The Progress in Bioprinting and Its Potential Impact on Health-Related Quality of Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Analysis
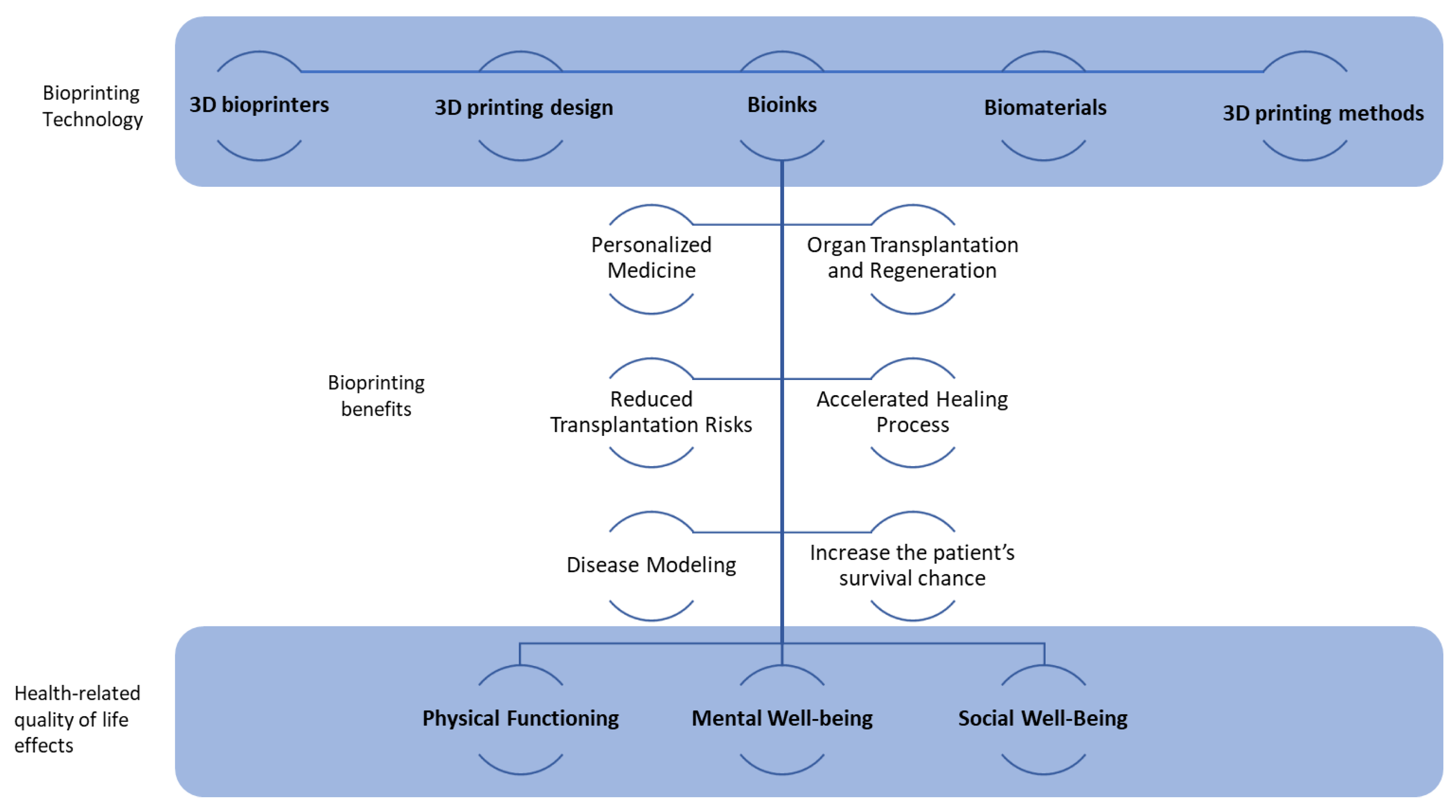
3. Results
4. Discussion
4.1. Personalised Part Production and Reducing Rejection Risks after Transplantation
4.2. Increase the Patient’s Chance of Survival
4.3. Reducing Patient Waiting Time
4.4. Homocellular Tissue Model Generation and Precise Fabrication Process with Accurate Specifications
4.5. Improves Skin Construction Speed and Save Patient Life
4.6. Eliminating/Reducing the Need for Organs Donor
4.7. Expanding Treatment/Transplantation Possibilities
4.8. Creating More Functional Implants
4.9. Beneficial for Cancer Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Park, S.A.; Kim, W.D.; Ha, T.; Xin, Y.Z.; Lee, J.; Lee, D. Current Advances in 3D Bioprinting Technology and Its Applications for Tissue Engineering. Polymers 2020, 12, 2958. [Google Scholar] [CrossRef]
- Agarwal, S.; Saha, S.; Balla, V.K.; Pal, A.; Barui, A.; Bodhak, S. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration—A Review. Front. Mech. Eng. 2020, 6, 589171. [Google Scholar] [CrossRef]
- Shukla, A.K.; Gao, G.; Kim, B.S. Applications of 3D Bioprinting Technology in Induced Pluripotent Stem Cells-Based Tissue Engineering. Micromachines 2022, 13, 155. [Google Scholar] [CrossRef]
- Crook, J.M.; Tomaskovic-Crook, E. Bioprinting 3D Human Induced Pluripotent Stem Cell Constructs for Multilineage Tissue Engineering and Modeling. Methods Mol. Biol. 2020, 2140, 251–258. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Mistewicz, K.; Nowacki, B.; In-Na, P.; Krushynska, A.; Mishra, Y.K.; Kim, H.J. A focused review on three-dimensional bioprinting technology for artificial organ fabrication. Biomater. Sci. 2022, 10, 5054–5080. [Google Scholar] [CrossRef]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef]
- Veeman, D.; Sai, M.S.; Sureshkumar, P.; Jagadeesha, T.; Natrayan, L.; Ravichandran, M.; Mammo, W.D. Additive Manufacturing of Biopolymers for Tissue Engineering and Regenerative Medicine: An Overview, Potential Applications, Advancements, and Trends. Int. J. Polym. Sci. 2021, 2021, 4907027. [Google Scholar] [CrossRef]
- Bozkurt, Y.; Karayel, E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J. Mater. Res. Technol. 2021, 14, 1430–1450. [Google Scholar] [CrossRef]
- Mills, P.A.S.; Mills, D.K. Reduced Supply in the Organ Donor Market and How 3D Printing Can Address This Shortage: A Critical Inquiry into the Collateral Effects of Driverless Cars. Appl. Sci. 2020, 10, 6400. [Google Scholar] [CrossRef]
- Karzyński, K.; Kosowska, K.; Ambrożkiewicz, F.; Berman, A.; Cichoń, J.; Klak, M.; Serwańska-Świętek, M.; Wszoła, M. Use of 3D bioprinting in biomedical engineering for clinical application. Med. Stud./Stud. Med. 2018, 34, 93–97. [Google Scholar] [CrossRef]
- Mermin-Bunnell, A. Integrating Bioprinted Organs into Our Healthcare System. Intersect Stanf. J. Sci. Technol. Soc. 2020, 14. [Google Scholar]
- Arias, E.; Huang, Y.H.; Zhao, L.; Seelaus, R.; Patel, P.; Cohen, M. Virtual Surgical Planning and Three-Dimensional Printed Guide for Soft Tissue Correction in Facial Asymmetry. J. Craniofacial Surg. 2019, 30, 846–850. [Google Scholar] [CrossRef]
- Zoabi, A.; Redenski, I.; Oren, D.; Kasem, A.; Zigron, A.; Daoud, S.; Moskovich, L.; Kablan, F.; Srouji, S. 3D Printing and Virtual Surgical Planning in Oral and Maxillofacial Surgery. J. Clin. Med. 2022, 11, 2385. [Google Scholar] [CrossRef]
- Altinörs, M.N.; Altinörs, M.N. Future Prospects of Organ Transplantation. In Organ Donation and Transplantation; Intechopen: London, UK, 2020; pp. 57–74. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Chohan, J.S. The role of additive manufacturing for biomedical applications: A critical review. J. Manuf. Process. 2021, 64, 828–850. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Acosta-Velez, G.F.; Wu, B.M. 3D Pharming: Direct Printing of Personalized Pharmaceutical Tablets. Polym. Sci. 2016, 2, 11. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- Ahmad, J.; Garg, A.; Mustafa, G.; Mohammed, A.A.; Ahmad, M.Z. 3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope. Pharmaceutics 2023, 15, 1448. [Google Scholar] [CrossRef]
- Khairuzzaman, A. Regulatory perspectives on 3D printing in pharmaceuticals. In AAPS Advances in the Pharmaceutical Sciences Series; Springer: Berlin/Heidelberg, Germany, 2018; Volume 31, pp. 215–236. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2021, 14, 1. [Google Scholar] [CrossRef]
- Zhou, J.; Vijayavenkataraman, S. 3D-printable conductive materials for tissue engineering and biomedical applications. Bioprinting 2021, 24, e00166. [Google Scholar] [CrossRef]
- Jonczyk, R.; Kurth, T.; Lavrentieva, A.; Walter, J.-G.; Scheper, T.; Stahl, F. Living Cell Microarrays: An Overview of Concepts. Microarrays 2016, 5, 11. [Google Scholar] [CrossRef]
- Sobek, J.; Bartscherer, K.; Jacob, A.; Hoheisel, J.; Angenendt, P. Microarray Technology as a Universal Tool for High-Throughput Analysis of Biological Systems. Comb. Chem. High Throughput Screen. 2006, 9, 365–380. [Google Scholar] [CrossRef]
- Jain, K.K. Innovative Diagnostic Technologies and Their Significance for Personalized Medicine. Mol. Diagn. Ther. 2010, 14, 141–147. [Google Scholar] [CrossRef]
- Brambilla, D.; Chiari, M.; Gori, A.; Cretich, M. Towards precision medicine: The role and potential of protein and peptide microarrays. Analyst 2019, 144, 5353–5367. [Google Scholar] [CrossRef]
- Yu, X.; Schneiderhan-Marra, N.; Joos, T.O. Protein Microarrays for Personalized Medicine. Clin. Chem. 2010, 56, 376. [Google Scholar] [CrossRef]
- Li, S.; Song, G.; Bai, Y.; Song, N.; Zhao, J.; Liu, J.; Hu, C. Applications of Protein Microarrays in Biomarker Discovery for Autoimmune Diseases. Front. Immunol. 2021, 12, 645632. [Google Scholar] [CrossRef]
- Shrestha, S.; Lekkala, V.K.R.; Acharya, P.; Siddhpura, D.; Lee, M.Y. Recent advances in microarray 3D bioprinting for high-throughput spheroid and tissue culture and analysis. Essays Biochem. 2021, 65, 481–489. [Google Scholar] [CrossRef]
- Mikhailovich, V.; Gryadunov, D.; Kolchinsky, A.; Makarov, A.A.; Zasedatelev, A. DNA microarrays in the clinic: Infectious diseases. BioEssays 2008, 30, 673–682. [Google Scholar] [CrossRef]
- Gorlov, I.P.; Gallick, G.E.; Gorlova, O.Y.; Amos, C.; Logothetis, C.J. GWAS Meets Microarray: Are the Results of Genome-Wide Association Studies and Gene-Expression Profiling Consistent? Prostate Cancer as an Example. PLoS ONE 2009, 4, e6511. [Google Scholar] [CrossRef]
- Lee, M.Y. Overview of microarray bioprinting technology. In Microarray Bioprinting Technology: Fundamentals and Practices; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–17. [Google Scholar] [CrossRef]
- Daub, M.; Zengerle, R. Bioprinting on Chip. In Encyclopedia of Microfluidics and Nanofluidics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–17. [Google Scholar] [CrossRef]
- Jovic, T.H.; Combellack, E.J.; Jessop, Z.M.; Whitaker, I.S. 3D Bioprinting and the Future of Surgery. Front. Surg. 2020, 7, 609836. [Google Scholar] [CrossRef]
- Spencer, A.R.; Shirzaei Sani, E.; Soucy, J.R.; Corbet, C.C.; Primbetova, A.; Koppes, R.A.; Annabi, N. Bioprinting of a Cell-Laden Conductive Hydrogel Composite. ACS Appl. Mater. Interfaces 2019, 11, 30518–30533. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Datta, S.; Barman, A. Future of Bioprinting in Healthcare: A Review. Int. J. Health Technol. Innov. 2023, 2, 5–15. [Google Scholar] [CrossRef]
- The WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Fayers, P.M.; Machin, D. Quality of Life The assessment, Analysis and Reporting of Patient-Reported Outcomes. John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9780849358081. [Google Scholar]
- Zhang, H.; Zheng, S.; Liu, Q.; Wei, P.; Gu, F.; Yu, J.; Wang, Z.; Li, J.; Xu, Y.; Tang, C.; et al. 3D-Printed Antibiotic-Loaded Bone Cement Spacers as Adjunctive Therapy for Hip Infections after Arthroplasty: A Clinical Assessment. Available online: https://ssrn.com/abstract=4491644 (accessed on 29 June 2023).
- Castrisos, G.; Gonzalez Matheus, I.; Sparks, D.; Lowe, M.; Ward, N.; Sehu, M.; Wille, M.L.; Phua, Y.; Medeiros Savi, F.; Hutmacher, D.; et al. Regenerative matching axial vascularisation of absorbable 3D-printed scaffold for large bone defects: A first in human series. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2108–2118. [Google Scholar] [CrossRef]
- Daly, L.K.; de Looze, J.W.M.; Forrestal, D.P.; Wagels, M.; Spurgin, A.L.; Hoey, J.D.; Holt, M.R.; Vasani, S.; Desselle, M.R. 3D printing for respiratory physiotherapy: A tale of three disciplines. Ann. 3D Print. Med. 2023, 9, 100096. [Google Scholar] [CrossRef]
- Oud, T.; Tuijtelaars, J.; Bogaards, H.; Nollet, F.; Brehm, M.A. Preliminary effectiveness of 3D-printed orthoses in chronic hand conditions: Study protocol for a non-randomised interventional feasibility study. BMJ Open 2023, 13, e069424. [Google Scholar] [CrossRef]
- Filipov, I.; Chirila, L.; Bolognesi, F.; Cristache, C.M. Buccally or Lingually Tilted Implants in the Lateral Atrophic Mandible: A Three-Year Follow-Up Study Focused on Neurosensory Impairment, Soft-Tissue-Related Impaction and Quality of Life Improvement. Medicina 2023, 59, 697. [Google Scholar] [CrossRef]
- Pamias-Romero, J.; Saez-Barba, M.; De-Pablo-García-Cuenca, A.; Vaquero-Martínez, P.; Masnou-Pratdesaba, J.; Bescós-Atín, C. Quality of Life after Mandibular Reconstruction Using Free Fibula Flap and Customized Plates: A Case Series and Comparison with the Literature. Cancers 2023, 15, 2582. [Google Scholar] [CrossRef]
- Panja, N.; Maji, S.; Choudhuri, S.; Ali, K.A.; Hossain, C.M. 3D Bioprinting of Human Hollow Organs. AAPS PharmSciTech 2022, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Melchels, F.P.W.; Ferreira, M.J.S.; Moxon, S.R.; Potjewyd, G.; Dargaville, T.R.; Kimber, S.J.; Domingos, M. Emulating Human Tissues and Organs: A Bioprinting Perspective Toward Personalized Medicine. Chem. Rev. 2020, 120, 11093–11139. [Google Scholar] [CrossRef]
- Reddy, V.S.; Ramasubramanian, B.; Telrandhe, V.M.; Ramakrishna, S. Contemporary standpoint and future of 3D bioprinting in tissue/organs printing. Curr. Opin. Biomed. Eng. 2023, 27, 100461. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pei, X.; Jiang, L.; Hu, C.; Sun, J.; Xing, F.; Zhou, C.; Fan, Y.; Zhang, X. Bionic design and 3D printing of porous titanium alloy scaffolds for bone tissue repair. Compos. Part B Eng. 2019, 162, 154–161. [Google Scholar] [CrossRef]
- Al-Dulimi, Z.; Wallis, M.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D printing technology as innovative solutions for biomedical applications. Drug Discov. Today 2021, 26, 360–383. [Google Scholar] [CrossRef]
- Bartolo, P.; Malshe, A.; Ferraris, E.; Koc, B. 3D bioprinting: Materials, processes, and applications. CIRP Ann. 2022, 71, 577–597. [Google Scholar] [CrossRef]
- Ghahri, T.; Salehi, Z.; Aghajanpour, S.; Eslaminejad, M.B.; Kalantari, N.; Akrami, M.; Dinarvand, R.; Jang, H.L.; Esfandyari-Manesh, M. Development of osteon-like scaffold-cell construct by quadruple coaxial extrusion-based 3D bioprinting of nanocomposite hydrogel. Biomater. Adv. 2023, 145, 213254. [Google Scholar] [CrossRef]
- Persaud, A.; Maus, A.; Strait, L.; Zhu, D. 3D Bioprinting with Live Cells. Eng. Regen. 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Tan, G.; Ioannou, N.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D printing in Ophthalmology: From medical implants to personalised medicine. Int. J. Pharm. 2022, 625, 122094. [Google Scholar] [CrossRef]
- Lotz, O.; McKenzie, D.R.; Bilek, M.M.; Akhavan, B. Biofunctionalized 3D printed structures for biomedical applications: A critical review of recent advances and future prospects. Prog. Mater. Sci. 2023, 137, 101124. [Google Scholar] [CrossRef]
- Campioni, I.; Scarano, A.; Mangione, F.; Fidanza, A.; Perinetti, T.; Logroscino, G.; Saracco, M. 3D Printing Applications in Orthopaedic Surgery: Clinical Experience and Opportunities. Appl. Sci. 2022, 12, 3245. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Canzi, P.; Marconi, S.; Montasser, M.A.; Bressani, D.; Gandini, P.; Sfondrini, M.F. Properties of CAD/CAM 3D Printing Dental Materials and Their Clinical Applications in Orthodontics: Where Are We Now? Appl. Sci. 2022, 12, 551. [Google Scholar] [CrossRef]
- Pan, R.L.; Martyniak, K.; Karimzadeh, M.; Gelikman, D.G.; DeVries, J.; Sutter, K.; Coathup, M.; Razavi, M.; Sawh-Martinez, R.; Kean, T.J. Systematic review on the application of 3D-bioprinting technology in orthoregeneration: Current achievements and open challenges. J. Exp. Orthop. 2022, 9, 95. [Google Scholar] [CrossRef]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 2022, 287, 287. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, L.; Ma, L.; Luo, Y.; Yang, H.; Cui, Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794. [Google Scholar] [CrossRef]
- Sarmah, J.K.; Dutta, A.; Sarmah, S.; Ankaleswar, B. Guar gum nanoparticles: A new paradigm in biomedical applications. In Nanoparticles: Preparation and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 119–144. [Google Scholar] [CrossRef]
- An, C.; Zhou, R.; Zhang, H.; Zhang, Y.; Liu, W.; Liu, J.; Bao, B.; Sun, K.; Ren, C.; Zhang, Y.; et al. Microfluidic-templated cell-laden microgels fabricated using phototriggered imine-crosslinking as injectable and adaptable granular gels for bone regeneration. Acta Biomater. 2023, 157, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Jahagirdar, V.; Gudapati, S.; Mouchli, M. Three-dimensional visualization and virtual reality simulation role in hepatic surgery: Further research warranted. World J. Gastrointest. Surg. 2022, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Luo, Y.J.; Wang, J.H.; Xu, W.Z.; Shi, Z.; Fu, J.Z.; Shu, Q. Patient-specific three-dimensional printed heart models benefit preoperative planning for complex congenital heart disease. World J. Pediatr. WJP 2019, 15, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, V.V.; Zaytsev, V.V.; Kupatadze, D.D.; Krivoshchekov, E.V.; Loboda, O.S.; Lezhnev, A.A. 3D printing technology in planning of surgical strategy for complex congenital heart defects. Kardiol. Serdechno-Sosud. Khirurgiya 2020, 13, 294–298. [Google Scholar] [CrossRef]
- Birla, R.K.; Williams, S.K. 3D bioprinting and its potential impact on cardiac failure treatment: Anindustry perspective. APL Bioeng. 2020, 4, 10903. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.; Ishtiaq, I.; Aslam, K.; Ali, U.; Mehak, S.; Khan, S.; Sajjad, S.; Babar, M. 3D bioprinting–a step towards heart tissue regeneration. J. Appl. Biotechnol. Bioeng. 2021, 8, 1–4. [Google Scholar] [CrossRef]
- Qasim, M.; Haq, F.; Kang, M.H.; Kim, J.H. 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int. J. Nanomed. 2019, 14, 1311–1333. [Google Scholar] [CrossRef]
- Roche, C.D.; Brereton, R.J.L.; Ashton, A.W.; Jackson, C.; Gentile, C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur. J. Cardio-Thorac. Surgery Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, 500–510. [Google Scholar] [CrossRef]
- Desanlis, A.; Albouy, M.; Rousselle, P.; Thépot, A.; Dos Santos, M.; Auxenfans, C.; Marquette, C. Validation of an implantable bioink using mechanical extraction of human skin cells: First steps to a 3D bioprinting treatment of deep second degree burn. J. Tissue Eng. Regen. Med. 2021, 15, 37–48. [Google Scholar] [CrossRef]
- Sedighi, M.; Shrestha, N.; Mahmoudi, Z.; Khademi, Z.; Ghasempour, A.; Dehghan, H.; Talebi, S.F.; Toolabi, M.; Préat, V.; Chen, B.; et al. Multifunctional Self-Assembled Peptide Hydrogels for Biomedical Applications. Polymers 2023, 15, 1160. [Google Scholar] [CrossRef]
- Kamolz, L.P.; Griffith, M.; Finnerty, C.; Kasper, C. Skin Regeneration, Repair, and Reconstruction. BioMed Res. Int. 2015, 2015, 1. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Esworthy, T.; Miao, S.; Zhou, X.; Lee, S.J.; Fisher, J.P.; Zhang, L.G. Recent Advances in 3D Printing: Vascular Network for Tissue and Organ Regeneration. Transl. Res. J. Lab. Clin. Med. 2019, 211, 46. [Google Scholar] [CrossRef]
- Gonzalez, J.; Garijo, I.; Sanchez, A. Organ Trafficking and Migration: A Bibliometric Analysis of an Untold Story. Int. J. Environ. Res. Public Health 2020, 17, 3204. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Nam, H.; Jang, J.; Lee, S.J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Barceló, X.; Eichholz, K.F.; Garcia, O.; Kelly, D.J. Tuning the Degradation Rate of Alginate-Based Bioinks for Bioprinting Functional Cartilage Tissue. Biomedicines 2022, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Leberfinger, A.N.; Dinda, S.; Wu, Y.; Koduru, S.V.; Ozbolat, V.; Ravnic, D.J.; Ozbolat, I.T. Bioprinting functional tissues. Acta Biomater. 2019, 95, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Guvendiren, M. Complex 3D bioprinting methods. APL Bioeng. 2021, 5, 11508. [Google Scholar] [CrossRef]
- Xu, H.Q.; Liu, J.C.; Zhang, Z.Y.; Xu, C.X. A review on cell damage, viability, and functionality during 3D bioprinting. Mil. Med. Res. 2022, 9, 70. [Google Scholar] [CrossRef]
- He, J.; Mao, M.; Li, X.; Chua, C.K. Bioprinting of 3D Functional Tissue Constructs. Int. J. Bioprint. 2021, 7, 395. [Google Scholar] [CrossRef]
- Ramos, T.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication-A Year in Review. Tissue Eng. Part C Methods 2020, 26, 91–106. [Google Scholar] [CrossRef]
- Condino, S.; Piazza, R.; Carbone, M.; Bath, J.; Troisi, N.; Ferrari, M.; Berchiolli, R. Bioengineering, augmented reality, and robotic surgery in vascular surgery: A literature review. Front. Surg. 2022, 9, 966118. [Google Scholar] [CrossRef]
- Agarwal, P.; Arora, G.; Panwar, A.; Mathur, V.; Srinivasan, V.; Pandita, D.; Vasanthan, K.S. Diverse Applications of Three-Dimensional Printing in Biomedical Engineering: A Review. 3D Print. Addit. Manuf. 2023. ahead of print. [Google Scholar] [CrossRef]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020-2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Rosemann, T.; Laux, G.; Szecsenyi, J. Osteoarthritis: Quality of life, comorbidities, medication and health service utilization assessed in a large sample of primary care patients. J. Orthop. Surg. Res. 2007, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, J.; Rollo, J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. Biomaterials and scaffold design: Key to tissue-engineering cartilage. Biotechnol. Appl. Biochem. 2007, 46, 73. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Kovalsky, D.; Meyer, S.C.; Chowdhary, A.; Lockstadt, H.; Techy, F.; Langel, C.; Limoni, R.; Yuan, P.S.; Kranenburg, A.; et al. Prospective Trial of Sacroiliac Joint Fusion Using 3D-Printed Triangular Titanium Implants. Med. Devices 2020, 13, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Rosca, A.M.; Tutuianu, R.; Titorencu, I. Advances in Skin Regeneration and Reconstruction. Front. Stem Cell Regen. Med. Res. 2020, 9, 143–187. [Google Scholar] [CrossRef]
- Varkey, M.; Visscher, D.O.; Van Zuijlen, P.P.M.; Atala, A.; Yoo, J.J. Skin bioprinting: The future of burn wound reconstruction? Burn. Trauma 2019, 7, s41038-019-0142-7. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Yakimova, M.S.; Aboushanab, S.A.S.; Ivantsova, M.N.; Kamel, M. Overview on 3D bioprinting technology: Potentials and current challenges. AIP Conf. Proc. 2020, 2313, 080034. [Google Scholar] [CrossRef]
- Menon, A.; Vijayavenkataraman, S. Novel vision restoration techniques: 3D bioprinting, gene and stem cell therapy, optogenetics, and the bionic eye. Artif. Organs 2022, 46, 1463–1474. [Google Scholar] [CrossRef]
- Wu, Y.; Su, H.; Li, M.; Xing, H. Digital light processing-based multi-material bioprinting: Processes, applications, and perspectives. J. Biomed. Mater. Res. Part A 2023, 111, 527–542. [Google Scholar] [CrossRef]
- Farhat, W.; Chatelain, F.; Marret, A.; Faivre, L.; Arakelian, L.; Cattan, P.; Fuchs, A. Trends in 3D bioprinting for esophageal tissue repair and reconstruction. Biomaterials 2021, 267, 120465. [Google Scholar] [CrossRef]
- Takeda, Y.; Lau, J.; Nouh, H.; Hirayama, H. A 3D printing replication technique for fabricating digital dentures. J. Prosthet. Dent. 2020, 124, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Dong, R.; Jin, H.T.; Tong, P.J.; Xiao, L.W. Application status of 3D printing patient-specific instrumentation in total knee arthroplasty. Zhongguo Gu Shang China J. Orthop. Traumatol. 2019, 32, 582–586. [Google Scholar] [CrossRef]
- Sun, Z. Patient-specific three-dimensional printing in cardiovascular disease. Australas. Med. J. 2020, 13, 136–141. [Google Scholar] [CrossRef]
- Bhuskute, H.; Shende, P.; Prabhakar, B. 3D Printed Personalized Medicine for Cancer: Applications for Betterment of Diagnosis, Prognosis and Treatment. AAPS PharmSciTech 2021, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Mercader, C.; Vilaseca, A.; Moreno, J.L.; López, A.; Sebastià, M.C.; Nicolau, C.; Ribal, M.J.; Peri, L.; Costa, M.; Alcaraz, A. Role of the three-dimensional printing technology incomplex laparoscopic renal surgery: A renal tumor in a horseshoe kidney. Int. Braz J. Urol Off. J. Braz. Soc. Urol. 2019, 45, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ting, Y.H.; Youssef, S.H.; Song, Y.; Garg, S. Three-dimensional printing for cancer applications: Research landscape and technologies. Pharmaceuticals 2021, 14, 787. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, I.; Ariz, A.; Bharti, D.; Haleem, A.; Javaid, M.; Bahl, S. 3D Printing Technology and its Significant Applications in the Context of Healthcare Education. J. Ind. Integr. Manag. 2023, 8, 113–130. [Google Scholar] [CrossRef]
- Schulze, M.; Gosheger, G.; Bockholt, S.; De Vaal, M.; Budny, T.; Tönnemann, M.; Pützler, J.; Bövingloh, A.S.; Rischen, R.; Hofbauer, V.; et al. Complex Bone Tumors of the Trunk—The Role of 3D Printing and Navigation in Tumor Orthopedics: A Case Series and Review of the Literature. J. Pers. Med. 2021, 11, 517. [Google Scholar] [CrossRef]
- Pavan Kalyan, B.; Kumar, L. 3D Printing: Applications in Tissue Engineering, Medical Devices, and Drug Delivery. AAPS PharmSciTech 2022, 23, 92. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Vaishya, R. 3D printing applications for the treatment of cancer. Clin. Epidemiol. Glob. Health 2020, 8, 1072–1076. [Google Scholar] [CrossRef]
- Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery. Pharmaceutics 2020, 12, 1077. [Google Scholar] [CrossRef]
- Sánchez Rodríguez, D.A.; Ramos-Murillo, A.I.; Godoy-Silva, R.D. Tissue engineering, 3D-Bioprinting, morphogenesis modelling and simulation of biostructures: Relevance, underpinning biological principles and future trends. Bioprinting 2021, 24, e00171. [Google Scholar] [CrossRef]
- Ngadimin, C.; Chew, K.Y.; Kong, T.Y. Taking the guesswork out of mandibular reconstruction: The role of computer-guided planning and 3D-printing of patient-specific implants. In British Journal of Surgery; Wiley: Hoboken, NJ, USA, 2019; Volume 106. [Google Scholar]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef]
- Weheida, S.M.; Lotfy, A.; El-Aziz, A.; Ahmed, S.; Elgwad, A.-E.A.; Kamal, A.I.; Elsherbiny, M.M. Effect of Protocol of Care on Self Care Practice for Patients Post Kidney Transplantation Operation. Tanta Sci. Nurs. J. 2023, 2314, 49. [Google Scholar] [CrossRef]
- Singh, G.; Pandey, P.M. Role of Imaging Data in Additive Manufacturing for Biomedical Applications. In Materials Horizons: From Nature to Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2020; pp. 69–94. [Google Scholar] [CrossRef]
- Gao, X.; Chen, X.; Zhang, G.; Yu, Z.; Wu, C.; Lin, H.; Chen, X. A versatile three-dimensional printing-technology-based digital design and experimental study for acetabular fracture surgery. Int. J. Clin. Exp. Med. 2020, 13, 3053–3060. [Google Scholar]
- Horas, K.; Hoffmann, R.; Faulenbach, M.; Heinz, S.M.; Langheinrich, A.; Schweigkofler, U. Advances in the Preoperative Planning of Revision Trauma Surgery Using 3D Printing Technology. J. Orthop. Trauma 2020, 34, E181–E186. [Google Scholar] [CrossRef]
- Alemayehu, D.G.; Zhang, Z.; Tahir, E.; Gateau, D.; Zhang, D.F.; Ma, X. Preoperative Planning Using 3D Printing Technology in Orthopedic Surgery. BioMed Res. Int. 2021, 2021, 7940242. [Google Scholar] [CrossRef]
- Tooulias, A.; Tsoulfas, G.; Papadopoulos, V.; Alexiou, M.; Karolos, I.-A.; Pikridas, C.; Tsioukas, V. Assisting Difficult Liver Operations Using 3D Printed Models. Livers 2021, 1, 138–146. [Google Scholar] [CrossRef]
- Chae, S.; Cho, D.-W. Biomaterial-based 3D bioprinting strategy for orthopedic tissue engineering. Acta Biomater. 2023, 156, 4–20. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Huang, J.F.; Lin, J.L.; Yang, D.J.; Xu, T.Z.; Chen, D.; Zan, X.; Wu, A.M. 3D bioprinting in orthopedics translational research. J. Biomater. Sci. Polym. Ed. 2019, 30, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Fortunato, G.M.; Hann, S.Y.; Ayan, B.; Vajanthri, K.Y.; Presutti, D.; Cui, H.; Chan, A.H.P.; Costantini, M.; Onesto, V.; et al. Recent advances in bioprinting technologies for engineering cardiac tissue. Mater. Sci. Eng. C 2021, 124, 11205. [Google Scholar] [CrossRef]
- Beer, N.; Kaae, S.; Genina, N.; Sporrong, S.K.; Alves, T.L.; Hoebert, J.; De Bruin, M.L.; Hegger, I. Magistral Compounding with 3D Printing: A Promising Way to Achieve Personalized Medicine. Ther. Innov. Regul. Sci. 2023, 57, 26. [Google Scholar] [CrossRef]
- Birbara, N.S.; Otton, J.M.; Pather, N. 3D Modelling and Printing Technology to Produce Patient-Specific 3D Models. Heart Lung Circ. 2019, 28, 302–313. [Google Scholar] [CrossRef]
- Weidert, S.; Andress, S.; Suero, E.; Becker, C.; Hartel, M.; Behle, M.; Willy, C. 3D printing in orthopedic and trauma surgery education and training: Possibilities and fields of application. Unfallchirurg 2019, 122, 444–451. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Bezstarosti, H.; Metsemakers, W.J.; van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; McNally, M.A.; Marais, L.C.; Verhofstad, M.H.J. Management of critical-sized bone defects in the treatment of fracture-related infection: A systematic review and pooled analysis. Arch. Orthop. Trauma Surg. 2021, 141, 1215–1230. [Google Scholar] [CrossRef]
- Lu, A.S.; Baranowski, T.; Hong, S.L.; Buday, R.; Thompson, D.; Beltran, A.; Dadabhoy, H.R.; Chen, T.-A. The Narrative Impact of Active Video Games on Physical Activity Among Children: A Feasibility Study. J. Med. Internet Res. 2016, 18, e272. [Google Scholar] [CrossRef]
- Lee, S.J.; Yan, D.; Zhou, X.; Cui, H.; Esworthy, T.; Hann, S.Y.; Keidar, M.; Zhang, L.G. Integrating cold atmospheric plasma with 3D printed bioactive nanocomposite scaffold for cartilage regeneration. Mater. Sci. Eng. C 2020, 111, 110844. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zhou, D.; Ma, X.; Yang, S.; Xu, T. Mechanical engineering of hair follicle regeneration by in situ bioprinting. Biomater. Adv. 2022, 142, 213127. [Google Scholar] [CrossRef]
- Fiorio, G. The Ontology of Vision. The Invisible, Consciousness of LivingMatter. Front. Psychol. 2016, 7, 89. [Google Scholar] [CrossRef][Green Version]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Steinmetz, J.D.; Saylan, M.; Mersha, A.M.; Weldemariam, A.H.; Wondmeneh, T.G.; Sreeramareddy, C.T.; Pinheiro, M.; Yaseri, M.; Yu, C.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Stevelink, S.A.M.; Malcolm, E.M.; Fear, N.T. Visual impairment, coping strategies and impact on daily life: A qualitative study among working-age UK ex-service personnel Health behavior, health promotion and society. BMC Public Health 2015, 15, 1118. [Google Scholar] [CrossRef]
- Jia, S.; Yang, J.; Lau, A.D.-S.; Chen, F.; Bu, Y.; Cai, E.; Wang, H.; Chieng, H.-E.; Sun, T.; Zhou, Z.; et al. Digital light processing-bioprinted poly-NAGA-GelMA-based hydrogel lenticule for precise refractive errors correction. Biofabrication 2023, 15, 035011. [Google Scholar] [CrossRef]
- Nivetha, S.; Thejashree, A.; Abinaya, R.; Harini, S.; Chowdary, G.M. Bionic Eyes—An Artificial Vision. In Lecture Notes on Data Engineering and Communications Technologies; Springer: Berlin/Heidelberg, Germany, 2020; Volume 35. [Google Scholar] [CrossRef]
- Alturkistani, R.; Kavin, A.; Devasahayam, S.; Thomas, R.; Colombini, E.L.; Cifuentes, C.A.; Homer-Vanniasinkam, S.; Wurdemann, H.A.; Moazen, M. Affordable passive 3D-printed prosthesis for persons with partial hand amputation. Prosthet. Orthot. Int. 2020, 44, 92–98. [Google Scholar] [CrossRef]
- Xia, J.; Li, Y.; Cai, D.; Shi, X.; Zhao, S.; Yang, X. Direct resin composite restoration of maxillary central incisors using a 3D-printed template: Two clinical cases. BMC Oral Health 2018, 18, 158. [Google Scholar] [CrossRef]
- Moiduddin, K.; Mian, S.H.; Umer, U.; Alkhalefah, H.; Ahmed, F.; Hashmi, F.H. Design, Analysis, and 3D Printing of a Patient-Specific Polyetheretherketone Implant for the Reconstruction of Zygomatic Deformities. Polymers 2023, 15, 886. [Google Scholar] [CrossRef]
- Ohara, K.; Isshiki, Y.; Hoshi, N.; Ohno, A.; Kawanishi, N.; Nagashima, S.; Inoue, M.; Kubo, D.; Yamaya, K.; Inoue, E.; et al. Patient satisfaction with conventional dentures vs. digital dentures fabricated using 3D-printing: A randomized crossover trial. J. Prosthodont. Res. 2022, 66, 623–629. [Google Scholar] [CrossRef]
- Samaila, E.M.; Negri, S.; Zardini, A.; Bizzotto, N.; Maluta, T.; Rossignoli, C.; Magnan, B. Value of three-dimensional printing of fractures in orthopaedic trauma surgery. J. Int. Med. Res. 2020, 48, 0300060519887299. [Google Scholar] [CrossRef]
- McKean, L.; Samaha, D.; Stillwell, M.; Tupler, R.; Sarkar, K.; Whitlow, C. Three-dimensional modeling with virtual reality as a tool for pre-operative planning in colorectal surgery. In Diseases of the Colon & Rectum; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020; Volume 63. [Google Scholar]
- Cornejo, J.; Cornejo-Aguilar, J.A.; Vargas, M.; Helguero, C.G.; Milanezi De Andrade, R.; Torres-Montoya, S.; Asensio-Salazar, J.; Rivero Calle, A.; Martínez Santos, J.; Damon, A.; et al. Anatomical Engineering and 3D Printing for Surgery and Medical Devices: International Review and Future Exponential Innovations. BioMed Res. Int. 2022, 2022, 6797745. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Kuang, G.; Yu, Y.; Zhao, Y. Photopolymerized 3D Printing Scaffolds with Pt(IV) Prodrug Initiator for Postsurgical Tumor Treatment. Research 2022, 2022, 9784510. [Google Scholar] [CrossRef]
- Hagan, C.T.; Bloomquist, C.; Warner, S.; Knape, N.M.; Kim, I.; Foley, H.; Wagner, K.T.; Mecham, S.; DeSimone, J.; Wang, A.Z. 3D printed drug-loaded implantable devices for intraoperative treatment of cancer. J. Control Release Off. J. Control Release Soc. 2022, 344, 147–156. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Kadimisetty, K.; Bhalerao, K.S.; Chen, T.; Rusling, J.F. 3D-Printed Immunosensor Arrays for Cancer Diagnostics. Sensors 2020, 20, 4514. [Google Scholar] [CrossRef]
- Anup, N.; Gadeval, A.; Tekade, R.K. A 3D-Printed Graphene BioFuse Implant for Postsurgical Adjuvant Therapy of Cancer: Proof of Concept in 2D- and 3D-Spheroid Tumor Models. ACS Appl. Bio Mater. 2023, 6, 1195–1212. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 2019, 92, 37–47. [Google Scholar] [CrossRef]
- Xue, W.; Yu, S.-Y.; Kuss, M.; Kong, Y.; Shi, W.; Chung, S.; Kim, S.-Y.; Duan, B. 3D bioprinted white adipose model forin vitrostudy of cancer-associated cachexia induced adipose tissue remodeling. Biofabrication 2022, 14, 034106. [Google Scholar] [CrossRef]

| Key Benefit/Topic | Area of Application/Significance | References |
|---|---|---|
| Personalized part production and reducing rejection risks after transplantation | Production of cell-containing constructs | Gu et al., 2020 [62] |
| Biomaterials development | Talebian et al., 2019; Zhang et al., 2021 [63,64] | |
| Tissue development | Talebian et al., 2019; Zhang et al., 2021 [63,64] | |
| Ability to print tissue analogue structures | Zhang et al., 2019; Sarmah et al., 2022 [65,66] | |
| Production of scaffolds with a homogeneous distribution of cells | Veeman et al., 2021; An et al., 2023 [7,67] | |
| Disease modeling | Shukla et al., 2022; Crook et al., 2020; Fonseca et al., 2020 [3,4,24] | |
| Increase the patient’s chance of survival | 3D and bioprinting has great potential to serve as an efficient and safe alternative to the traditional methods and materials | Ahmed et al., 2022 [68] |
| 3D and bioprinting show promise in becoming effective alternatives | Xu et al., 2019 [69] | |
| 3D printing in the field of medicine through medical engineering | Suvorov et al., 2020 [70] | |
| 3D printing enhances the integration of the implanted tissues | Birla et al., 2020; Shahzadi et al., 2021; Qasim et al., 2019; Roche et al., 2020; Agarwal et al., 2021 [71,72,73,74] | |
| The focus of 3D printing is to facilitation of improved functional outcomes in patient care | Desanlis et al., 2021; Sedighi et al., 2023; Kamolz et al., 2022; Hann et al., 2019 [75,76,77,78] | |
| Reducing patient wait time | Bioprinting could allow for more lives to be saved and shorter wait times for organs | Gonzalez et al., 2020 [79] |
| 3D bioprinting decreases the waitlist for organs transplant | Jeong et al., 2020; Barceló et al., 2022; Leberfinger et al., 2019 [80,81,82] | |
| Bioprinting is expected to reduce the cost and time of preclinical discovery | Ji et al., 2021; Xu et al., 2022; He et al., 2021 [83,84,85] | |
| 3D bioprinting technology has high precision and fast construction speed | Ramos et al., 2020; Condino et al., 2022 [86,87] | |
| 3D bioprinting has potential to generate a new class of bioactive medical implants | Jovic et al., 2020 [34] | |
| 3D bioprinting saves time | Agarwal et al., 2023 [88] | |
| Homocellular tissue model generation and precise fabrication process with accurate specifications | 3D bioprinting is able to restore lost function caused by disease or damage | Singh et al., 2019 [89] |
| 3D bioprinting opens new possibilities for personalized treatments and regenerative medicine | Rosemann et al., 2007 [90] | |
| Tissue-engineered 3D scaffold would provide the necessary structural support and physical environment for cells to attach, grow, and differentiate | Raghunath et al., 2007 [91] | |
| 3D-printed triangular titanium implants significantly improved pain, disability, and patients quality of life | Patel et al., 2020 [92] | |
| Improves skin construction speed and saves patient life | 3D bioprinting holds promising applications to save patient life | Rosca et al., 2020 [93] |
| 3D bioprinting reduces donor requirements | Kamolz et al., 2022 [77] | |
| 3D-printed skin possesses enormous potential as grafts for wound healing, burned skin replacement, and in vitro human skin models for product and drug testing | Varkey et al., 2019; Kamolz et al., 2022 [77,94] | |
| 3D-bioprinting-based strategies can be used alone or in combination to promote faster wound healing and fulfill patient needs in terms of effectiveness, cost-effectiveness, and cosmetic appearance | Kolimi et al., 2022; Chouhan et al., 2019 [95,96] | |
| Eliminating/reducing the need for organ donors | Production of artificial tissues and organs may completely replace the damaged organ | Yakimova et al., 2020 [97] |
| 3D bioprinting may eliminate the need for organ donors and reduce organ trafficking | Mills & Mills, 2020 [9] | |
| Offering medical solutions tailored to each individual patient | 3D bioprinting, stem cell therapy, gene therapy, implantable devices, etc. have potential to restore functional vision for the visually impaired | Shukla et al., 2022; Ji et al., 2021; Menon et al., 2022 [3,83,98] |
| Digital light processing (DLP) bioprinting enables the production of structures with high precision | Wu et al., 2023 [99] | |
| Using 3D design and printing technologies allow for the creation of customized prosthesis for different amputation configurations | Farhat et al., 2021 [100] | |
| Creating more functional implants | 3D implants can reduced the surgical time and hospitalization period due to no donor-site morbidity | Takeda et al., 2020 [101] |
| 3D bioprinting lends a high degree of control over vascular network patterning during the design and initial building of the construct | Pan et al., 2022; Fang et al., 2019 [59,102] | |
| 3D constructs would transform the ability to personalize pharmaceutical and disease management | Sun et al., 2020 [103] | |
| 3D bioprinting has potential to produce patient-specific body parts, such as organs and limbs, with the capability of revolutionizing personalized medicine and surgery | Jovic et al., 2020 [34] | |
| Beneficial for cancer treatment | 3D printing technology helps in the treatment of cancer | Bhuskute et al., 2022 [104] |
| 3D-printed models eliminates various risk factors during the surgery | Mercader et al., 2019 [105] | |
| 3D-printed patient-specific tumor models can help healthcare professionals make better treatment decisions | Li et al., 2021; Tasneem et al., 2021; Schulze et al., 2021 [106,107,108] | |
| 3D-printed tumor models can used for developing personalized anti-cancer drugs | Pavan & Kumar, 2022 [109] | |
| 3D-printed models also help in improving the diagnosis of cancer treatments | Haleem et al., 2020 [110] | |
| 3D printing will be applied to provide the proper dose to kill cancer cells without damaging the healthy tissues | Bhuskute et al., 2022; Wu et al., 2020 [104,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaneva, A.; Shopova, D.; Bakova, D.; Mihaylova, A.; Kasnakova, P.; Hristozova, M.; Semerdjieva, M. The Progress in Bioprinting and Its Potential Impact on Health-Related Quality of Life. Bioengineering 2023, 10, 910. https://doi.org/10.3390/bioengineering10080910
Yaneva A, Shopova D, Bakova D, Mihaylova A, Kasnakova P, Hristozova M, Semerdjieva M. The Progress in Bioprinting and Its Potential Impact on Health-Related Quality of Life. Bioengineering. 2023; 10(8):910. https://doi.org/10.3390/bioengineering10080910
Chicago/Turabian StyleYaneva, Antoniya, Dobromira Shopova, Desislava Bakova, Anna Mihaylova, Petya Kasnakova, Maria Hristozova, and Maria Semerdjieva. 2023. "The Progress in Bioprinting and Its Potential Impact on Health-Related Quality of Life" Bioengineering 10, no. 8: 910. https://doi.org/10.3390/bioengineering10080910
APA StyleYaneva, A., Shopova, D., Bakova, D., Mihaylova, A., Kasnakova, P., Hristozova, M., & Semerdjieva, M. (2023). The Progress in Bioprinting and Its Potential Impact on Health-Related Quality of Life. Bioengineering, 10(8), 910. https://doi.org/10.3390/bioengineering10080910






