Using a Xenogeneic Acellular Dermal Matrix Membrane to Enhance the Reparability of Bone Marrow Mesenchymal Stem Cells for Cartilage Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the ADM Membrane
2.2. Assessment of Cellular Content
2.3. Characterization of the Scaffolds
2.4. In Vitro Experiment
2.5. In Vivo Experiment
2.5.1. Animal Surgery Procedure
2.5.2. Synovial Fluid Analysis and Macrography
2.5.3. Histological Assessment of Repaired Tissue
2.5.4. Nanoindentation Assessment
2.6. Statistical Analysis
3. Results
3.1. Gross Observation and SEM Images of ADM Scaffolds
3.2. Evaluation of ADM
3.3. Physicochemical Properties of the ADM Scaffold
3.4. Distribution, Viability, and Chondrogenesis of BMSCs Seeded on ADM Scaffolds
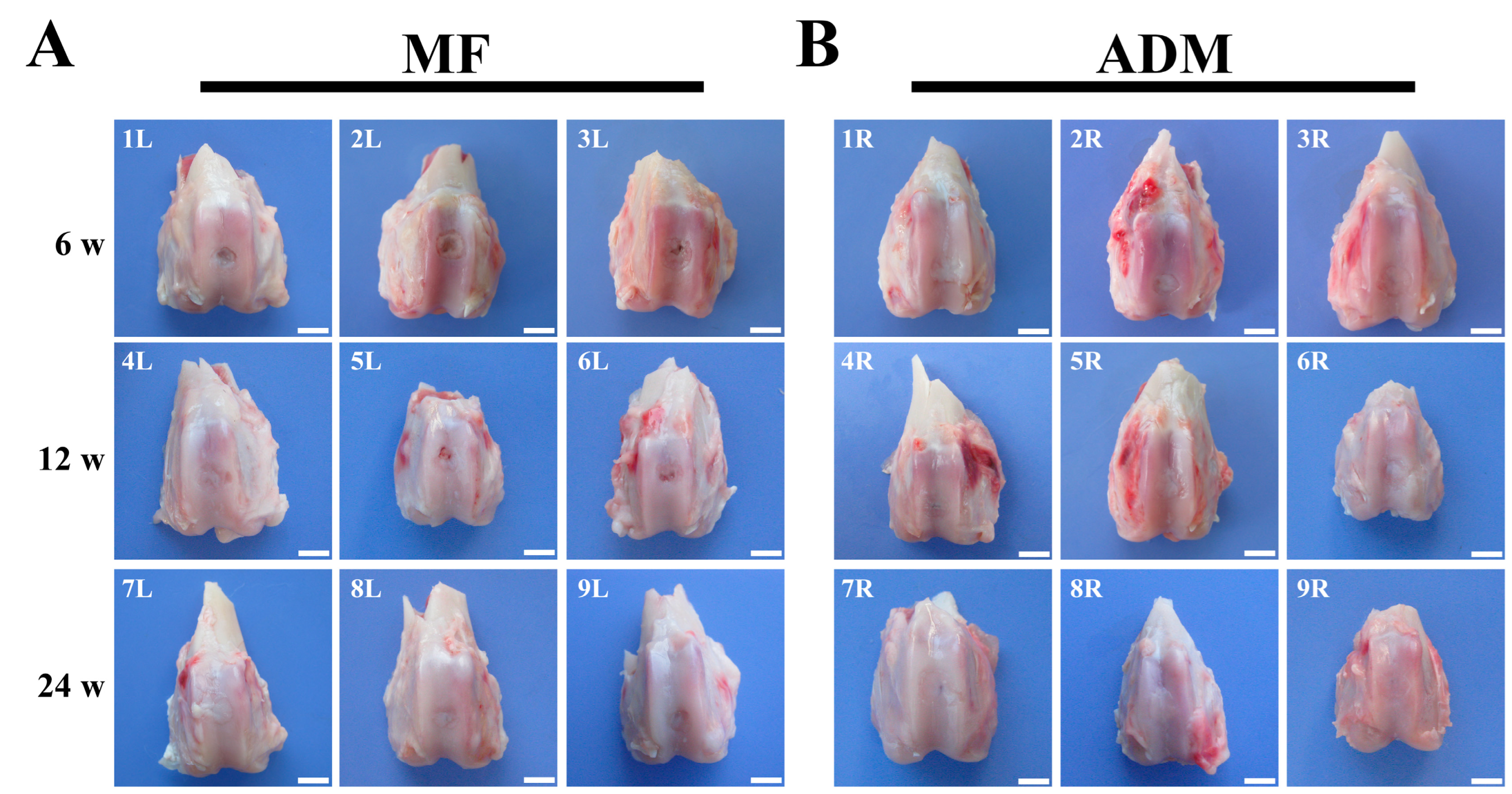
3.5. Abrasion and Inflammation Detection of the Repaired Knees
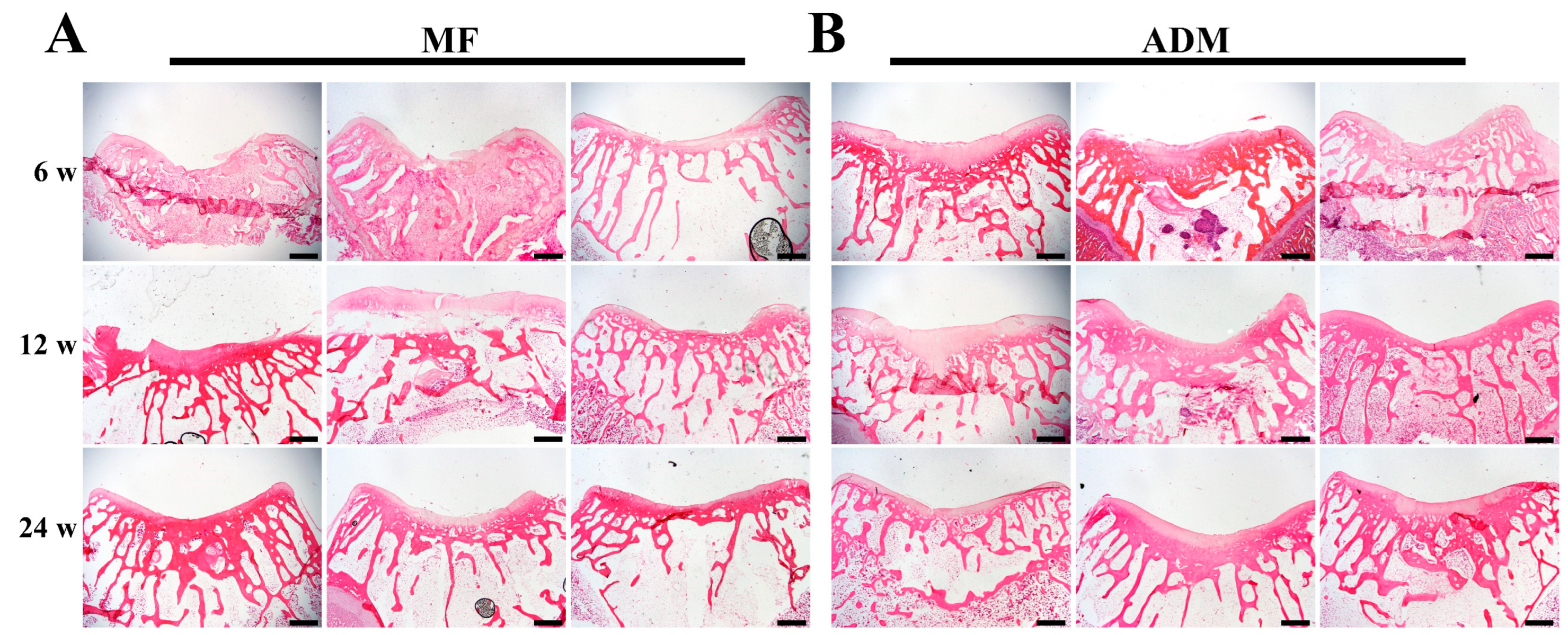
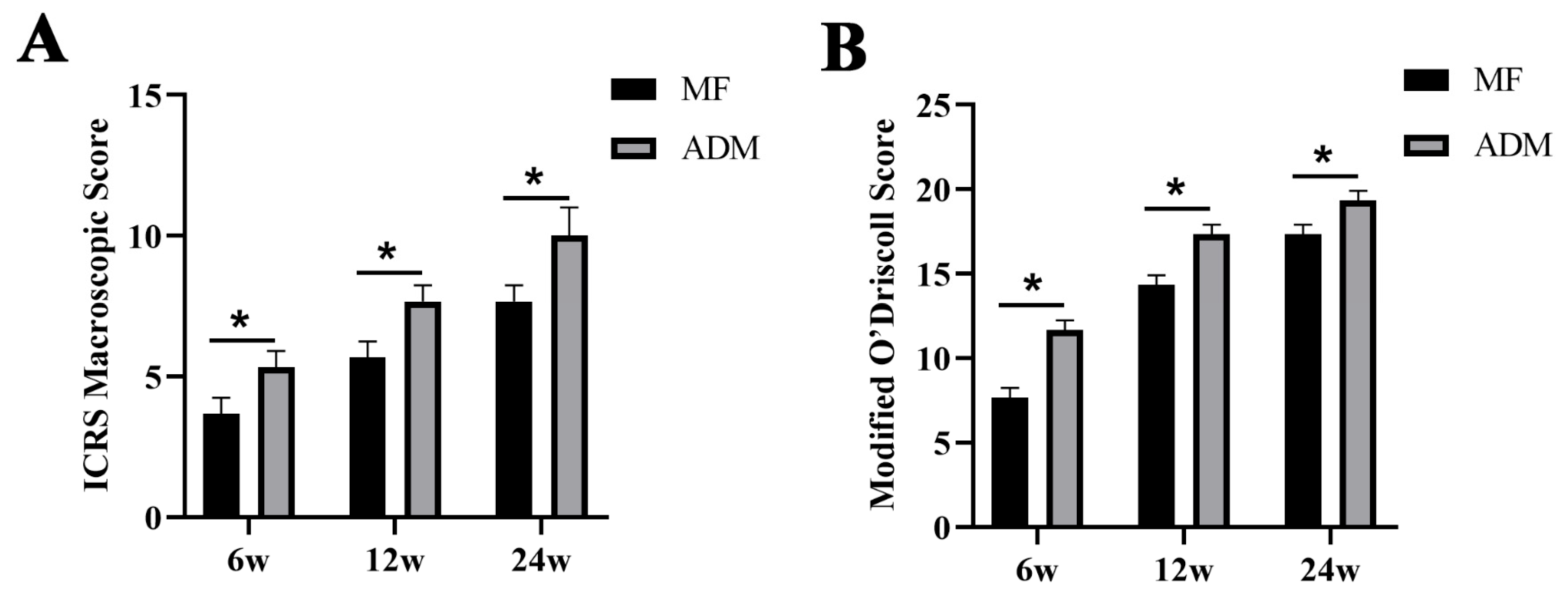
3.6. Gross Observation and Histological Evaluation of Cartilage Repair
3.7. Cartilage-Specific Staining
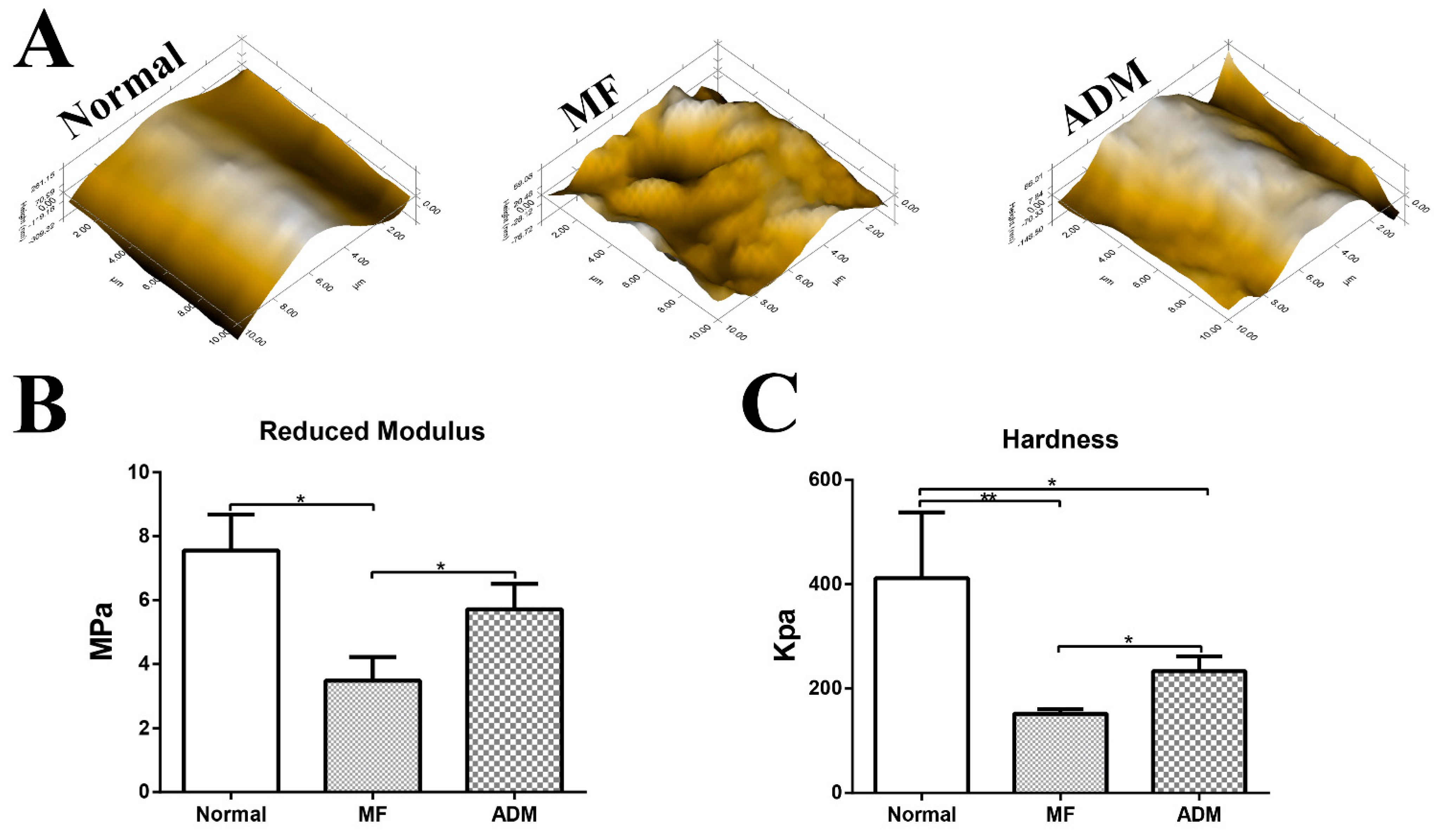
3.8. Biomechanical Properties of the Repaired Cartilage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.K.; Huwe, L.W.; Paschos, N.; Aryaei, A.; Gegg, C.A.; Hu, J.C.; Athanasiou, K.A. Tension stimulation drives tissue formation in scaffold-free systems. Nat. Mater. 2017, 16, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.L.; Schenck, R.C., Jr.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 2016, 8, 153–160. [Google Scholar] [CrossRef]
- DeFroda, S.F.; Bokshan, S.L.; Yang, D.S.; Daniels, A.H.; Owens, B.D. Trends in the Surgical Treatment of Articular Cartilage Lesions in the United States from 2007 to 2016. J. Knee Surg. 2021, 34, 1609–1616. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Li, T.Z.; Jin, C.Z.; Choi, B.H.; Kim, M.S.; Kim, Y.J.; Park, S.R.; Yoon, J.H.; Min, B.-H. Using Cartilage Extracellular Matrix (CECM) Membrane to Enhance the Reparability of the Bone Marrow Stimulation Technique for Articular Cartilage Defect in Canine Model. Adv. Funct. Mater. 2012, 22, 4292–4300. [Google Scholar] [CrossRef]
- Dai, L.; He, Z.; Jiang, Y.; Zhang, X.; Ren, S.; Zhu, J.; Shao, Z.; Huang, H.; Zhang, J.; Fu, X.; et al. One-step strategy for cartilage repair using acellular bone matrix scaffold based in situ tissue engineering technique in a preclinical minipig model. Am. J. Transl. Res. 2019, 11, 6650–6659. [Google Scholar]
- Ringe, J.; Sittinger, M. Regenerative medicine: Selecting the right biological scaffold for tissue engineering. Nat. Rev. Rheumatol. 2014, 10, 388–389. [Google Scholar] [CrossRef]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4, 141ra193. [Google Scholar] [CrossRef]
- Bahrami, N.; Bordbar, S.; Hasanzadeh, E.; Goodarzi, A.; Ai, A.; Mohamadnia, A. The effect of decellularized cartilage matrix scaffolds combined with endometrial stem cell-derived osteocytes on osteochondral tissue engineering in rats. In vitro cellular & developmental biology. Animal 2022, 58, 480–490. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Chen, M.; Liu, Y.; Cui, X.; Liang, J.; Wang, Q.; Fan, Y.; Zhang, X. Childhood Cartilage ECM Enhances the Chondrogenesis of Endogenous Cells and Subchondral Bone Repair of the Unidirectional Collagen-dECM Scaffolds in Combination with Microfracture. ACS Appl. Mater. Interfaces 2021, 13, 57043–57057. [Google Scholar] [CrossRef]
- Cui, P.; Pan, P.; Qin, L.; Wang, X.; Chen, X.; Deng, Y.; Zhang, X. Nanoengineered hydrogels as 3D biomimetic extracellular matrix with injectable and sustained delivery capability for cartilage regeneration. Bioact. Mater. 2023, 19, 487–498. [Google Scholar] [CrossRef]
- Das, P.; Mishra, R.; Devi, B.; Rajesh, K.; Basak, P.; Roy, M.; Roy, P.; Lahiri, D.; Nandi, S.K. Decellularized xenogenic cartilage extracellular matrix (ECM) scaffolds for the reconstruction of osteochondral defects in rabbits. J. Mater. Chem. B 2021, 9, 4873–4894. [Google Scholar] [CrossRef]
- Nie, X.; Chuah, Y.J.; Zhu, W.; He, P.; Peck, Y.; Wang, D.A. Decellularized tissue engineered hyaline cartilage graft for articular cartilage repair. Biomaterials 2020, 235, 119821. [Google Scholar] [CrossRef]
- Ma, A.; Jiang, L.; Song, L.; Hu, Y.; Dun, H.; Daloze, P.; Yu, Y.; Jiang, J.; Zafarullah, M.; Chen, H. Reconstruction of cartilage with clonal mesenchymal stem cell-acellular dermal matrix in cartilage defect model in nonhuman primates. Int. Immunopharmacol. 2013, 16, 399–408. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhou, G.; Liu, Y.; Cao, Y. Biological Evaluation of Acellular Cartilaginous and Dermal Matrixes as Tissue Engineering Scaffolds for Cartilage Regeneration. Front. Cell Dev. Biol. 2020, 8, 624337. [Google Scholar] [CrossRef]
- Longoni, A.; Utomo, L.; Robinson, A.; Levato, R.; Rosenberg, A.; Gawlitta, D. Acceleration of Bone Regeneration Induced by a Soft-Callus Mimetic Material. Adv. Sci. 2022, 9, e2103284. [Google Scholar] [CrossRef]
- Sutherland, A.J.; Converse, G.L.; Hopkins, R.A.; Detamore, M.S. The bioactivity of cartilage extracellular matrix in articular cartilage regeneration. Adv. Health Mater. 2015, 4, 29–39. [Google Scholar] [CrossRef]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.B.; Souza, M.V.; Conceicao, L.G.; Viloria, M.I.V.; Bedoya, S.A.O. Evaluation of dermal collagen stained with picrosirius red and examined under polarized light microscopy. Bras. Dermatol. 2018, 93, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Hu, X.; Huang, H.; Liu, Z.; Yuan, L.; Shao, Z.; Jiang, Y.; Zhang, J.; Fu, X.; Duan, X.; et al. Microfracture combined with functional pig peritoneum-derived acellular matrix for cartilage repair in rabbit models. Acta Biomater. 2017, 53, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, Q.; Yu, H.; Cheng, J.; Wu, N.; Shi, W.; Zhao, F.; Shao, Z.; Meng, Q.; Chen, H.; et al. Cryo-self-assembled silk fibroin sponge as a biodegradable platform for enzyme-responsive delivery of exosomes. Bioact. Mater. 2022, 8, 505–514. [Google Scholar] [CrossRef]
- Hendriks, J.A.; Moroni, L.; Riesle, J.; de Wijn, J.R.; van Blitterswijk, C.A. The effect of scaffold-cell entrapment capacity and physico-chemical properties on cartilage regeneration. Biomaterials 2013, 34, 4259–4265. [Google Scholar] [CrossRef]
- Man, Z.; Yin, L.; Shao, Z.; Zhang, X.; Hu, X.; Zhu, J.; Dai, L.; Huang, H.; Yuan, L.; Zhou, C.; et al. The effects of co-delivery of BMSC-affinity peptide and rhTGF-beta1 from coaxial electrospun scaffolds on chondrogenic differentiation. Biomaterials 2014, 35, 5250–5260. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Aigner, T.; Brittberg, M.; Bullough, P.; Hollander, A.; Hunziker, E.; Kandel, R.; Nehrer, S.; Pritzker, K.; Roberts, S.; et al. Histological assessment of cartilage repair: A report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J. Bone Jt. Surg. Am. 2003, 85 (Suppl. S2), 45–57. [Google Scholar]
- Mainil-Varlet, P.; Van Damme, B.; Nesic, D.; Knutsen, G.; Kandel, R.; Roberts, S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am. J. Sports Med. 2010, 38, 880–890. [Google Scholar] [CrossRef]
- Rutgers, M.; van Pelt, M.J.; Dhert, W.J.; Creemers, L.B.; Saris, D.B. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthr. Cartil. 2010, 18, 12–23. [Google Scholar] [CrossRef]
- Orth, P.; Madry, H. Complex and elementary histological scoring systems for articular cartilage repair. Histol. Histopathol. 2015, 30, 911–919. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Song, J.; Li, X.; Zhang, X.; Zhou, Z.; Chen, D.; Ma, P.X.; Peng, W.; Wang, W.; et al. Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomater. 2018, 79, 202–215. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, B.; Liu, W.; Li, X.; Liang, K.; Fan, Z.; Li, J.J.; Niu, Y.; He, Z.; Li, H.; et al. In situ self-assembled organoid for osteochondral tissue regeneration with dual functional units. Bioact. Mater. 2023, 27, 200–215. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, T.; Gao, C.; Cao, F.; Li, H.; Liao, Z.; Fu, L.; Li, P.; Chen, W.; Sun, Z.; et al. 3D-Bioprinted Difunctional Scaffold for In Situ Cartilage Regeneration Based on Aptamer-Directed Cell Recruitment and Growth Factor-Enhanced Cell Chondrogenesis. ACS Appl. Mater. Interfaces 2021, 13, 23369–23383. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Chen, J.; Qu, Y.; Qi, H.; Wang, Q.; Yang, Z.; Wu, A.; Wang, F.; Li, P. Naringin in the repair of knee cartilage injury via the TGF-β/ALK5/Smad2/3 signal transduction pathway combined with an acellular dermal matrix. J. Orthop. Transl. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Hu, X.; Shao, Z.; Zhu, J.; Dai, L.; Man, Z.; Yuan, L.; Chen, H.; Zhou, C.; et al. A functional biphasic biomaterial homing mesenchymal stem cells for in vivo cartilage regeneration. Biomaterials 2014, 35, 9608–9619. [Google Scholar] [CrossRef]
- Lee, K.B.; Wang, V.T.; Chan, Y.H.; Hui, J.H. A novel, minimally-invasive technique of cartilage repair in the human knee using arthroscopic microfracture and injections of mesenchymal stem cells and hyaluronic acid--a prospective comparative study on safety and short-term efficacy. Ann. Acad. Med. Singap. 2012, 41, 511–517. [Google Scholar]
- Nie, X.; Wang, D.A. Decellularized orthopaedic tissue-engineered grafts: Biomaterial scaffolds synthesised by therapeutic cells. Biomater. Sci. 2018, 6, 2798–2811. [Google Scholar] [CrossRef]
- Ye, K.; Traianedes, K.; Robins, S.A.; Choong, P.F.M.; Myers, D.E. Osteochondral repair using an acellular dermal matrix-pilot in vivo study in a rabbit osteochondral defect model. J. Orthop. Res. 2018, 36, 1919–1928. [Google Scholar] [CrossRef]
- Tognetti, L.; Pianigiani, E.; Ierardi, F.; Lorenzini, G.; Casella, D.; Liso, F.G.; De Pascalis, A.; Cinotti, E.; Rubegni, P. The use of human acellular dermal matrices in advanced wound healing and surgical procedures: State of the art. Dermatol. Ther. 2021, 34, e14987. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Robertson, W.B.; Zhao, J.; Chen, W.; Xu, J. Emerging Trend in the Pharmacotherapy of Osteoarthritis. Front. Endocrinol. 2019, 10, 431. [Google Scholar] [CrossRef] [PubMed]






| Physical and Chemical Properties | |||
|---|---|---|---|
| Diameter | 3.92 ± 0.21 mm | ESR | 1.90 ± 0.14% |
| Thickness | 0.98 ± 0.19 mm | Porosity | 0.59 ± 0.06% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Meng, Q.; Hu, X.; Cheng, J.; Shao, Z.; Yang, Y.; Ao, Y. Using a Xenogeneic Acellular Dermal Matrix Membrane to Enhance the Reparability of Bone Marrow Mesenchymal Stem Cells for Cartilage Injury. Bioengineering 2023, 10, 916. https://doi.org/10.3390/bioengineering10080916
Shi W, Meng Q, Hu X, Cheng J, Shao Z, Yang Y, Ao Y. Using a Xenogeneic Acellular Dermal Matrix Membrane to Enhance the Reparability of Bone Marrow Mesenchymal Stem Cells for Cartilage Injury. Bioengineering. 2023; 10(8):916. https://doi.org/10.3390/bioengineering10080916
Chicago/Turabian StyleShi, Weili, Qingyang Meng, Xiaoqing Hu, Jin Cheng, Zhenxing Shao, Yuping Yang, and Yingfang Ao. 2023. "Using a Xenogeneic Acellular Dermal Matrix Membrane to Enhance the Reparability of Bone Marrow Mesenchymal Stem Cells for Cartilage Injury" Bioengineering 10, no. 8: 916. https://doi.org/10.3390/bioengineering10080916
APA StyleShi, W., Meng, Q., Hu, X., Cheng, J., Shao, Z., Yang, Y., & Ao, Y. (2023). Using a Xenogeneic Acellular Dermal Matrix Membrane to Enhance the Reparability of Bone Marrow Mesenchymal Stem Cells for Cartilage Injury. Bioengineering, 10(8), 916. https://doi.org/10.3390/bioengineering10080916







