinic | 173.53(152.42~194.63) | 184.45(162.33~206.56) | 0.191(0.21) | <0.001 ### (1.11) | ||
| Lab | 144.79(122.52~167.06) | 124.69(101.22~148.16) | 0.036 * (0.37) | ||||
| Clinic Group | Lab Group | Independent t-Test | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean% ± SD% | Mean ± SD | Mean% ± SD% | p | Cohen’s d | |
| USE | ||||||
| Usefulness | 34.64 ± 14.86 | 61.85% ± 26.54% | 43.00 ± 8.71 | 76.79% ± 15.55% | 0.137 | 0.68 |
| Ease of Use | 48.09 ± 19.42 | 62.46% ± 25.22% | 58.00 ± 13.16 | 75.32% ± 17.09% | 0.192 | 0.59 |
| Ease of Learning | 19.55 ± 8.64 | 69.81% ± 30.86% | 22.70 ± 4.30 | 81.07% ± 15.34% | 0.300 | 0.46 |
| Satisfaction | 29.36 ± 14.75 | 59.93% ± 30.10% | 37.60 ± 8.00 | 76.73% ± 16.33% | 0.127 | 0.68 |
| IMI | ||||||
| Interest/Enjoyment | 4.80 ± 1.66 | 68.51% ± 23.73% | 5.70 ± 0.90 | 81.43% ± 12.91% | 0.143 | 0.67 |
| Perceived Competence | 4.52 ± 1.20 | 64.61% ± 17.09% | 4.93 ± 1.22 | 70.36% ± 17.42% | 0.455 | 0.33 |
| Effort/Importance | 5.18 ± 1.12 | 74.03% ± 16.06% | 5.83 ± 0.97 | 83.21% ± 13.89% | 0.179 | 0.61 |
| Pressure/Tension | 2.82 ± 1.18 | 40.26% ± 16.83% | 2.40 ± 1.02 | 34.29% ± 14.50% | 0.397 | 0.38 |
| Perceived Choice | 4.70 ± 0.65 | 67.21% ± 9.29% | 4.48 ± 1.13 | 63.93% ± 16.10% | 0.581 | 0.25 |
| Value/Usefulness | 4.75 ± 1.70 | 67.86% ± 24.28% | 6.28 ± 0.68 | 89.64% ± 9.74% | 0.016 * | 1.16 |
| Relatedness | 4.84 ± 1.41 | 69.16% ± 20.09% | 5.80 ± 0.79 | 82.86% ± 11.27% | 0.073 | 0.83 |
References
- Appleby, E.; Gill, S.T.; Hayes, L.K.; Walker, T.L.; Walsh, M.; Kumar, S. Effectiveness of telerehabilitation in the management of adults with stroke: A systematic review. PLoS ONE 2019, 14, e0225150. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Chan, S.Y.; Sum, M.W.C.; Wong, E.; Chui, Y.P.M. In patient stroke rehabilitation efficiency: Influence of organization of service delivery and staff numbers. BMC Health Serv. Res. 2008, 8, 86. [Google Scholar] [CrossRef]
- Chen, Y.; Abel, K.T.; Janecek, J.T.; Chen, Y.; Zheng, K.; Cramer, S.C. Home-based technologies for stroke rehabilitation: A systematic review. Int. J. Med. Inform. 2019, 123, 11–22. [Google Scholar] [CrossRef]
- Basteris, A.; Nijenhuis, S.M.; Stienen, A.H.; Buurke, J.H.; Prange, G.B.; Amirabdollahian, F. Training modalities in robot-mediated upper limb rehabilitation in stroke: A framework for classification based on a systematic review. J. NeuroEngi. Rehabil. 2014, 11, 111. [Google Scholar] [CrossRef]
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. NeuroEng. Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef]
- Yap, H.K.; Lim, J.H.; Nasrallah, F.; Yeow, C.-H. Design and preliminary feasibility study of a soft robotic glove for hand function assistance in stroke survivors. Front. Neurosci. 2017, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Thimabut, W.; Terachinda, P.; Kitisomprayoonkul, W. Effectiveness of a Soft Robotic Glove to Assist Hand Function in Stroke Patients: A Cross-Sectional Pilot Study. Rehabil. Res. Pract. 2022, 2022, 3738219. [Google Scholar] [CrossRef] [PubMed]
- Bessler, J.; Prange-Lasonder, G.B.; Schaake, L.; Saenz, J.F.; Bidard, C.; Fassi, I.; Valori, M.; Lassen, A.B.; Buurke, J.H. Safety Assessment of Rehabilitation Robots: A Review Identifying Safety Skills and Current Knowledge Gaps. Front. Robot. AI 2021, 8, 2878. [Google Scholar] [CrossRef]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef]
- Sierra Marín, S.D.; Gomez-Vargas, D.; Céspedes, N.; Múnera, M.; Roberti, F.; Barria, P.; Ramamoorthy, S.; Becker, M.; Carelli, R.; Cifuentes, C.A. Expectations and perceptions of healthcare professionals for robot deployment in hospital environments during the COVID-19 pandemic. Front. Robot. AI 2021, 8, 612746. [Google Scholar] [CrossRef]
- Standing, C.; Standing, S.; McDermott, M.L.; Gururajan, R.; Kiani Mavi, R. The paradoxes of telehealth: A review of the literature 2000–2015. Syst. Res. Behav. Sci. 2018, 35, 90–101. [Google Scholar] [CrossRef]
- Nam, C.; Rong, W.; Li, W.; Cheung, C.; Ngai, W.; Cheung, T.; Pang, M.; Li, L.; Hu, J.; Wai, H.; et al. An Exoneuromusculoskeleton for Self-Help Upper Limb Rehabilitation after Stroke. Soft Robot. 2022, 9, 14–35. [Google Scholar] [CrossRef]
- Hu, X.L.; Tong, K.-Y.; Song, R.; Zheng, X.J.; Leung, W.W. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabilit. Neural Repair 2009, 23, 837–846. [Google Scholar] [CrossRef]
- Rong, W.; Li, W.; Pang, M.; Hu, J.; Wei, X.; Yang, B.; Wai, H.; Zheng, X.; Hu, X. A Neuromuscular Electrical Stimulation (NMES) and robot hybrid system for multi-joint coordinated upper limb rehabilitation after stroke. J. NeuroEng. Rehabil. 2017, 14, 34. [Google Scholar] [CrossRef]
- Hu, X.-L.; Tong, R.K.-Y.; Ho, N.S.; Xue, J.-J.; Rong, W.; Li, L.S. Wrist rehabilitation assisted by an electromyography-driven neuromuscular electrical stimulation robot after stroke. Neurorehabilit. Neural Repair 2015, 29, 767–776. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Lin, K.-C.; Cheng, H.-J.; Wu, C.-Y.; Hsieh, Y.-W.; Chen, C.-K. Effects of combining robot-assisted therapy with neuromuscular electrical stimulation on motor impairment, motor and daily function, and quality of life in patients with chronic stroke: A double-blinded randomized controlled trial. J. NeuroEng. Rehabil. 2015, 12, 96. [Google Scholar] [CrossRef]
- Nam, C.; Zhang, B.; Chow, T.; Ye, F.; Huang, Y.; Guo, Z.; Li, W.; Rong, W.; Hu, X.; Poon, W. Home-based self-help telerehabilitation of the upper limb assisted by an electromyography-driven wrist/hand exoneuromusculoskeleton after stroke. J. NeuroEng. Rehabil. 2021, 18, 137. [Google Scholar] [CrossRef]
- Ashworth, B. Preliminary trial of carisoprodal in multiple sclerosis. Practitioner 1964, 192, 540–542. [Google Scholar]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Hensel, A.; Angermeyer, M.C.; Riedel-Heller, S.G. Measuring cognitive change in older adults: Reliable change indices for the Mini-Mental State Examination. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1298–1303. [Google Scholar] [CrossRef]
- Pike, S.; Lannin, N.A.; Cameron, L.; Palit, M.; Cusick, A. Chronic stroke survivors with upper limb spasticity: Linking experience to the ICF. Disabil. Rehabil. 2022, 44, 3925–3937. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-J.; Tong, K.-Y.; Hu, X.-L. The responsiveness and correlation between Fugl-Meyer Assessment, Motor Status Scale, and the Action Research Arm Test in chronic stroke with upper-extremity rehabilitation robotic training. Int. J. Rehabil. Res. 2011, 34, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lyle, R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef]
- Mackintosh, S. Functional Independence Measure. J. Physiother. 2009, 55, 65. [Google Scholar] [CrossRef]
- Wolf, S.L.; Lecraw, D.E.; Barton, L.A.; Jann, B.B. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp. Neurol. 1989, 104, 125–132. [Google Scholar] [CrossRef]
- Gregson, J.M.; Leathley, M.; Moore, A.P.; Sharma, A.K.; Smith, T.L.; Watkins, C.L. Reliability of the Tone Assessment Scale and the modified Ashworth scale as clinical tools for assessing poststroke spasticity. Arch. Phys. Med. Rehabil. 1999, 80, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tong, K.; Song, R.; Zheng, X.; Lui, K.; Leung, W.; Ng, S.; Au-Yeung, S. Quantitative evaluation of motor functional recovery process in chronic stroke patients during robot-assisted wrist training. J. Electromyogr. Kinesiol. 2009, 19, 639–650. [Google Scholar] [CrossRef]
- Hingtgen, B.; McGuire, J.R.; Wang, M.; Harris, G.F. An upper extremity kinematic model for evaluation of hemiparetic stroke. J. Biomech. 2006, 39, 681–688. [Google Scholar] [CrossRef]
- Murphy, M.A.; Willén, C.; Sunnerhagen, K.S. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabilit. Neural Repair 2011, 25, 71–80. [Google Scholar] [CrossRef]
- Lund, A.M. Measuring usability with the USE questionnaire. Usability Interface 2001, 8, 3–6. [Google Scholar]
- McAuley, E.; Duncan, T.; Tammen, V.V. Psychometric properties of the Intrinsic Motivation Inventory in a competitive sport setting: A confirmatory factor analysis. Res. Q. Exerc. Sport 1989, 60, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Vanmulken, D.A.; Spooren, A.I.; Bongers, H.M.; Seelen, H.A. Robot-assisted task-oriented upper extremity skill training in cervical spinal cord injury: A feasibility study. Spinal Cord 2015, 53, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, E.; Lefeber, N.; Willaert, W.; De Neef, F.; Bruyndonckx, L.; Spooren, A.; Michielsen, M.; Ramon, T.; Kerckhofs, E. Motivation, expectations, and usability of a driven gait orthosis in stroke patients and their therapists. Top. Stroke Rehabil. 2017, 24, 299–308. [Google Scholar] [CrossRef]
- Guillén-Climent, S.; Garzo, A.; Muñoz-Alcaraz, M.N.; Casado-Adam, P.; Arcas-Ruiz-Ruano, J.; Mejías-Ruiz, M.; Mayordomo-Riera, F.J. A usability study in patients with stroke using MERLIN, a robotic system based on serious games for upper limb rehabilitation in the home setting. J. NeuroEng. Rehabil. 2021, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B.; Chen, H.J. A comparative study of various tests for normality. J. Am. Stat. Assoc. 1968, 63, 1343–1372. [Google Scholar] [CrossRef]
- Qian, Q.; Nam, C.; Guo, Z.; Huang, Y.; Hu, X.; Ng, S.C.; Zheng, Y.; Poon, W. Distal versus proximal—An investigation on different supportive strategies by robots for upper limb rehabilitation after stroke: A randomized controlled trial. J. NeuroEng. Rehabil. 2019, 16, 64. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Tran, V.D.; Dario, P.; Posteraro, F. Wrist Robot-assisted Rehabilitation Treatment in Subacute and Chronic Stroke Patients: From Distal to Proximal Motor Recovery. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Izumi, S.-I. Maladaptive plasticity for motor recovery after stroke: Mechanisms and approaches. Neural Plast. 2012, 2012, 359728. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Hu, G.-C. Post-stroke Spasticity: A Review of Epidemiology, Pathophysiology, and Treatments. Int. J. Gerontol. 2018, 12, 280–284. [Google Scholar] [CrossRef]
- Valenzuela, M.; Varacallo, M. Anatomy, Shoulder and Upper Limb, Hand Lumbrical Muscles. In StatPearls; StatPearls Publishing Copyright © 2023; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gribble, P.L.; Mullin, L.I.; Cothros, N.; Mattar, A. Role of cocontraction in arm movement accuracy. J. Neurophysiol. 2003, 89, 2396–2405. [Google Scholar] [CrossRef]
- Rohrer, B.; Fasoli, S.; Krebs, H.I.; Hughes, R.; Volpe, B.; Frontera, W.R.; Stein, J.; Hogan, N. Movement smoothness changes during stroke recovery. J. Neurosci. 2002, 22, 8297–8304. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, J.W.; Carmichael, S.T.; Corbett, D.; Wittenberg, G.F. Getting neurorehabilitation right: What can be learned from animal models? Neurorehabilit. Neural Repair 2012, 26, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.; Mehta, S.; Pereira, S.; McIntyre, A.; Janzen, S.; Allen, L.; Lobo, L.; Viana, R. Time to Rethink Long-Term Rehabilitation Management of Stroke Patients. Top. Stroke Rehabil. 2012, 19, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, P. Upper limb motor impairment after stroke. Phys. Med. Rehabil. Clin. 2015, 26, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef]
- Stewart, M.A. Effective physician-patient communication and health outcomes: A review. CMAJ Can. Med. Assoc. J. 1995, 152, 1423. [Google Scholar]
- Almathami, H.K.Y.; Win, K.T.; Vlahu-Gjorgievska, E. Barriers and facilitators that influence telemedicine-based, real-time, online consultation at patients’ homes: Systematic literature review. J. Med. Internet Res. 2020, 22, e16407. [Google Scholar] [CrossRef]


| Clinic Group | Lab Group | |
|---|---|---|
| Participants source | Outpatients referred by rehabilitation doctors | Volunteers from local communities |
| Evaluation | ||
| Venue | Neurorehabilitation lab at PolyU | |
| Assessor | The same blinded assessor | |
| Mandatory courses | ||
| Venue | A treatment room, CRSSC | Neurorehabilitation lab at PolyU |
| Operator | Registered OT, OTA | Research staff |
| Supervision duration | The first 30 min/session | 60 min |
| Self-help training | ||
| Venue | Participants’ homes | |
| Training frequency | 3–5 sessions/week | |
| Session duration | 60–90 min/session | 60 min/session |
| Remote training supervisor and contact | Registered OT | Research staff |
| Remote availability | 9 am to 6 pm, Monday to Friday | Flexible whenever needed |
| Withdrawal | Yes, at any time point in the program | |
| System Maintenance | Referred by the CE to technicians of the research team | Technicians of the research team |
| Charge to patient | HKD375 at the CRSSC | Free |
| Characteristics | Clinic Group (n = 12) | Lab Group (n = 12) | p |
|---|---|---|---|
| Age a in years (mean ± SD) | 53.33 ± 11.47 | 58.42 ± 13.47 | 0.203 |
| Time since stroke a in years (mean ± SD) | 3.32 ± 2.22 | 12.42 ± 10.88 | 0.003 * |
| Gender b (male/female) | 7/5 | 6/6 | 0.682 |
| Hemiplegic side c (left/right) | 6/6 | 9/3 | 0.400 |
| Stroke type c (ischemic/hemorrhagic) | 3/9 | 6/6 | 0.400 |
| Parameters | Clinic Group (n = 12) | Lab Group (n = 12) |
|---|---|---|
| Frequency (session/week) | 3.33 ± 0.47 | 3.75 ± 0.72 |
| Duration (min/session) | 91.30 ± 8.50 | 62.80 ± 1.93 |
| Complete movement cycles (cycle/session) | 184.23 ± 30.46 | 115.20 ± 9.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qing, W.; Nam, C.-Y.; Shum, H.M.-H.; Chan, M.K.-L.; Yu, K.-P.; Ng, S.S.-W.; Yang, B.; Hu, X. The Translation of Mobile-Exoneuromusculoskeleton-Assisted Wrist–Hand Poststroke Telerehabilitation from Laboratory to Clinical Service. Bioengineering 2023, 10, 976. https://doi.org/10.3390/bioengineering10080976
Qing W, Nam C-Y, Shum HM-H, Chan MK-L, Yu K-P, Ng SS-W, Yang B, Hu X. The Translation of Mobile-Exoneuromusculoskeleton-Assisted Wrist–Hand Poststroke Telerehabilitation from Laboratory to Clinical Service. Bioengineering. 2023; 10(8):976. https://doi.org/10.3390/bioengineering10080976
Chicago/Turabian StyleQing, Wanyi, Ching-Yi Nam, Harvey Man-Hok Shum, Marko Ka-Leung Chan, King-Pong Yu, Serena Sin-Wah Ng, Bibo Yang, and Xiaoling Hu. 2023. "The Translation of Mobile-Exoneuromusculoskeleton-Assisted Wrist–Hand Poststroke Telerehabilitation from Laboratory to Clinical Service" Bioengineering 10, no. 8: 976. https://doi.org/10.3390/bioengineering10080976
APA StyleQing, W., Nam, C.-Y., Shum, H. M.-H., Chan, M. K.-L., Yu, K.-P., Ng, S. S.-W., Yang, B., & Hu, X. (2023). The Translation of Mobile-Exoneuromusculoskeleton-Assisted Wrist–Hand Poststroke Telerehabilitation from Laboratory to Clinical Service. Bioengineering, 10(8), 976. https://doi.org/10.3390/bioengineering10080976








