Deammonification Potential of Pig Slurries and Vapor Condensates from Sewage Sludge Drying—Substrate Quality and Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Pig Slurry Collection
2.2. Collection of Vapor Condensates
2.3. Physiochemical Characterization
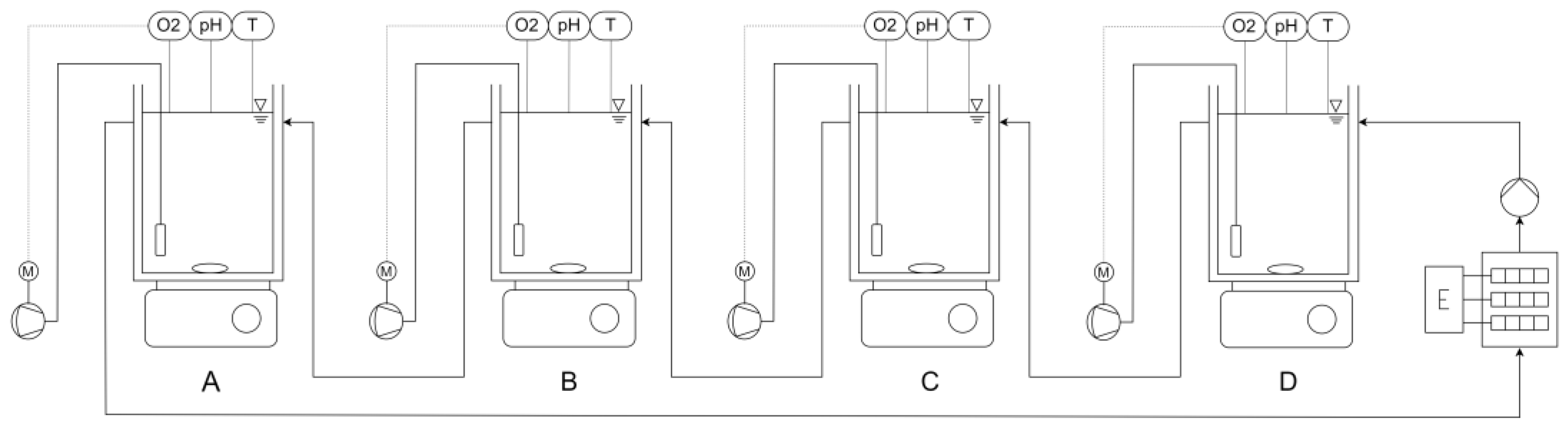
2.4. Inhibition on Nitritation
Experimental Setting for C-1
3. Results
3.1. Characterization and of Examination of Pig Slurry
3.1.1. Composition of Pig Slurries
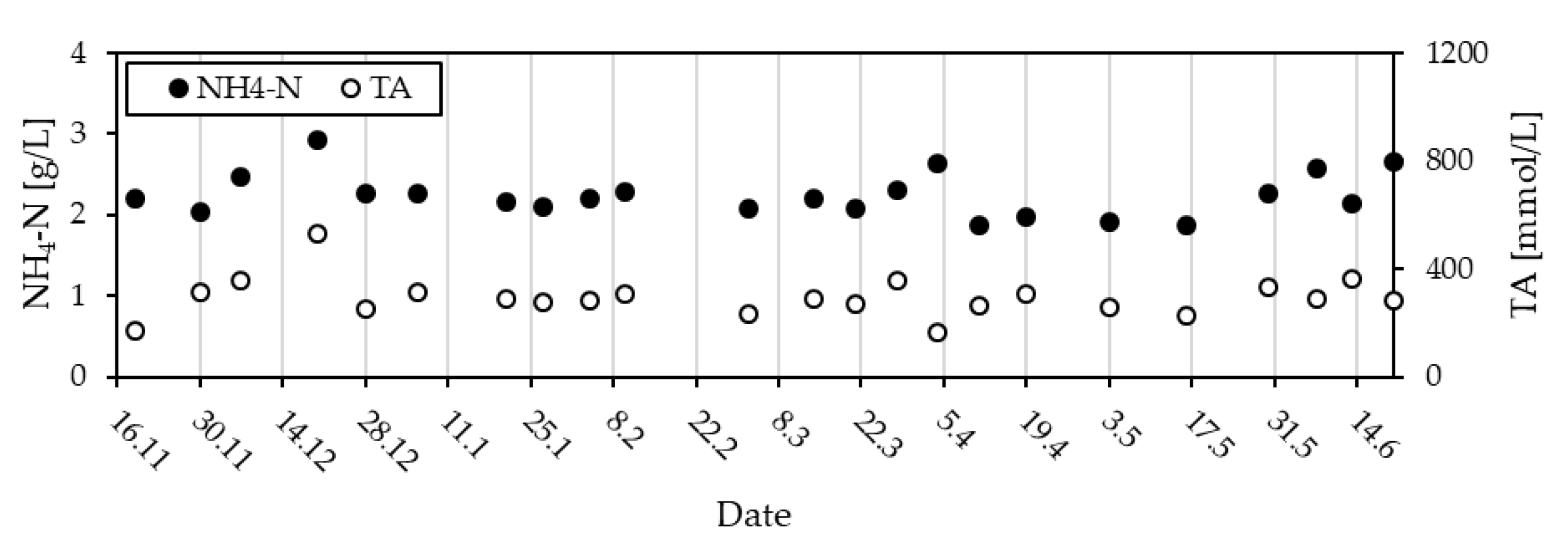
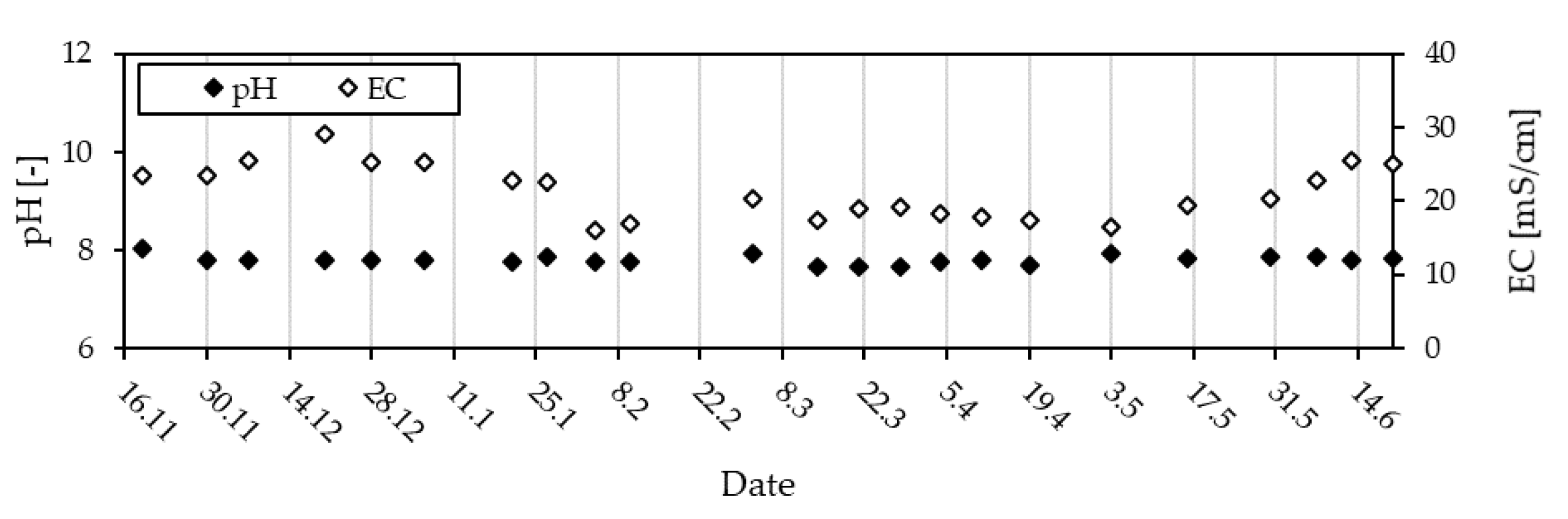
3.1.2. Pig Slurry Monitoring
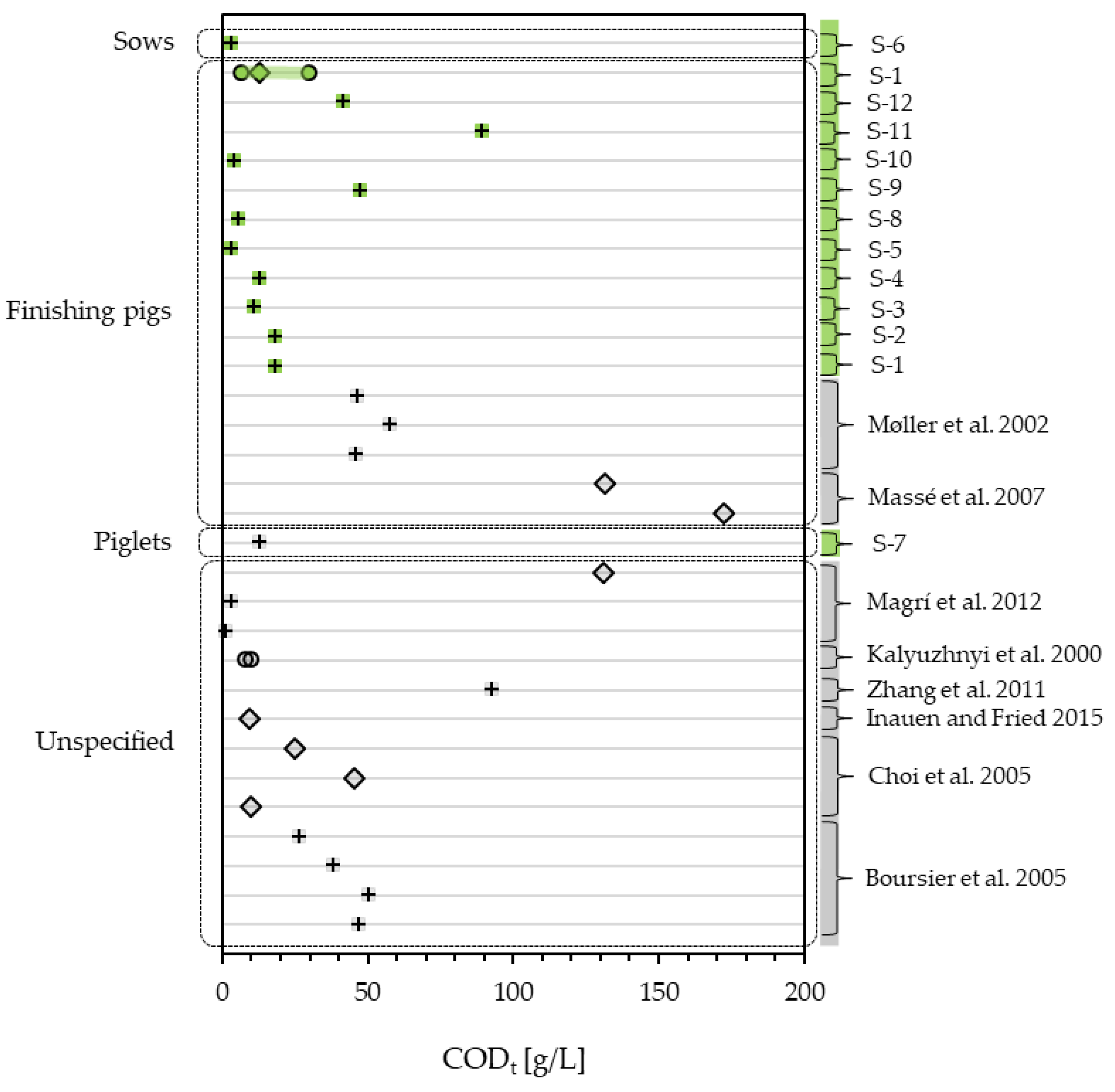
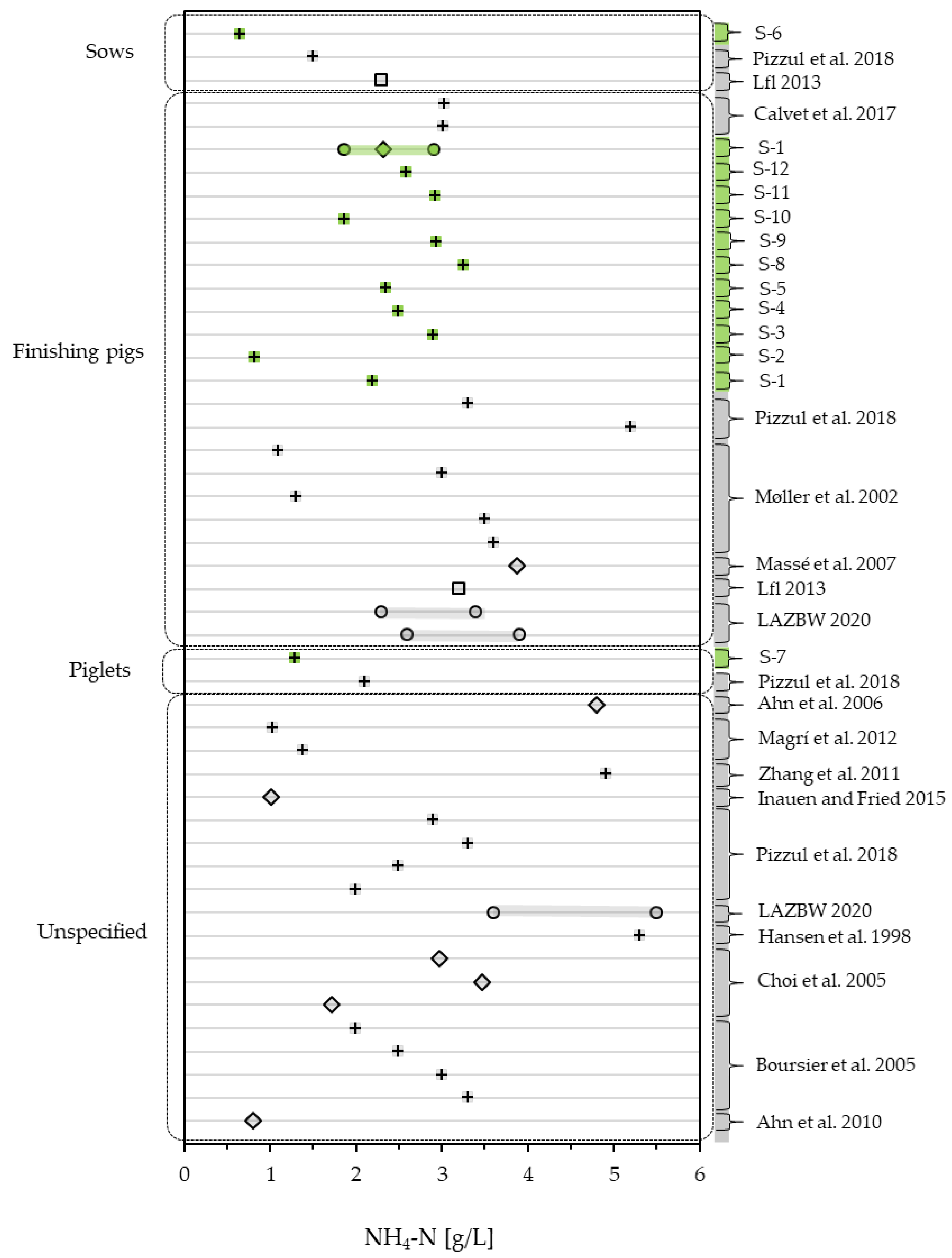
3.1.3. Slurry Quality—An Overview of CODt, CODs and NH4-N
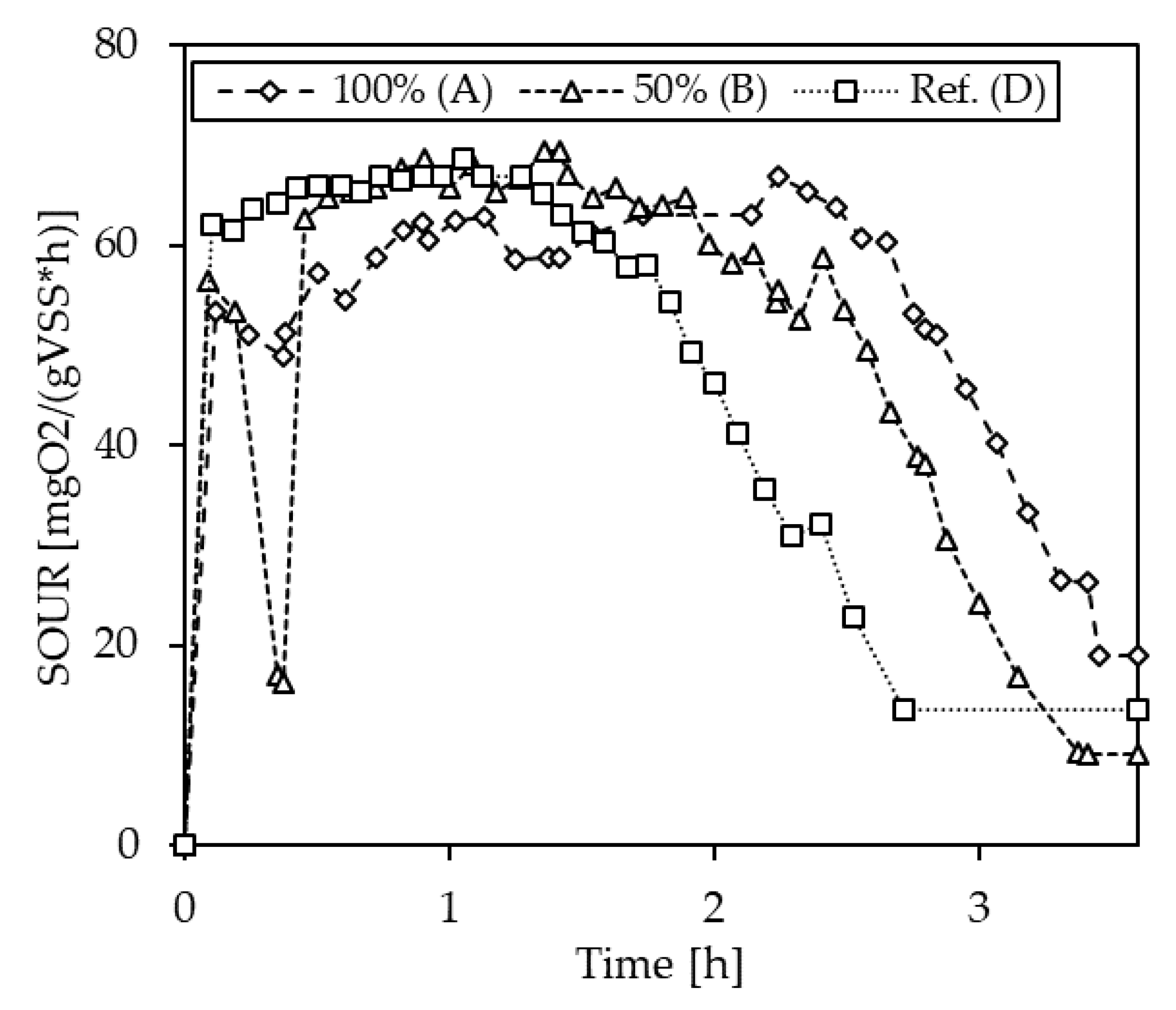
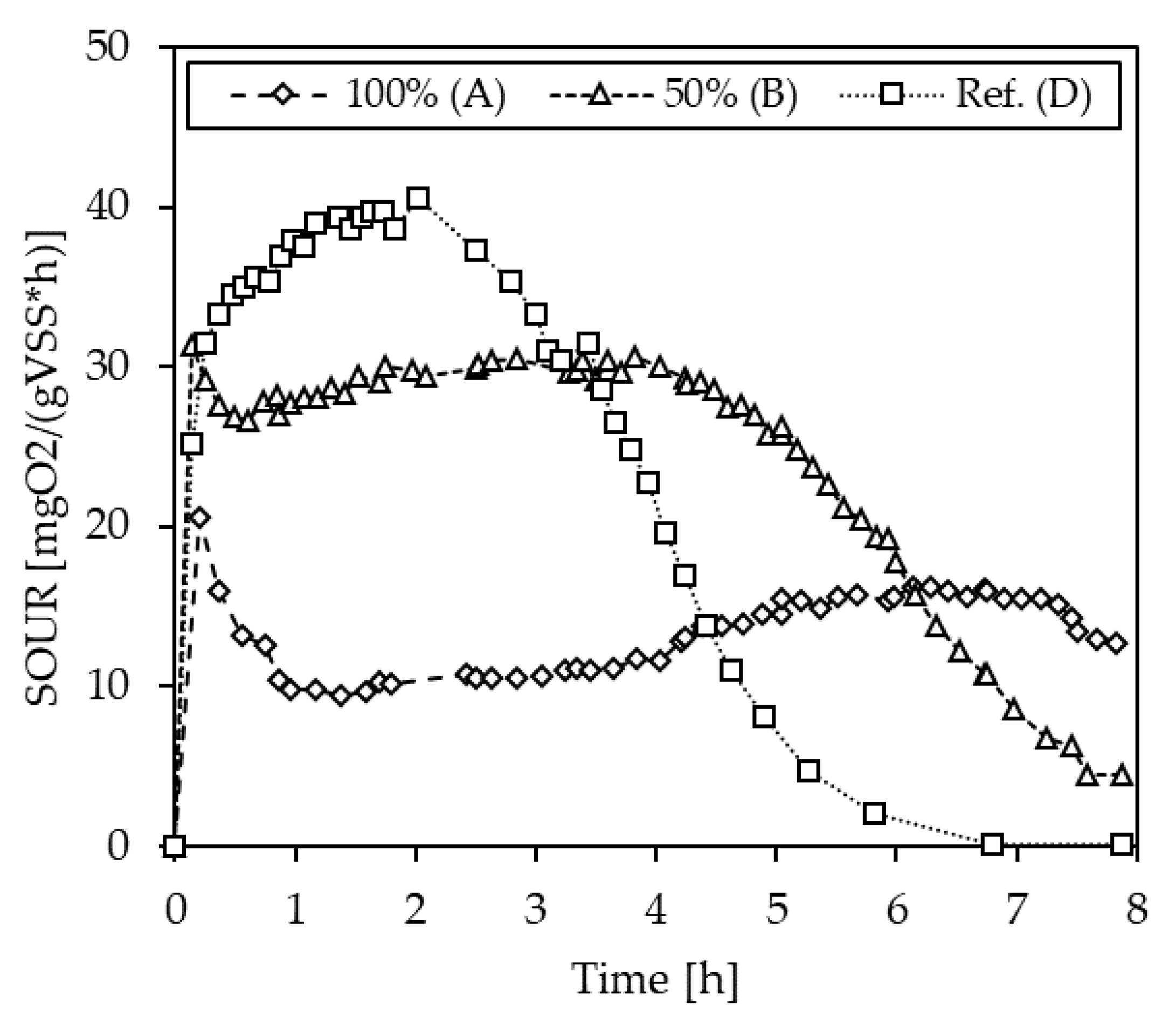
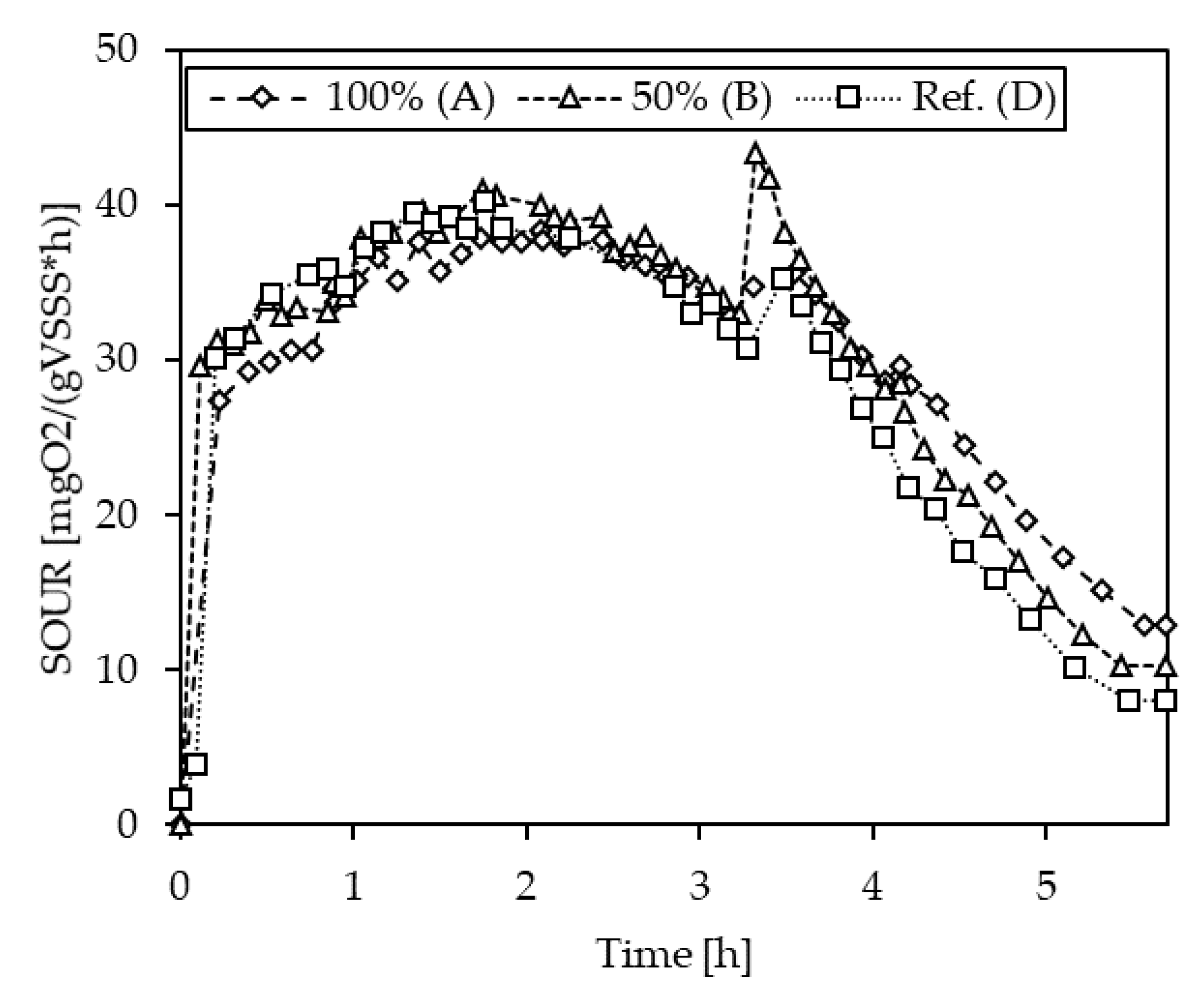
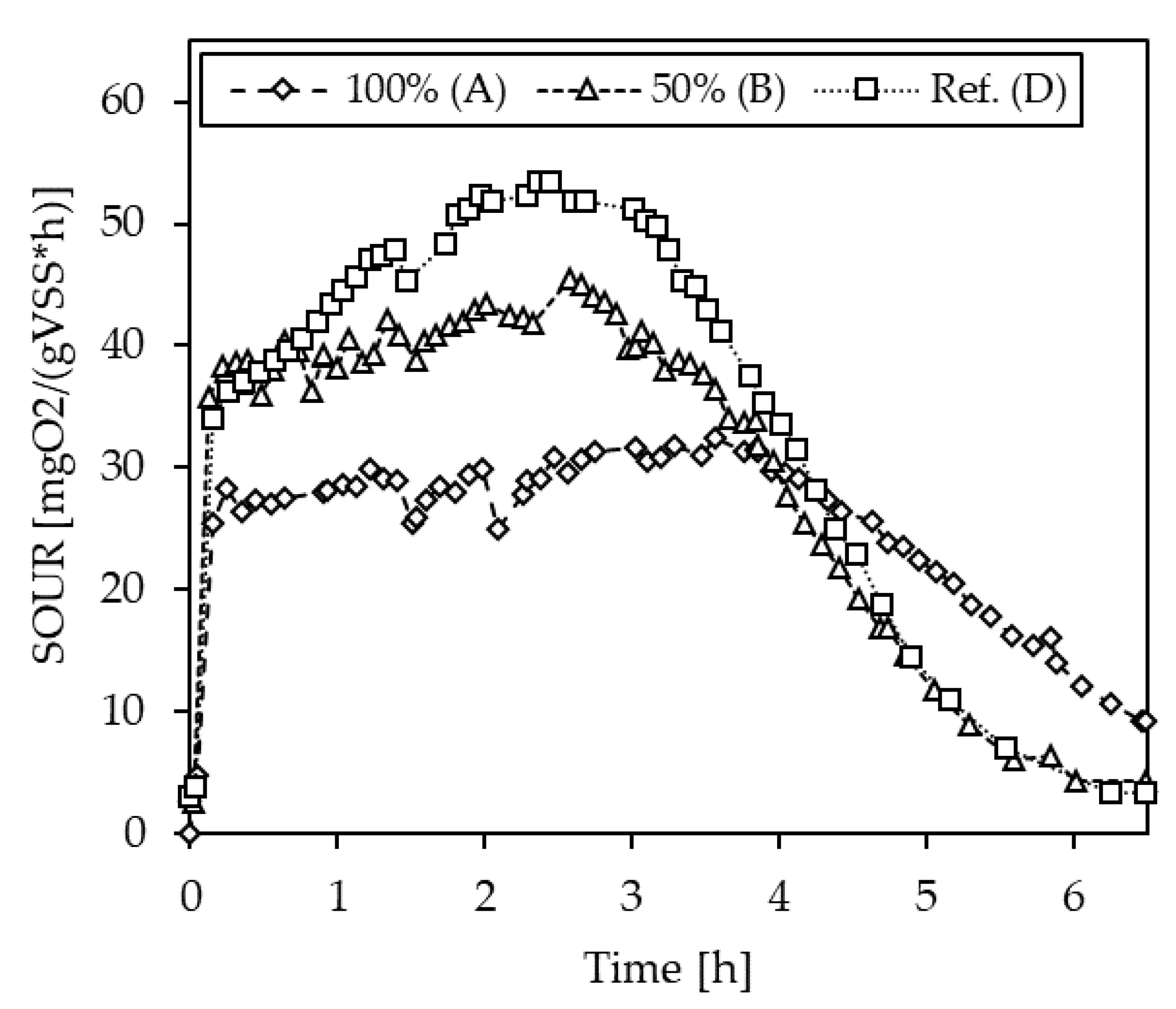
3.1.4. Inhibition Testing with Pig Slurries
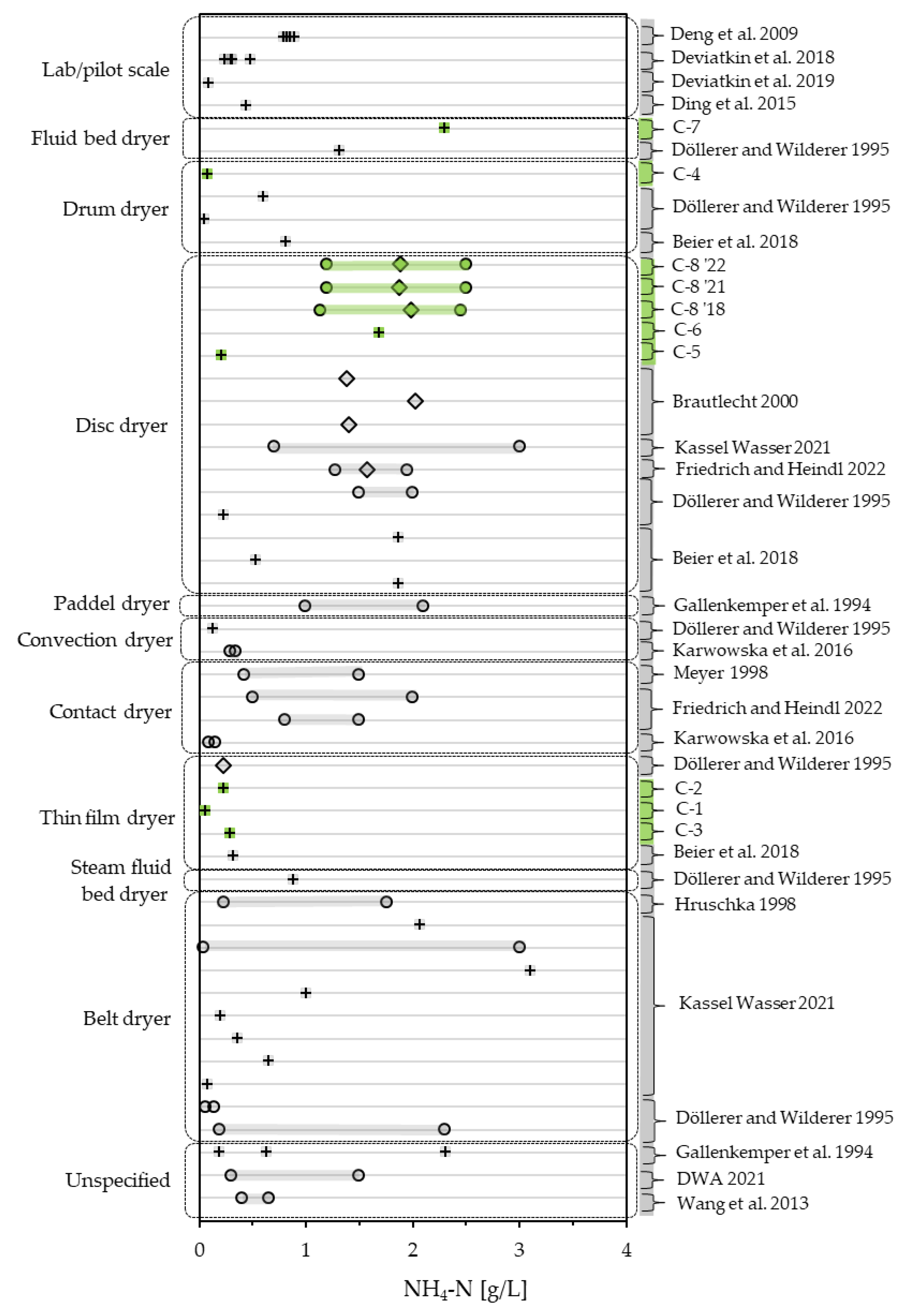
3.2. Characterization and of Examination of Vapor Condensates
3.2.1. Composition of Vapor Condensates
3.2.2. Condensate Monitoring
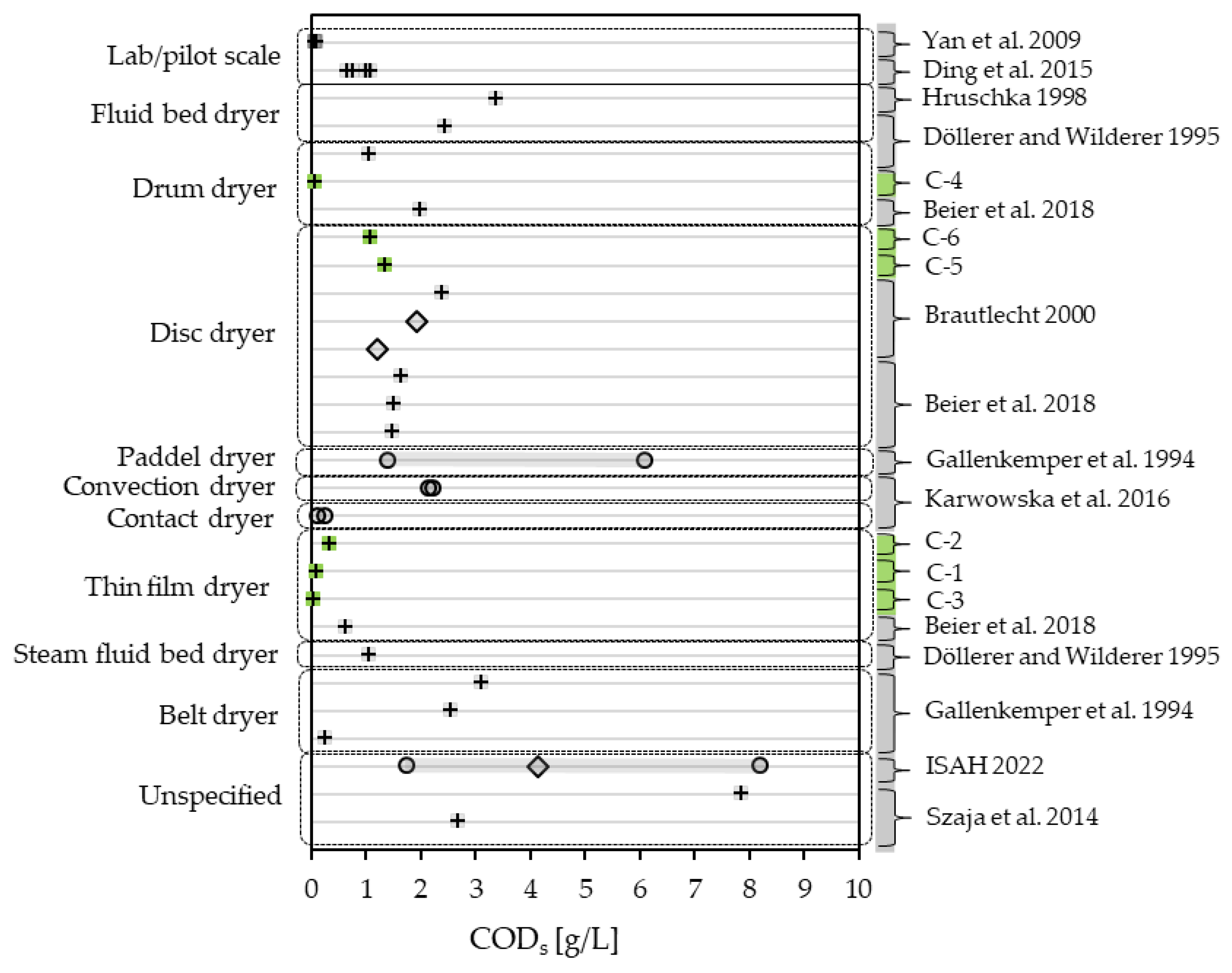
3.2.3. Condensate Quality—An Overview of CODt, CODs and NH4-N
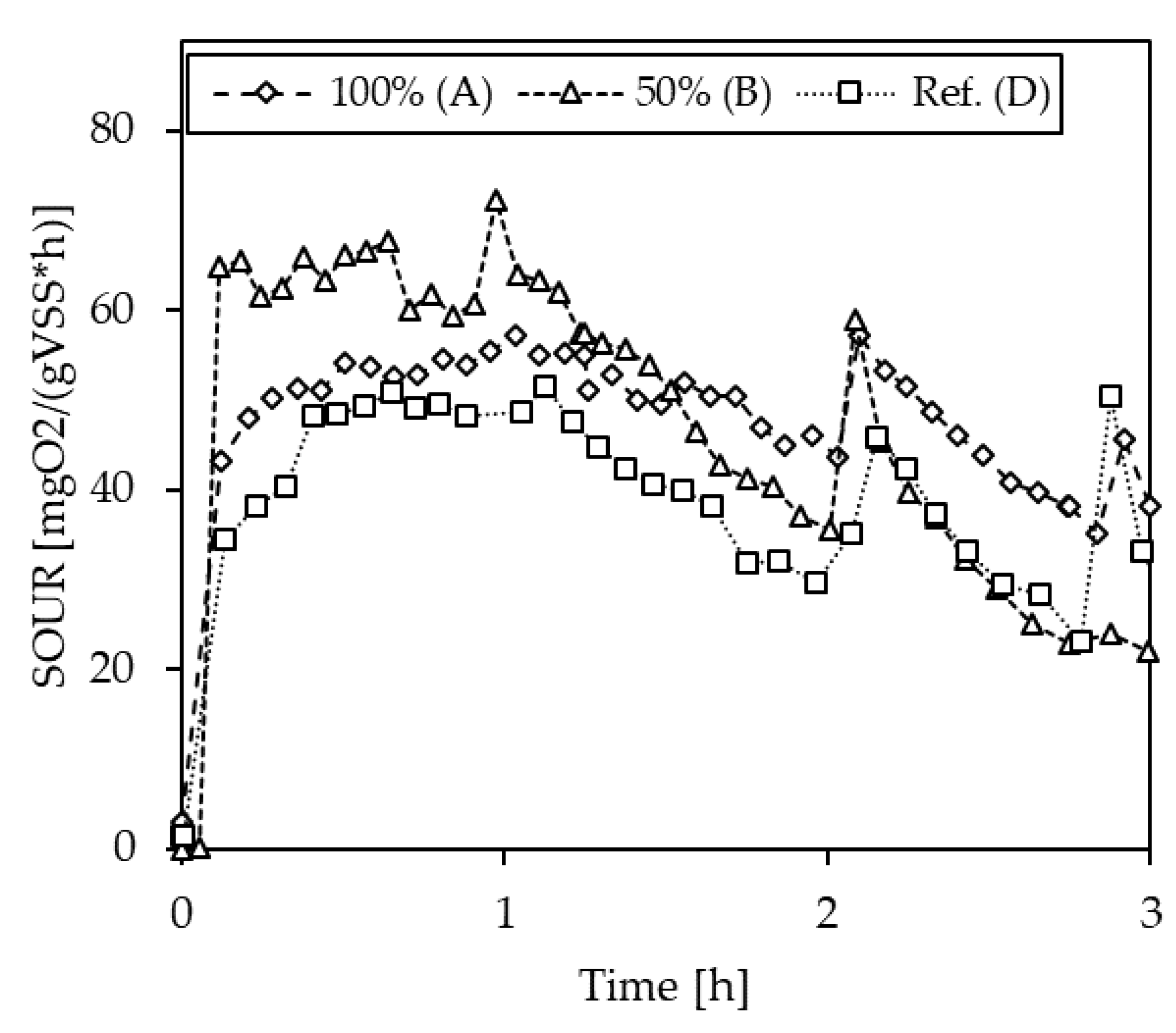
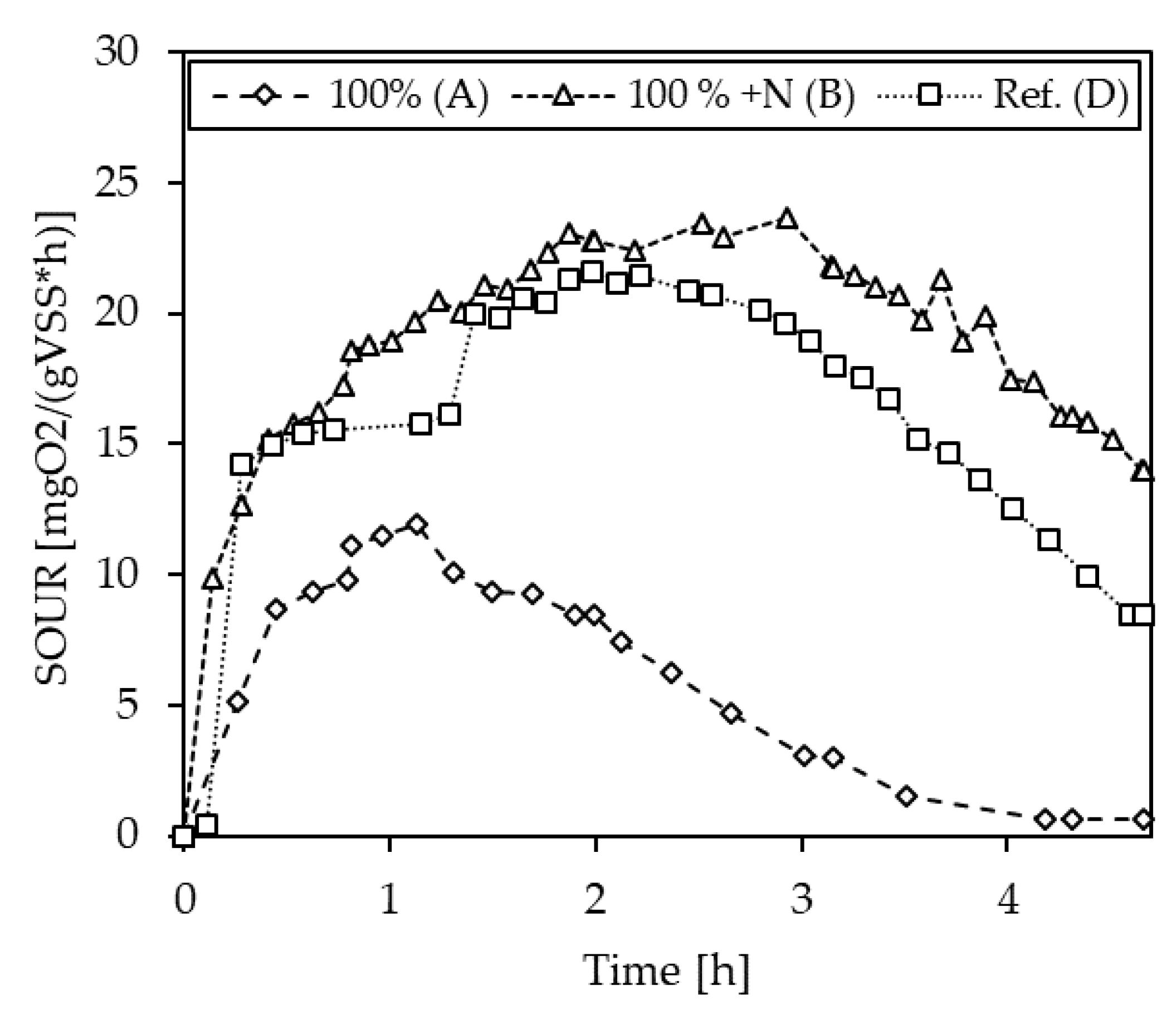
3.2.4. Inhibition Testing with Vapor Condensates
4. Discussion
4.1. Discussion of Substrate Quality
4.2. Discussion of AOB Inhibition
4.3. Discussion of Deammonification Potential
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Abbreviation | Parameter | Unit |
|---|---|---|
| COD | Chemical Oxygen Demand | [mg/L; g/L] |
| CODt | Total Chemical Oxygen Demand | [mg/L; g/L] |
| CODs | Soluble Chemical Oxygen Demand | [mg/L; g/L] |
| CODb | Biodegradable Chemical Oxygen Demand | [mg/L; g/L] |
| BOD5 | Biological Oxygen Demand after 5 days | [mg/L; g/L] |
| TOC | Total Organic Carbon | [mg/L; g/L] |
| TN | Total Nitrogen | [mg/L; g/L] |
| TKN | Total Kjeldahl Nitrogen | [mg/L] |
| NH4-N | Ammonium nitrogen | [mg/L; g/L] |
| NO2-N | Nitrite nitrogen | [mg/L] |
| NO3-N | Nitrate nitrogen | [mg/L] |
| FA | Free Ammonia | [mg/L] |
| TA | Total Alkalinity | [mmol/L] |
| TP | Total Phosphorous | [mg/L] |
| PO4-P | Orthophosphate phosphorous | [mg/L] |
| TS | Total Solids | [g/kg] |
| VS | Volatile Solids | [g/kg] |
| TSS | Total Suspended Solids | [g/L] |
| VSS | Volatile Suspended Solids | [g/L] |
| LOI | Loss On Ignition | [%] |
| pH | pH value | [-] |
| EC | Electrical Conductivity | [mS/cm] |
| DO | Dissolved Oxygen | [mg/L] |
| OUR | Oxygen Uptake Rate | [mg/L/h] |
| SOUR | Specific Oxygen Uptake Rate | [mg/gVSS/h] |
| S-1 | S-2 | S-3 | S-4 | S-5 | S-6 | S-7 | S-8 | S-9 | S-10 | S-11 | S-12 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TS | [g/kg] | 15.9 | 51.8 | 12.5 | - | 125.1 | 5.4 | 39.5 | 78.3 | 46.3 | 92.2 | 100.6 | 42.1 |
| LOI | [%] | 69.2 | 69.5 | 51.2 | - | 71.5 | 61.1 | 67.1 | 81.4 | 72.8 | 59.1 | 81.9 | 65.6 |
| pH | [-] | 7.96 | 7.16 | - | - | 7.31 | 7.48 | 7.67 | 7.40 | 8.05 | 8.07 | 7.34 | 7.91 |
| CODt | [g/L] | 18.51 | 18.36 | 11.09 | 12.84 | 3.13 | 3.16 | 12.80 | 5.67 | 47.70 | 3.94 | 89.50 | 41.60 |
| CODs | [g/L] | 2.53 | 3.24 | 6.16 | 5.72 | 0.79 | 1.29 | 3.67 | 0.36 | 0.50 | 3.48 | 19.40 | 8.28 |
| TN | [g/L] | 4.17 | 2.54 | 3.56 | 2.93 | 5.54 | 0.72 | 2.05 | 5.77 | 5.32 | 4.75 | 8.84 | 4.26 |
| NH4-N | [g/L] | 2.20 | 0.82 | 2.91 | 2.50 | 2.35 | 0.65 | 1.29 | 3.26 | 2.94 | 1.86 | 2.92 | 2.58 |
| NO2-N | [mg/L] | 0.5 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| NO3-N | [mg/L] | 13.2 | 3.7 | 23.4 | 22.9 | 24.5 | 16.3 | 18.8 | 13.7 | 9.1 | 9.1 | 16.8 | 35.8 |
| TP | [mg/L] | 346 | 1010 | 109 | 124 | 2720 | 70 | 267 | 1360 | 1130 | 3320 | 1830 | 1030 |
| PO4-P | [mg/L] | 50 | 27 | 45 | - | 138 | 51 | 112 | 268 | 161 | 175 | 284 | 220 |
| TA | [mmol/L] | 383 | 75 | 198 | 201 | 286 | 70 | 129 | 263 | 256 | 209 | 206 | 259 |
| CODs:CODt | [%] | 13.7 | 17.7 | 55.5 | 44.6 | 25.3 | 40.8 | 28.7 | 6.3 | 1.0 | 88.3 | 21.7 | 43.1 |
| CODs:NH4-N | [-] | 1.1 | 4.0 | 2.1 | 2.3 | 0.3 | 2.0 | 2.8 | 0.1 | 0.2 | 1.9 | 6.6 | 3.2 |
| NH4-:TN | [%] | 52.8 | 32.3 | 81.6 | 85.3 | 42.3 | 91.1 | 63.1 | 56.5 | 55.3 | 39.2 | 33.0 | 60.6 |
| TA:NH4-N | [mmol/mg] | 0.17 | 0.09 | 0.07 | 0.08 | 0.12 | 0.11 | 0.10 | 0.09 | 0.11 | 0.07 | 0.10 | 0.12 |


| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C-7 | C-818 * | C-821 * | C-822 * | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TSS | [mg/L] | 60 | 50 | 0.5 | 16.9 | 700 | 62.7 | - | 94 | 76 | 103 |
| LOI | [%] | 47.8 | 78.2 | ND | 33.3 | 68.0 | 83.0 | - | 80.2 | 85.2 | 86.0 |
| pH | [-] | 7.27 | 9.70 | 10.18 | 8.92 | 8.64 | 9.57 | 9.3 | 9.5 | 9.7 | 9.7 |
| EC | [mS/cm] | 1.564 | 0.818 | 0.714 | 1.230 | 1.998 | 4.310 | 10.039 | 7.412 | 6.443 | 6.269 |
| CODt | [mg/L] | 108 | 348 | 54 | 70 | 3158 | 1560 | 8950 | 1613 | 1967 | 1227 |
| CODs | [mg/L] | 104 | 324 | 53 | 60 | 1347 | 1072 | - | - | - | - |
| TN | [mg/L] | 66 | 261 | 343 | 91 | 302 | 1780 | 2600 | - | - | - |
| NH4-N | [mg/L] | 59 | 228 | 286 | 76 | 207 | 1690 | 2300 | 1992 | 1879 | 1894 |
| NO2-N | [mg/L] | 0.02 | 0.03 | 0.36 | 0.80 | 0.68 | 0.32 | - | - | - | - |
| NO3-N | [mg/L] | 1.02 | 1.33 | 2.90 | 0.34 | 4.50 | 0.92 | - | - | - | - |
| TP | [mg/L] | 38.40 | 2.43 | 0.01 | 0.45 | 1.51 | 1.74 | 120 | 1.7 | 1.1 | 1.6 |
| PO4-P | [mg/L] | 38.40 | 2.40 | ND | 0.33 | 1.42 | 1.41 | 40 | - | - | - |
| TA | [mmol/L] | 11.0 | 13.5 | 22.8 | 11.8 | 18.5 | 73.4 | - | - | - | - |
| CODs:CODt | [%] | 96.3 | 93.1 | 98.3 | 86.2 | 42.7 | 68.7 | - | - | - | - |
| CODs:NH4-N | [-] | 1.8 | 1.4 | 0.2 | 0.8 | 6.5 | 0.6 | - | - | - | - |
| NH4-:TN | [%] | 89.4 | 87.4 | 83.4 | 83.6 | 68.5 | 94.9 | 88.5 | - | - | - |
| TA:NH4-N | [mmol/mg] | 0.19 | 0.06 | 0.08 | 0.15 | 0.09 | 0.04 | - | - | - | - |
References
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic Digestion of Swine Manure: Inhibition by Ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.-W.; Jahng, D. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef] [PubMed]
- Pizzul, L.; Rodhe, L.; Burzynska, I.; Kieronczyk, M.; Mazur, K.; Neumann, S.; Tamm, K.; Jakovickis, R.; Sekowski, M. Titration, Buffer Capacity and Acid Consumption of Animal Slurries in Baltic Sea Region Countries: Research Results; 2018. Available online: http://balticslurry.eu/wp-content/uploads/2016/06/Titration-buffer-capacity-and-acid-consumption-of-animal-slurries-in-Baltic-Sea-Region-countries-.pdf (accessed on 30 May 2023).
- Kassel Wasser. Klärschlammverwertung und Phosphorrecycling für die Region Nord-Ost-Hessen (RePhoNOH): Machbarkeitsstudie, 2021. Available online: https://umwelt.hessen.de/sites/umwelt.hessen.de/files/2021-08/endbericht-rephonoh-geschuetzt-.pdf (accessed on 30 May 2023).
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of Nitrification by Ammonia and Nitrous Acid. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar]
- Bonassa, G.; Bolsan, A.C.; Hollas, C.E.; Venturin, B.; Candido, D.; Chini, A.; De Prá, M.C.; Antes, F.G.; Campos, J.L.; Kunz, A. Organic carbon bioavailability: Is it a good driver to choose the best biological nitrogen removal process? Sci. Total Environ. 2021, 786, 147390. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Rind, S.; Rani, K. Systematic review: External carbon source for biological denitrification for wastewater. Biotechnol. Bioeng. 2023, 120, 642–658. [Google Scholar] [CrossRef]
- Samuelsson, P.; Halvarsson, B.; Carlsson, B. Cost-efficient operation of a denitrifying activated sludge process. Water Res. 2007, 41, 2325–2332. [Google Scholar] [CrossRef]
- Vineyard, D.; Hicks, A.; Karthikeyan, K.G.; Barak, P. Economic analysis of electrodialysis, denitrification, and anammox for nitrogen removal in municipal wastewater treatment. J. Clean. Prod. 2020, 262, 121145. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hamza, R.A.; Elbeshbishy, E. Enhancement of denitrification efficiency using municipal and industrial waste fermentation liquids as external carbon sources. Sci. Total Environ. 2022, 816, 151578. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences—An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef]
- Brock, T.D.; Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms, 15th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2017; ISBN 0134261925. [Google Scholar]
- Schneider, Y.; Beier, M.; Rosenwinkel, K.-H. Determination of the nitrous oxide emission potential of deammonification under anoxic conditions. Water Environ. Res. 2011, 83, 2199–2210. [Google Scholar] [CrossRef]
- Peng, L.; Carvajal-Arroyo, J.M.; Seuntjens, D.; Prat, D.; Colica, G.; Pintucci, C.; Vlaeminck, S.E. Smart operation of nitritation/denitritation virtually abolishes nitrous oxide emission during treatment of co-digested pig slurry centrate. Water Res. 2017, 127, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-S.; Min, K.-S.; Lee, Y.-O. Piggery Waste Treatment using Partial Nitritation and Anaerobic Ammonium Oxidation. J. Korean Soc. Water Environ. 2006, 22, 599–604. [Google Scholar]
- Molinuevo, B.; García, M.C.; Karakashev, D.; Angelidaki, I. Anammox for ammonia removal from pig manure effluents: Effect of organic matter content on process performance. Bioresour. Technol. 2009, 100, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Yamamoto, T.; Misaka, M.; Isaka, K.; Sumino, T.; Bhatti, Z.; Furukawa, K. High-rate nitrogen removal from livestock manure digester liquor by combined partial nitritation-anammox process. Biodegradation 2010, 21, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.; Vázquez-Padín, J.R.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Is the CANON reactor an alternative for nitrogen removal from pre-treated swine slurry? Biochem. Eng. J. 2012, 65, 23–29. [Google Scholar] [CrossRef]
- Staunton, E.T.; Aitken, M.D. Coupling Nitrogen Removal and Anaerobic Digestion for Energy Recovery from Swine Waste: 2 Nitritation/Anammox. Environ. Eng. Sci. 2015, 32, 750–760. [Google Scholar] [CrossRef]
- Pichel, A.; Moreno, R.; Figueroa, M.; Campos, J.L.; Mendez, R.; Mosquera-Corral, A.; Del Val Rio, A. How to cope with NOB activity and pig manure inhibition in a partial nitritation-anammox process? Sep. Purif. Technol. 2019, 212, 396–404. [Google Scholar] [CrossRef]
- Chini, A.; Bolsan, A.C.; Hollas, C.E.; Antes, F.G.; Fongaro, G.; Treichel, H.; Kunz, A. Evaluation of deammonification reactor performance and microrganisms community during treatment of digestate from swine sludge CSTR biodigester. J. Environ. Manag. 2019, 246, 19–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, L.; Chen, Y.; Cao, W.; Zhang, Y. Effects of hydroxylamine on treatment of anaerobic digestate of pig manure in partial nitrification-anaerobic ammonium oxidation. Bioresour. Technol. 2022, 363, 128015. [Google Scholar] [CrossRef]
- de Prá, M.C.; Kunz, A.; Bortoli, M.; Perondi, T.; Chini, A. Simultaneous removal of TOC and TSS in swine wastewater using the partial nitritation process. J. Chem. Technol. Biotechnol. 2012, 87, 1641–1647. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, R.; Sui, Q.; Ritigala, T.; Wei, Y.; Cheng, X.; Ren, J.; Yu, D.; Chen, M.; Wang, T. Coupling anammox with denitrification in a full-scale combined biological nitrogen removal process for swine wastewater treatment. Bioresour. Technol. 2021, 329, 124906. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, F.; Shen, J.; Guo, Y.; Wang, S.; Qiang, H.; Qin, Y.; Li, Y.-Y. Control strategy and performance of simultaneous removal of nitrogen and organic matter in treating swine manure digestate using one reactor with airlift and micro-granule. Bioresour. Technol. 2022, 355, 127199. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Shen, J.; Chen, F.; Guo, Y.; Qin, Y.; Li, Y.-Y. Increasing nitrogen and organic matter removal from swine manure digestate by including pre-denitrification and recirculation in single-stage partial nitritation/anammox. Bioresour. Technol. 2023, 367, 128229. [Google Scholar] [CrossRef] [PubMed]
- Beier, M.; Mahnig, N.; Mikeska, M.; Rosenwinkel, K.-H. Entwicklung und Integration innovativer Kläranlagentechnologien für den Transformazionsprozess in Richtung Technikwende: Teilprojekt 2. Gefördert im Rahmen der BMBF-Fördermaßnahme ERWAS, FKZ: 02WER1319B, 2018, 205–230. Available online: https://bmbf.nawam-erwas.de/sites/default/files/abschlussbericht_e-klaer_geschuetzt.pdf (accessed on 30 May 2023).
- Brautlecht, P. Technische und Ökonomische Aspekte Kommunaler Klärschlammtrocknungsanlagen (Bd. 181); Institut für Siedlungswasserwirtschaft der RWTH Aachen: Aachen, Germany, 2000. [Google Scholar]
- Döllerer, J.; Wilderer, P.A. Reinigung von Brüdenkondensaten aus der Klärschlammtrocknung durch Einsatz des Sequencing-Batch-Biofilm-Reaktor-Verfahrens. Korresp. Abwasser 1995, 42, 69–73. [Google Scholar]
- Karwowska, B.; Sperczyńska, E.; Wiśniowska, E. Characteristics of reject waters and condensates generated during drying of sewage sludge from selected wastewater treatment plants. Desalination Water Treat. 2016, 57, 1176–1183. [Google Scholar] [CrossRef]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresour. Technol. 2002, 85, 189–196. [Google Scholar] [CrossRef]
- Choi, E.; Kim, D.; Eum, Y.; Yun, Z.; Min, K.-S. Full-Scale Experience for Nitrogen Removal from Piggery Waste. Water Environ. Res. 2005, 77, 381–389. [Google Scholar] [CrossRef]
- Meyer, J.-U. Entwicklung und Bilanzierung von Verfahrensketten zur Klärschlammentsorgung; Cuvillier: Göttingen, Germany, 1998; ISBN 3-89712-060-7. [Google Scholar]
- Deutsches Institut für Normung e.V. Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung; Summarische Wirkungs- und Stoffkenngrößen (Gruppe H); Bestimmung des Gesamttrockenrückstandes, des Filtrattrockenrückstandes und des Glührückstandes, 1987–01 (38409-1); Beuth Verlag: Berlin, Germany, 1987. [Google Scholar]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Kolisch, G.; Rolfs, T. Integrated sidestream treatment for enhanced enlargement of sewage plants. Water Sci. Technol. 2000, 41, 155–162. [Google Scholar] [CrossRef]
- Ahn, H.K.; Smith, M.C.; Kondrad, S.L.; White, J.W. Evaluation of biogas production potential by dry anaerobic digestion of switchgrass—Animal manure mixtures. Appl. Biochem. Biotechnol. 2010, 160, 965–975. [Google Scholar] [CrossRef]
- Magrí, A.; Vanotti, M.B.; Szögi, A.A.; Cantrell, K.B. Partial nitritation of swine wastewater in view of its coupling with the anammox process. J. Environ. Qual. 2012, 41, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Masse, L.; Massé, D.I.; Beaudette, V.; Muir, M. Size Distribution and Composition of Particles in Raw and Anaerobically Digested Swine Manure. Trans. ASAE 2005, 48, 1943–1949. [Google Scholar] [CrossRef]
- Boursier, H.; Béline, F.; Paul, E. Piggery wastewater characterisation for biological nitrogen removal process design. Bioresour. Technol. 2005, 96, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Inauen, S.; Fried, N. BLUT, MILCH, MOLKE UND GÜLLE IN ABWASSER 2015. Available online: https://www.gr.ch/DE/institutionen/verwaltung/ekud/anu/ANU_Dokumente/ANU-402-26d_ALT_BlutJaucheSchotte_AquaGas.pdf (accessed on 30 May 2023).
- Kalyuzhnyi, S.; Sklyar, V.; Rodriguez-Martinez, J.; Archipchenko, I.; Barboulina, I.; Orlova, O.; Epov, A.; Nekrasova, V.; Nozhevnikova, A.; Kovalev, A.; et al. Integrated mechanical, biological and physico-chemical treatment of liquid manure streams. Water Sci. Technol. 2000, 41, 175–182. [Google Scholar] [CrossRef]
- Massé, D.I.; Croteau, F.; Masse, L. The fate of crop nutrients during digestion of swine manure in psychrophilic anaerobic sequencing batch reactors. Bioresour. Technol. 2007, 98, 2819–2823. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid—Liquid separation of animal slurry in theory and practice. A review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Do, T.H.; Kim, S.D.; Hwang, S. The effect of calcium on the anaerobic digestion treating swine wastewater. Biochem. Eng. J. 2006, 30, 33–38. [Google Scholar] [CrossRef]
- Mondor, M.; Masse, L.; Ippersiel, D.; Lamarche, F.; Massé, D.I. Use of electrodialysis and reverse osmosis for the recovery and concentration of ammonia from swine manure. Bioresour. Technol. 2008, 99, 7363–7368. [Google Scholar] [CrossRef]
- LAZBW. Gülledüngung im Grünland. Merkblätter für Die Umweltgerechte Landbewirtschaftung. 2020. Available online: https://gruenland-online.de/medien/Merkblatt27_Guelleduengung_im_Gruenland_StandNov2020.pdf (accessed on 30 May 2023).
- LfL. Jahresbericht 2012, 2013. Available online: https://www.lfl.bayern.de/mam/cms07/iab/dateien/2_iab-jb-2012-24-06.pdf (accessed on 30 May 2023).
- Calvet, S.; Hunt, J.; Misselbrook, T.H. Low frequency aeration of pig slurry affects slurry characteristics and emissions of greenhouse gases and ammonia. Biosyst. Eng. 2017, 159, 121–132. [Google Scholar] [CrossRef]
- Viana, M.B.; Freitas, A.V.; Leitão, R.C.; Pinto, G.A.; Santaella, S.T. Anaerobic digestion of crude glycerol: A review. Environ. Technol. Rev. 2012, 1, 81–92. [Google Scholar] [CrossRef]
- Hruschka, H. Verfahren der Klaerschlammtrocknung auf bayerischen Klaeranlagen-Stand und Auswirkungen auf den praktischen Betrieb. In Wohin mit dem Klärschlamm; Springer-VDI-Verlag: Düsseldorf, Germany, 1998; pp. 117–128. [Google Scholar]
- Friedrich, D.; Heindl, A. Einsatz des Scheibentrockners zur Trocknung von Klärschlamm: Teil 2: Fördertechnik, Brüden und Kondensat. Korresp. Abwasser Abfall 2022, 69, 45–51. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Ji, M. Characteristics of Emitted Odor and Discharged Condensate Water of Sludge Thermal Drying Project in Shenzhen Nanshan Thermal Power Plant. AMR 2013, 777, 127–132. [Google Scholar] [CrossRef]
- DWA. Klärschlammtrocknung: Merkblatt M-379, 1. Auflage; DWA: Hennef, Germany, 2021; ISBN 978-3-96862-097-8. [Google Scholar]
- Deng, W.-Y.; Yan, J.-H.; Li, X.-D.; Wang, F.; Zhu, X.-W.; Lu, S.-Y.; Cen, K.-F. Emission characteristics of volatile compounds during sludges drying process. J. Hazard. Mater. 2009, 162, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-H.; Deng, W.-Y.; Li, X.-D.; Wang, F.; Chi, Y.; Lu, S.-Y.; Cen, K.-F. Experimental and Theoretical Study of Agitated Contact Drying of Sewage Sludge under Partial Vacuum Conditions. Dry. Technol. 2009, 27, 787–796. [Google Scholar] [CrossRef]
- ISAH, Institute of Sanitary Engineering and Waste Management. Unpublished Data on Vapor Condensates from Sewage Sludge Drying in Drying Facility A. Report; Institute of Sanitary Engineering and Waste Management: Hannover, Germany, 2022. [Google Scholar]
- Szaja, A.; Aguilar, A.J.; Łagód, G. Chemical oxygen demand fractionation of reject water from municipal wastewater treatment plant. Proc. ECOpole 2014, 8, 449–454. [Google Scholar] [CrossRef]
- Gallenkemper, B.; Eitner, R.; Dauber, S.; Tränkler, J. Klärschlammentsorgung: Grundlagen und ausgewählte Verfahren; Economica Verl.: Bonn, Germany, 1994; ISBN 3-87081-331-8. [Google Scholar]
- Ding, W.; Li, L.; Liu, J. Investigation of the effects of temperature and sludge characteristics on odors and VOC emissions during the drying process of sewage sludge. Water Sci. Technol. 2015, 72, 543–552. [Google Scholar] [CrossRef]
- Deviatkin, I.; Lyu, L.; Chen, S.; Havukainen, J.; Wang, F.; Horttanainen, M.; Mänttäri, M. Technical implications and global warming potential of recovering nitrogen released during continuous thermal drying of sewage sludge. Waste Manag. 2019, 90, 132–140. [Google Scholar] [CrossRef]
- Deviatkin, I.; Havukainen, J.; Horttanainen, M. Possibilities for enhanced nitrogen recovery from digestate through thermal drying. J. Mater. Cycles Waste Manag. 2018, 20, 1016–1025. [Google Scholar] [CrossRef]
- KTBL. Faustzahlen für die Landwirtschaft; Kuratorium für Technik und Bauwesen in der Landwirtschaft, 15. Auflage; Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V. (KTBL): Darmstadt, Germany, 2018; ISBN 9783945088593. [Google Scholar]
- Trabue, S.L.; Kerr, B.J.; Scoggin, K.D.; Andersen, D.; van Weelden, M. Swine diets impact manure characteristics and gas emissions: Part I protein level. Sci. Total Environ. 2021, 755, 142528. [Google Scholar] [CrossRef]
- Horttanainen, M.; Deviatkin, I.; Havukainen, J. Nitrogen release from mechanically dewatered sewage sludge during thermal drying and potential for recovery. J. Clean. Prod. 2017, 142, 1819–1826. [Google Scholar] [CrossRef]
- DWA. Biologische Stickstoffelimination von Schlammwässern der Anaeroben Schlammstabilisierung: Merkblatt M-349, 1. Auflage; DWA: Hennef, Germany, 2019; ISBN 978-3-88721-824-9. [Google Scholar]



| Sample | Animal Type | Feed | Stable | Point of Collection |
|---|---|---|---|---|
| S-1 | Finishing pig | Standard | Slatted floor | Storage tank |
| S-2 | Finishing pig | Standard | Slatted floor | Storage tank |
| S-3 | Finishing pig | Standard | Slatted floor | Storage tank |
| S-4 | Finishing pig | Standard | Slatted floor | Storage tank |
| S-5 | Finishing pig | Standard | Slatted floor | Central channel |
| S-6 | Sow | Standard | Slatted floor | Central channel |
| S-7 | Piglet | Standard | Slatted floor | Central channel |
| S-8 | Finishing pig | Standard | Slatted floor | Central channel |
| S-9 | Finishing pig | Standard | Slatted floor | Storage tank |
| S-10 | Finishing pig | Standard | Slatted floor | Central channel |
| S-11 | Finishing pig | Standard | Slatted floor (animal welfare) | Central channel |
| S-12 | Finishing pig | N-P-reduced | Slatted floor | Storage tank |
| Sample | TS Sludge | Co-Substrates | Dryer Type | Temperature | Degree of Drying |
|---|---|---|---|---|---|
| [%] | [°C] | [%] | |||
| C-1 * | 25 | Fats (food industry) | Thin film dryer | 225–230 | 50–60 |
| + linear dryer | 95–100 | 75–80 | |||
| C-2 * | 25 | no | Thin film dryer + disc dryer | 190 | 80–85 |
| C-3 | 21–32 | Yes (unknown) | Thin film dryer | 170 | 42.5 |
| C-4 | 25 | no | Drum dryer | 360 | 93 |
| C-5 | 25.7 | Fats (food industry) | Disc dryer | 110–120 | 93 |
| C-6 | 20.5 | Fats, wet waste, glycerol | Disc dryer | 168 | 39 |
| C-7 ** | - | - | Fluid bed dryer | 150 | 98 |
| C-8 *** | 20.5 | Fats, wet waste, glycerol | Disc dryer | 168 | 39 |
| Reactor | A | B | C | D |
|---|---|---|---|---|
| VSS [g/L] | 1.2–1.3 | 1.2–1.3 | 1.2–1.3 | 1.2–1.3 |
| SL [gN/gVSS] | 0.06 | 0.06 | 0.06 | 0.06 |
| DO [mg/L] | 2–4 | 2–4 | 2–4 | 2–4 |
| OUR calculation [mg/L] | 2.2–3.8 | 2.2–3.8 | 2.2–3.8 | 2.2–3.8 |
| Temperatur [°C] | 26 | 26 | 26 | 26 |
| pH | 7.5–8 | 7.5–8 | 7.5–8 | 7.5–8 |
| Slurry/condensate | 100% | 50% | 100% | 0% |
| TW with NH4Cl | 0% | 50% | 0% | 100% |
| ATU | - | - | 86 µmol/L | - |
| pH | EC | TS | LOI | CODt | CODs | NH4-N | NO2-N | NO3-N | TA | |
|---|---|---|---|---|---|---|---|---|---|---|
| [-] | [mS/cm] | [g/kg] | [%] | [g/L] | [g/L] | [g/L] | [mg/L] | [mg/L] | [mmol/L] | |
| Min. | 7.65 | 16.00 | 11.3 | 40.1 | 6.775 | 2.750 | 1.870 | 0.00 | 5.41 | 166 |
| Max. | 8.08 | 29.25 | 17.7 | 68.9 | 29.750 | 8.610 | 2.916 | 2.79 | 67.00 | 532 |
| Mean | 7.83 | 22.41 | 14.7 | 50.5 | 13.144 | 5.373 | 2.327 | 0.29 | 29.91 | 291 |
| SD | 0.10 | 3.93 | 2.1 | 8.8 | 4.471 | 1.669 | 0.290 | 0.52 | 13.3 | 65 |
| %RSD | 1.3% | 17.6% | 14.4% | 17.4% | 34.0% | 31.6% | 12.5% | 176.7% | 44.6% | 22.3% |
| n | 30 | 30 | 7 | 7 | 26 | 24 | 30 | 30 | 30 | 30 |
| Max. SOUR | Deviation to Ref. | ||
|---|---|---|---|
| [mgO2/gVSS/h] | [%] | ||
| S-1 | A (100%) | 14.0 | −2.3 |
| B (50%) | 18.4 | 28.0 | |
| D (Ref.) | 14.4 | - | |
| S-2 | A (100%) | 63.4 | −4.2 |
| B (50%) | 66.5 | 0.4 | |
| D (Ref.) | 66.2 | - | |
| S-4 | A (100%) | 55.3 | 12.7 |
| B (50%) | 66.0 | 34.5 | |
| D (Ref.) | 49.0 | - |
| pH | EC | TSS | LOI | CODt | BOD5 | TKN | NH4-N | ||
|---|---|---|---|---|---|---|---|---|---|
| [-] | [mS/cm] | [mg/L] | [%] | [mg/L] | [mg/L] | [mg/L] | [mg/L] | ||
| 2018 | Min. | 9.0 | 3.420 | 0 | 2.6 | 747 | 120 | 1150 | 1140 |
| Max. | 9.9 | 11.800 | 1250 | 99.4 | 3300 | 680 | 2510 | 2450 | |
| Mean | 9.5 | 7.412 | 94 | 80.3 | 1613 | 317 | 2077 | 1992 | |
| SD | 0.2 | 1.880 | 163 | 14.8 | 537 | 123 | 328 | 317 | |
| %RSD | 2.0% | 26.3% | 173.0% | 18.4% | 33.3% | 38.9% | 15.8% | 15.9% | |
| n | 42 | 41 | 343 | 343 | 41 | 36 | 37 | 35 | |
| 2021 | Min. | 9.2 | 3.360 | 0 | 3.4 | 836 | 100 | 1490 | 1200 |
| Max. | 10.1 | 10.600 | 3820 | 99.3 | 3180 | 490 | 2730 | 2500 | |
| Mean | 9.7 | 6.443 | 76 | 85.2 | 1967 | 281 | 1997 | 1879 | |
| SD | 0.2 | 1.641 | 285 | 13.4 | 487 | 93 | 268 | 274 | |
| %RSD | 1.8% | 25.5% | 374.7% | 15.7% | 24.7% | 33.1% | 13.4% | 14.6% | |
| n | 38 | 38 | 317 | 321 | 38 | 37 | 38 | 38 | |
| 2022 | Min. | 9.4 | 3.050 | 1 | 8.6 | 308 | 74 | 1290 | 1200 |
| Max. | 9.9 | 9.430 | 3280 | 100.0 | 3550 | 610 | 2590 | 2500 | |
| Mean | 9.7 | 6.269 | 103 | 86.0 | 1227 | 275 | 2034 | 1894 | |
| SD | 0.1 | 1.580 | 327 | 13.8 | 668 | 128 | 338 | 324 | |
| %RSD | 1.4% | 25.2% | 316.9% | 16.0% | 54.4% | 46.6% | 16.6% | 17.1% | |
| n | 39 | 39 | 312 | 312 | 39 | 32 | 38 | 36 |
| Max. SOUR | Deviation to Ref. | ||
|---|---|---|---|
| [mgO2/gVSS/h] | [%] | ||
| C-1 | A (100%) | 10.1 | −51.2 |
| B (100% + N) | 23.0 | 11.0 | |
| D (Ref.) | 20.7 | - | |
| C-2 | A (100%) | 15.6 | −60.5 |
| B (50%) | 29.7 | −24.5 | |
| D (Ref.) | 39.4 | - | |
| C-3 | A (100%) | 36.5 | −5.8 |
| B (50%) | 38.2 | −1.3 | |
| D (Ref.) | 38.7 | - | |
| C-6 | A (100%) | 30.8 | −40.3 |
| B (50%) | 42.0 | −18.7 | |
| D (Ref.) | 51.7 | - | |
| C-7 * | 100% | - | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiter, J.; Beier, M. Deammonification Potential of Pig Slurries and Vapor Condensates from Sewage Sludge Drying—Substrate Quality and Inhibition. Bioengineering 2023, 10, 826. https://doi.org/10.3390/bioengineering10070826
Reiter J, Beier M. Deammonification Potential of Pig Slurries and Vapor Condensates from Sewage Sludge Drying—Substrate Quality and Inhibition. Bioengineering. 2023; 10(7):826. https://doi.org/10.3390/bioengineering10070826
Chicago/Turabian StyleReiter, Johannes, and Maike Beier. 2023. "Deammonification Potential of Pig Slurries and Vapor Condensates from Sewage Sludge Drying—Substrate Quality and Inhibition" Bioengineering 10, no. 7: 826. https://doi.org/10.3390/bioengineering10070826
APA StyleReiter, J., & Beier, M. (2023). Deammonification Potential of Pig Slurries and Vapor Condensates from Sewage Sludge Drying—Substrate Quality and Inhibition. Bioengineering, 10(7), 826. https://doi.org/10.3390/bioengineering10070826







