Outcomes Using Focused Shockwave for Treatment of Bone Stress Injury in Runners
Abstract
1. Introduction
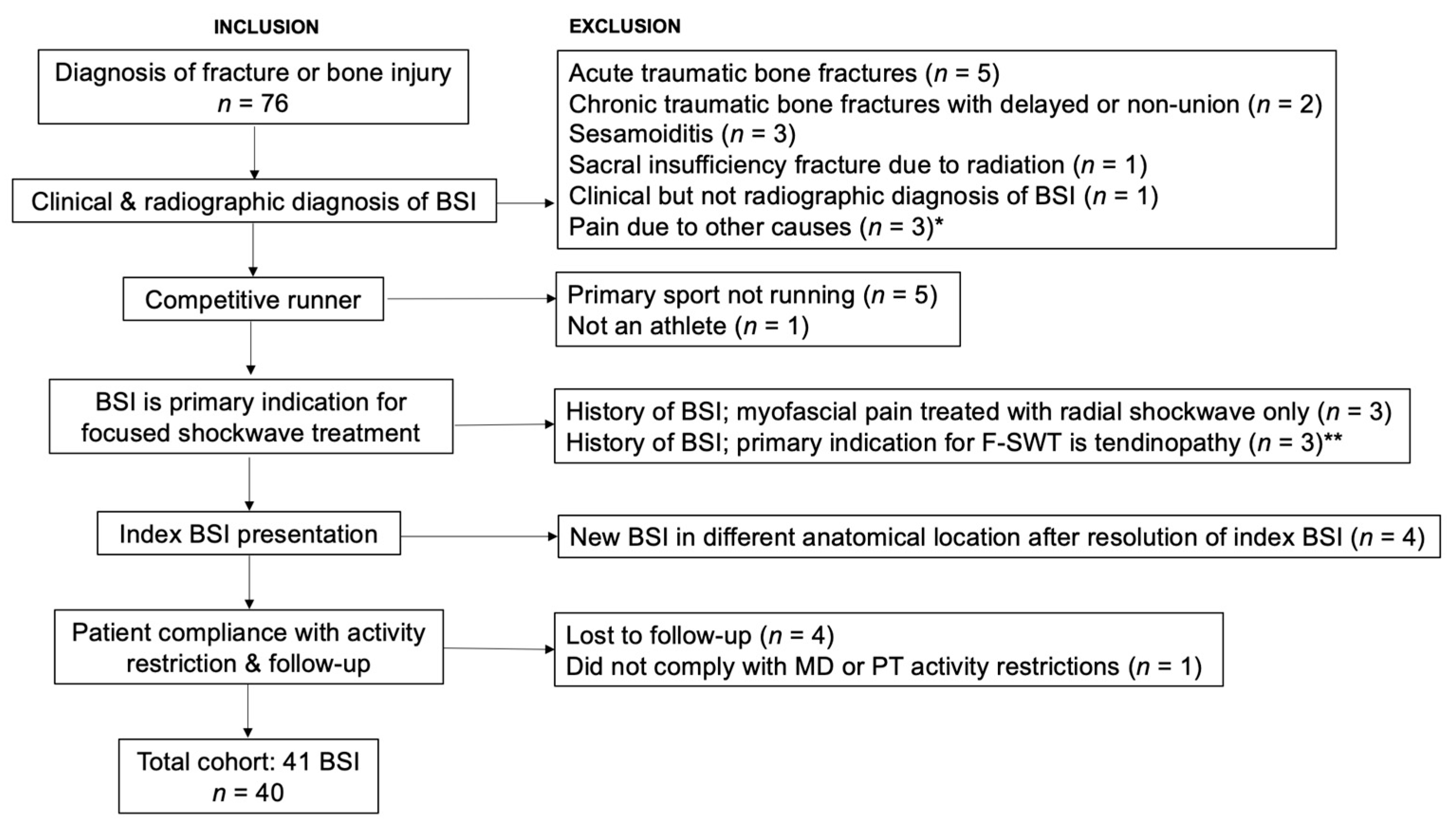
2. Materials and Methods
2.1. Study Procedures
2.2. Treatment Protocol
2.3. Data Processing
2.4. Statistics
3. Results
3.1. Patient Demographics
3.2. Injury Characteristics
3.3. Treatment Characteristics
3.4. Radiographic Outcomes
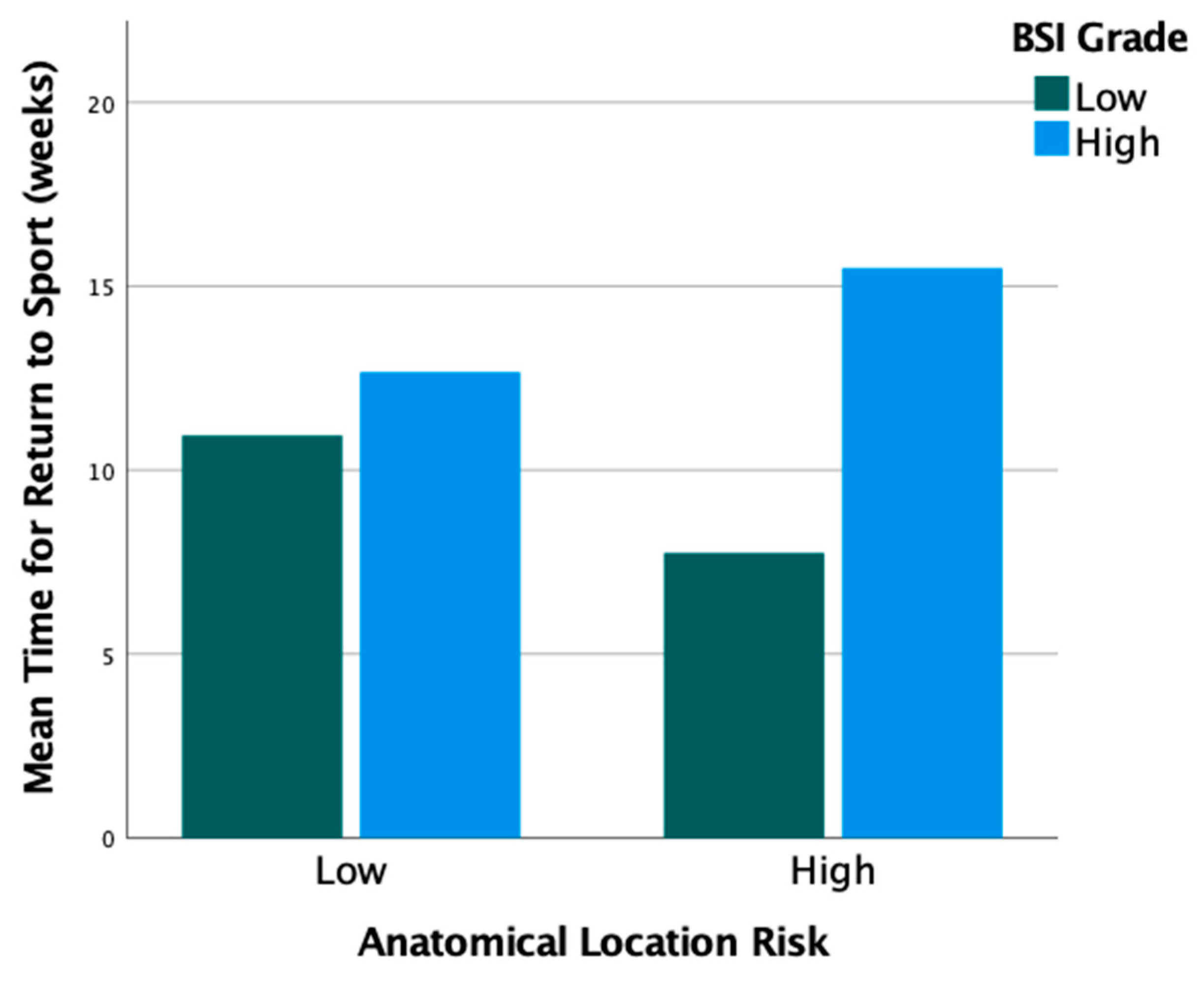
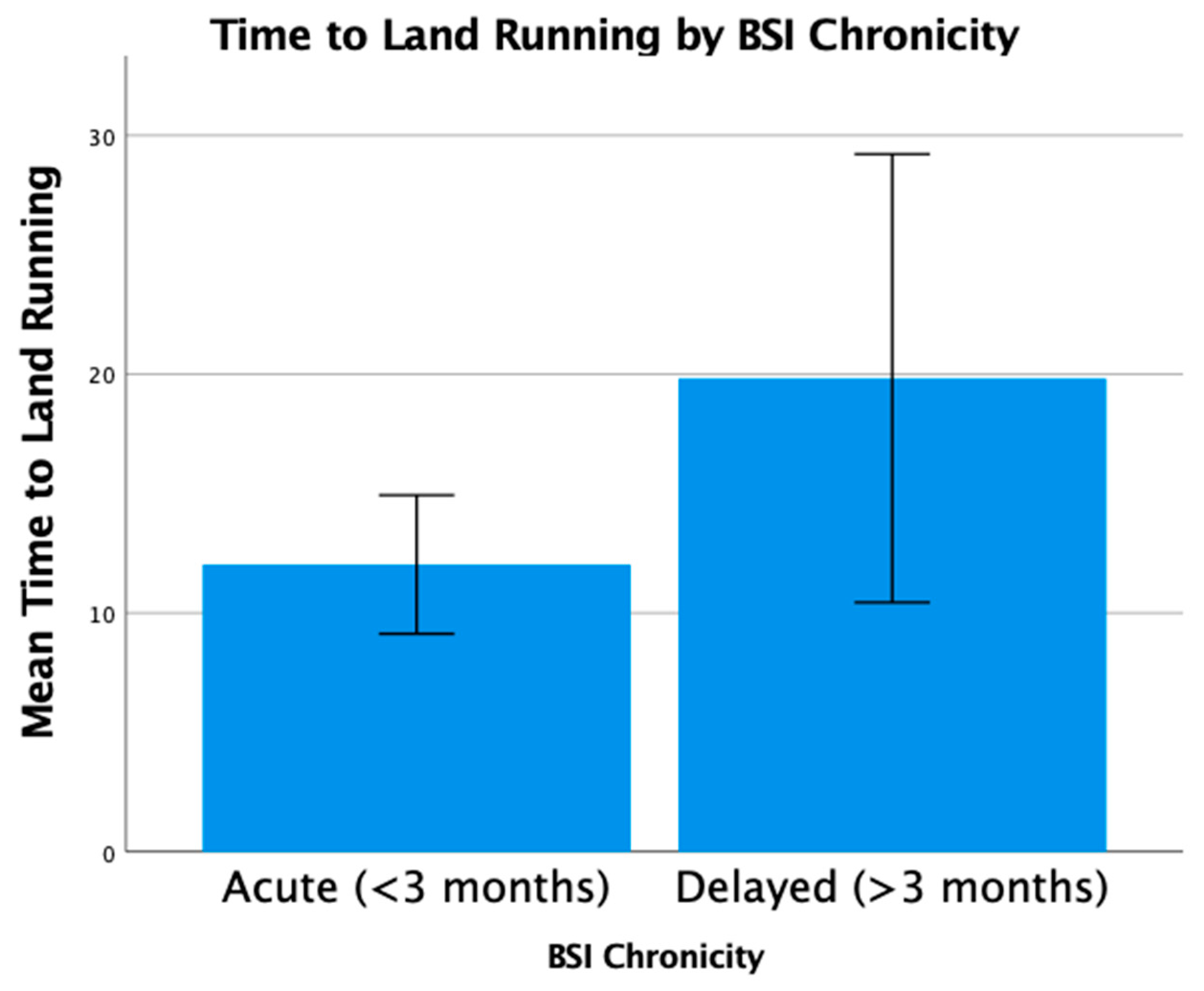
3.5. Clinical Outcomes
3.6. Long-Term Outcomes
4. Discussion
4.1. Summary of Findings
4.2. Review of Literature
4.3. Study Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoenig, T.; Ackerman, K.E.; Beck, B.R.; Bouxsein, M.L.; Burr, D.B.; Hollander, K.; Popp, K.L.; Rolvien, T.; Tenforde, A.S.; Warden, S.J. Bone Stress Injuries. Nat. Rev. Dis. Primer 2022, 8, 26. [Google Scholar] [CrossRef]
- Nattiv, A.; Kennedy, G.; Barrack, M.T.; Abdelkerim, A.; Goolsby, M.A.; Arends, J.C.; Seeger, L.L. Correlation of MRI Grading of Bone Stress Injuries with Clinical Risk Factors and Return to Play: A 5-Year Prospective Study in Collegiate Track and Field Athletes. Am. J. Sport. Med. 2013, 41, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Fredericson, M.; Bergman, A.G.; Hoffman, K.L.; Dillingham, M.S. Tibial Stress Reaction in Runners. Correlation of Clinical Symptoms and Scintigraphy with a New Magnetic Resonance Imaging Grading System. Am. J. Sport. Med. 1995, 23, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.; Agel, J.; Heikes, C.; Griffiths, H. Stress Injuries to Bone in College Athletes: A Retrospective Review of Experience at a Single Institution. Am. J. Sport. Med. 2003, 31, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, T.; Tenforde, A.S.; Strahl, A.; Rolvien, T.; Hollander, K. Does Magnetic Resonance Imaging Grading Correlate with Return to Sports after Bone Stress Injuries? A Systematic Review and Meta-Analysis. Am. J. Sport. Med. 2022, 50, 834–844. [Google Scholar] [CrossRef]
- Fredericson, M.; Kussman, A.; Misra, M.; Barrack, M.T.; De Souza, M.J.; Kraus, E.; Koltun, K.J.; Williams, N.I.; Joy, E.; Nattiv, A. The Male Athlete Triad-A Consensus Statement from the Female and Male Athlete Triad Coalition Part II: Diagnosis, Treatment, and Return-To-Play. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2021, 31, 349–366. [Google Scholar] [CrossRef]
- De Souza, M.J.; Nattiv, A.; Joy, E.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference Held in San Francisco, CA, May 2012, and 2nd International Conference Held in Indianapolis, IN, May 2013. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2014, 24, 96–119. [Google Scholar] [CrossRef]
- Hoenig, T.; Eissele, J.; Strahl, A.; Popp, K.L.; Stürznickel, J.; Ackerman, K.E.; Hollander, K.; Warden, S.J.; Frosch, K.-H.; Tenforde, A.S.; et al. Return to Sport Following Low-Risk and High-Risk Bone Stress Injuries: A Systematic Review and Meta-Analysis. Br. J. Sport. Med. 2023, 57, 427–432. [Google Scholar] [CrossRef]
- Taki, M.; Iwata, O.; Shiono, M.; Kimura, M.; Takagishi, K. Extracorporeal Shock Wave Therapy for Resistant Stress Fracture in Athletes: A Report of 5 Cases. Am. J. Sport. Med. 2007, 35, 1188–1192. [Google Scholar] [CrossRef]
- Mollon, B.; da Silva, V.; Busse, J.W.; Einhorn, T.A.; Bhandari, M. Electrical Stimulation for Long-Bone Fracture-Healing: A Meta-Analysis of Randomized Controlled Trials. J. Bone Jt. Surg. Am. 2008, 90, 2322–2330. [Google Scholar] [CrossRef]
- Moretti, B.; Notarnicola, A.; Garofalo, R.; Moretti, L.; Patella, S.; Marlinghaus, E.; Patella, V. Shock Waves in the Treatment of Stress Fractures. Ultrasound Med. Biol. 2009, 35, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Chaussy, C.; Schmiedt, E.; Jocham, D.; Brendel, W.; Forssmann, B.; Walther, V. First Clinical Experience with Extracorporeally Induced Destruction of Kidney Stones by Shock Waves. J. Urol. 1982, 127, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Haupt, G.; Haupt, A.; Ekkernkamp, A.; Gerety, B.; Chvapil, M. Influence of Shock Waves on Fracture Healing. Urology 1992, 39, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Furia, J.P.; Rompe, J.D.; Cacchio, A.; Maffulli, N. Shock Wave Therapy as a Treatment of Nonunions, Avascular Necrosis, and Delayed Healing of Stress Fractures. Foot Ankle Clin. 2010, 15, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Cacchio, A.; Giordano, L.; Colafarina, O.; Rompe, J.D.; Tavernese, E.; Ioppolo, F.; Flamini, S.; Spacca, G.; Santilli, V. Extracorporeal Shock-Wave Therapy Compared with Surgery for Hypertrophic Long-Bone Nonunions. J. Bone Jt. Surg. Am. 2009, 91, 2589–2597. [Google Scholar] [CrossRef]
- Birnbaum, K.; Wirtz, D.C.; Siebert, C.H.; Heller, K.D. Use of Extracorporeal Shock-Wave Therapy (ESWT) in the Treatment of Non-Unions. A Review of the Literature. Arch. Orthop. Trauma Surg. 2002, 122, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.S.; Roth, M.; Kapil, N. Stress Fractures: Diagnosis, Treatment, and Prevention. Am. Fam. Physician 2011, 83, 39–46. [Google Scholar]
- Fredericson, M.; Jennings, F.; Beaulieu, C.; Matheson, G.O. Stress Fractures in Athletes. Top. Magn. Reson. Imaging TMRI 2006, 17, 309–325. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Watanabe, L.M.; Moreno, T.J.; Fredericson, M. Use of an Antigravity Treadmill for Rehabilitation of a Pelvic Stress Injury. PM&R 2012, 4, 629–631. [Google Scholar] [CrossRef]
- Boden, B.P.; Osbahr, D.C.; Jimenez, C. Low-Risk Stress Fractures. Am. J. Sport. Med. 2001, 29, 100–111. [Google Scholar] [CrossRef]
- Boden, B.P.; Osbahr, D.C. High-Risk Stress Fractures: Evaluation and Treatment. J. Am. Acad. Orthop. Surg. 2000, 8, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Shepstone, L.; Donell, S.T.; Thomas, T.L. Shock Wave Therapy for Chronic Achilles Tendon Pain: A Randomized Placebo-Controlled Trial. Clin. Orthop. 2005, 440, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Durst, H.B.; Blatter, G.; Kuster, M.S. Osteonecrosis of the Humeral Head after Extracorporeal Shock-Wave Lithotripsy. J. Bone Jt. Surg. Br. 2002, 84, 744–746. [Google Scholar] [CrossRef]
- Erduran, M.; Akseki, D.; Ulusal, A.E. A Complication Due to Shock Wave Therapy Resembling Calcaneal Stress Fracture. Foot Ankle Int. 2013, 34, 599–602. [Google Scholar] [CrossRef]
- Saxena, A.; Behan, S.A.; Valerio, D.L.; Frosch, D.L. Navicular Stress Fracture Outcomes in Athletes: Analysis of 62 Injuries. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2017, 56, 943–948. [Google Scholar] [CrossRef]
- Maisonhaute, E.; Prado, C.; White, P.C.; Compton, R.G. Surface Acoustic Cavitation Understood via Nanosecond Electrochemistry. Part III: Shear Stress in Ultrasonic Cleaning. Ultrason. Sonochem. 2002, 9, 297–303. [Google Scholar] [CrossRef]
- Apfel, R.E. Acoustic Cavitation: A Possible Consequence of Biomedical Uses of Ultrasound. Br. J. Cancer Suppl. 1982, 5, 140–146. [Google Scholar]
- Bulut, O.; Eroglu, M.; Ozturk, H.; Tezeren, G.; Bulut, S.; Koptagel, E. Extracorporeal Shock Wave Treatment for Defective Nonunion of the Radius: A Rabbit Model. J. Orthop. Surg. Hong Kong 2006, 14, 133–137. [Google Scholar] [CrossRef]
- Narasaki, K.; Shimizu, H.; Beppu, M.; Aoki, H.; Takagi, M.; Takashi, M. Effect of Extracorporeal Shock Waves on Callus Formation during Bone Lengthening. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2003, 8, 474–481. [Google Scholar] [CrossRef]
- Aicher, A.; Heeschen, C.; Sasaki, K.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Low-Energy Shock Wave for Enhancing Recruitment of Endothelial Progenitor Cells: A New Modality to Increase Efficacy of Cell Therapy in Chronic Hind Limb Ischemia. Circulation 2006, 114, 2823–2830. [Google Scholar] [CrossRef]
- Martini, L.; Giavaresi, G.; Fini, M.; Torricelli, P.; de Pretto, M.; Schaden, W.; Giardino, R. Effect of Extracorporeal Shock Wave Therapy on Osteoblastlike Cells. Clin. Orthop. 2003, 413, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Wurtz, T.; Wang, C.-J.; Kuo, Y.-R.; Yang, K.D.; Huang, H.-C.; Wang, F.-S. Recruitment of Mesenchymal Stem Cells and Expression of TGF-Beta 1 and VEGF in the Early Stage of Shock Wave-Promoted Bone Regeneration of Segmental Defect in Rats. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2004, 22, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Shimokawa, H.; Oi, K.; Tatewaki, H.; Uwatoku, T.; Abe, K.; Matsumoto, Y.; Kajihara, N.; Eto, M.; Matsuda, T.; et al. Extracorporeal Cardiac Shock Wave Therapy Markedly Ameliorates Ischemia-Induced Myocardial Dysfunction in Pigs In Vivo. Circulation 2004, 110, 3055–3061. [Google Scholar] [CrossRef]
- Wang, C.-J. An Overview of Shock Wave Therapy in Musculoskeletal Disorders. Chang Gung Med. J. 2003, 26, 220–232. [Google Scholar]
- Warden, S.J. Prophylactic Use of NSAIDs by Athletes: A Risk/Benefit Assessment. Phys. Sportsmed. 2010, 38, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; McKinnon, C.J.; Taylor, K.M.; Kardouni, J.R.; Bulathsinhala, L.; Guerriere, K.I.; Popp, K.L.; Bouxsein, M.L.; Proctor, S.P.; Matheny, R.W. Nonsteroidal Anti-Inflammatory Drug Prescriptions Are Associated with Increased Stress Fracture Diagnosis in the US Army Population. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2019, 34, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Changstrom, B.G.; Brou, L.; Khodaee, M.; Braund, C.; Comstock, R.D. Epidemiology of Stress Fracture Injuries among US High School Athletes, 2005–2006 through 2012–2013. Am. J. Sport. Med. 2015, 43, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rizzone, K.H.; Ackerman, K.E.; Roos, K.G.; Dompier, T.P.; Kerr, Z.Y. The Epidemiology of Stress Fractures in Collegiate Student-Athletes, 2004–2005 Through 2013–2014 Academic Years. J. Athl. Train. 2017, 52, 966–975. [Google Scholar] [CrossRef]
- Rauh, M.J.; Barrack, M.; Nichols, J.F. Associations between the Female Athlete Triad and Injury among High School Runners. Int. J. Sport. Phys. Ther. 2014, 9, 948–958. [Google Scholar]
- Tenforde, A.S.; Carlson, J.L.; Chang, A.; Sainani, K.L.; Shultz, R.; Kim, J.H.; Cutti, P.; Golden, N.H.; Fredericson, M. Association of the Female Athlete Triad Risk Assessment Stratification to the Development of Bone Stress Injuries in Collegiate Athletes. Am. J. Sport. Med. 2017, 45, 302–310. [Google Scholar] [CrossRef]
- Nieves, J.W.; Formica, C.; Ruffing, J.; Zion, M.; Garrett, P.; Lindsay, R.; Cosman, F. Males Have Larger Skeletal Size and Bone Mass than Females, despite Comparable Body Size. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Sayres, L.C.; McCurdy, M.L.; Sainani, K.L.; Fredericson, M. Identifying Sex-Specific Risk Factors for Stress Fractures in Adolescent Runners. Med. Sci. Sport. Exerc. 2013, 45, 1843–1851. [Google Scholar] [CrossRef]
- Nattiv, A. Stress Fractures and Bone Health in Track and Field Athletes. J. Sci. Med. Sport 2000, 3, 268–279. [Google Scholar] [CrossRef]
- Kelsey, J.L.; Bachrach, L.K.; Procter-Gray, E.; Nieves, J.; Greendale, G.A.; Sowers, M.; Brown, B.W.; Matheson, K.A.; Crawford, S.L.; Cobb, K.L. Risk Factors for Stress Fracture among Young Female Cross-Country Runners. Med. Sci. Sport. Exerc. 2007, 39, 1457–1463. [Google Scholar] [CrossRef]
- Matheson, G.O.; Clement, D.B.; McKenzie, D.C.; Taunton, J.E.; Lloyd-Smith, D.R.; MacIntyre, J.G. Stress Fractures in Athletes. A Study of 320 Cases. Am. J. Sport. Med. 1987, 15, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Popp, K.L.; Ackerman, K.E.; Rudolph, S.E.; Johannesdottir, F.; Hughes, J.M.; Tenforde, A.S.; Bredella, M.A.; Xu, C.; Unnikrishnan, G.; Reifman, J.; et al. Changes in Volumetric Bone Mineral Density Over 12 Months After a Tibial Bone Stress Injury Diagnosis: Implications for Return to Sports and Military Duty. Am. J. Sport. Med. 2021, 49, 226–235. [Google Scholar] [CrossRef] [PubMed]
| Category | Characteristic | % | n |
|---|---|---|---|
| Sex | Female | 70% | 28 |
| Male | 30% | 12 | |
| Level of competition | High School | 15% | 6 |
| Collegiate | 25% | 10 | |
| Recreational | 48% | 19 | |
| Elite | 13% | 5 | |
| Athlete Triad Risk Score | Low | 35% | 14 |
| Moderate | 48% | 19 | |
| High | 18% | 7 | |
| Location | Posteromedial tibia | 34% | 14 |
| Metatarsal shaft or head | 12% | 5 | |
| Metatarsal base | 10% | 4 | |
| Cuboid | 10% | 4 | |
| Fibula | 10% | 4 | |
| Calcaneus | 5% | 2 | |
| Sacrum | 5% | 2 | |
| Anterior tibial cortex | 5% | 2 | |
| Femoral shaft | 2% | 1 | |
| Lesser trochanter | 2% | 1 | |
| Inferior pubic ramus | 2% | 1 | |
| Navicular | 2% | 1 | |
| Characteristic | Units | Mean | ±Standard Deviation |
| Age | years | 30 | ± 13 |
| Body mass index (BMI) | kg/m2 | 21 | ± 2 |
| Pre-injury training volume | km/week | 72 | ± 40 |
| Imaging Method | n | Mean ± SD (Weeks) | Range (Weeks) | Findings |
|---|---|---|---|---|
| XR | 2 | 5 ± 1 | 4–5 |
|
| MRI | 7 | 15 ± 7 | 10–27 |
|
| CT | 7 | 10 ± 6 | 4–22 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beling, A.; Saxena, A.; Hollander, K.; Tenforde, A.S. Outcomes Using Focused Shockwave for Treatment of Bone Stress Injury in Runners. Bioengineering 2023, 10, 885. https://doi.org/10.3390/bioengineering10080885
Beling A, Saxena A, Hollander K, Tenforde AS. Outcomes Using Focused Shockwave for Treatment of Bone Stress Injury in Runners. Bioengineering. 2023; 10(8):885. https://doi.org/10.3390/bioengineering10080885
Chicago/Turabian StyleBeling, Alexandra, Amol Saxena, Karsten Hollander, and Adam S. Tenforde. 2023. "Outcomes Using Focused Shockwave for Treatment of Bone Stress Injury in Runners" Bioengineering 10, no. 8: 885. https://doi.org/10.3390/bioengineering10080885
APA StyleBeling, A., Saxena, A., Hollander, K., & Tenforde, A. S. (2023). Outcomes Using Focused Shockwave for Treatment of Bone Stress Injury in Runners. Bioengineering, 10(8), 885. https://doi.org/10.3390/bioengineering10080885







