The Contribution of Various In Vitro Methodologies to Comprehending the Filling Ability of Root Canal Pastes in Primary Teeth
Abstract
1. Introduction
2. Materials and Methods
2.1. Teeth Selection
2.2. Teeth Preparation
2.3. Root Canal Obturation
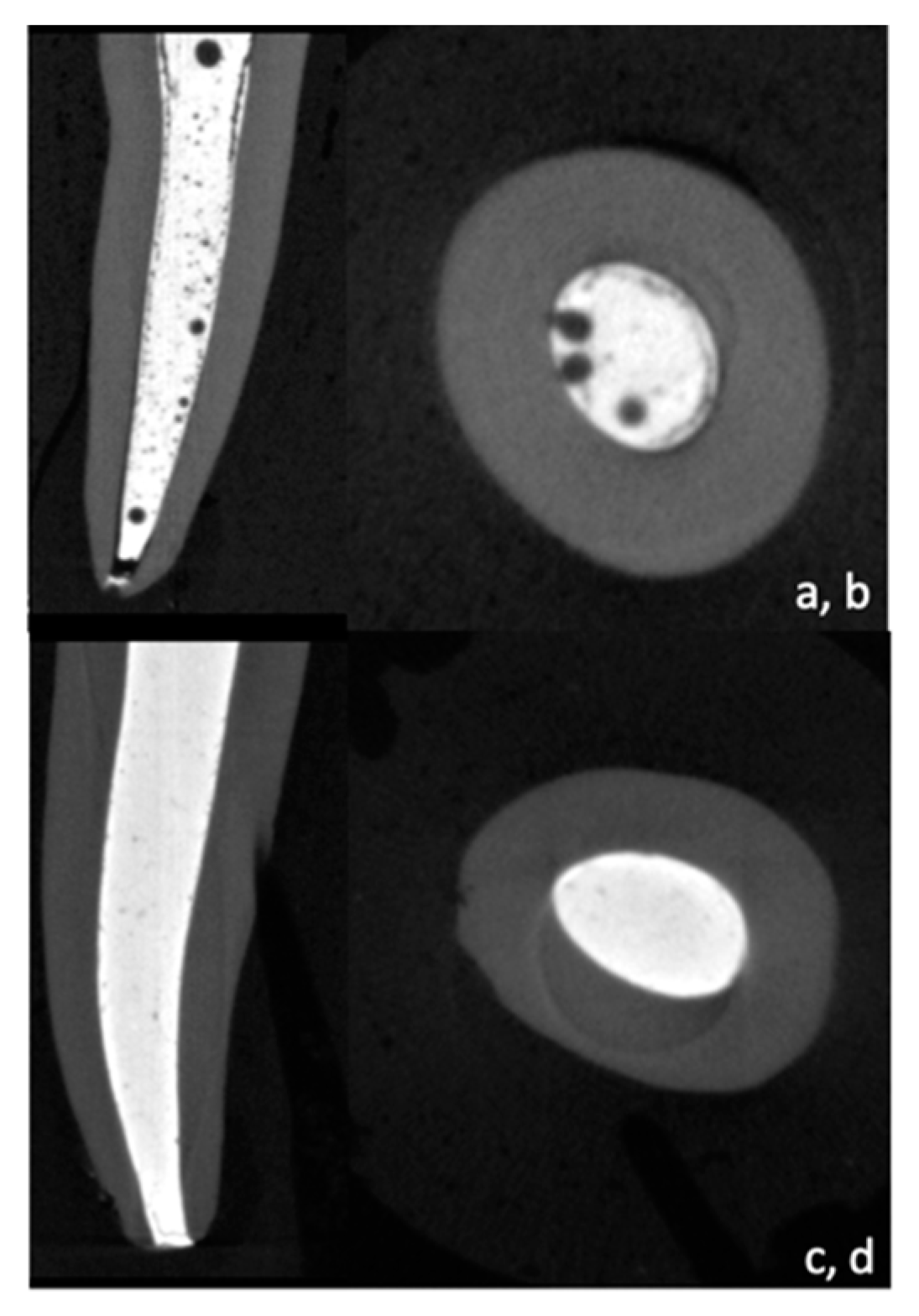
2.4. Micro CT Scanning
- Complete root with filling
- Complete filling with voids
- Filling without voids
- C: Canal (filling + voids)
- F: Filling without voids
- V: Voids
- Cc, Fc, and Vc for the coronal part
- Cm, Fm, and Vm for the middle part
- Ca, Fa, and Va for the apical part
2.5. Sectioning
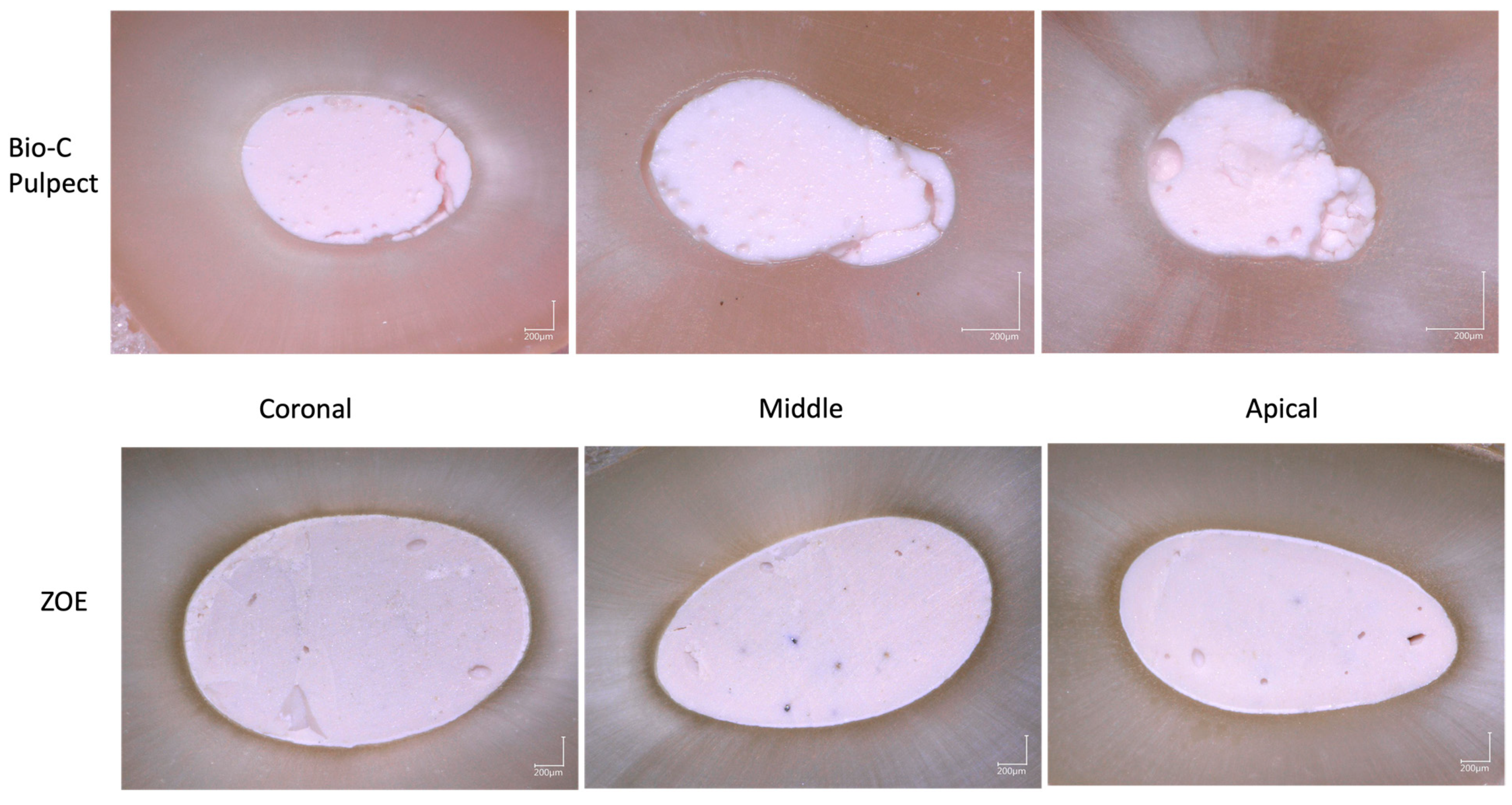
2.6. Digital Microscopy Observations
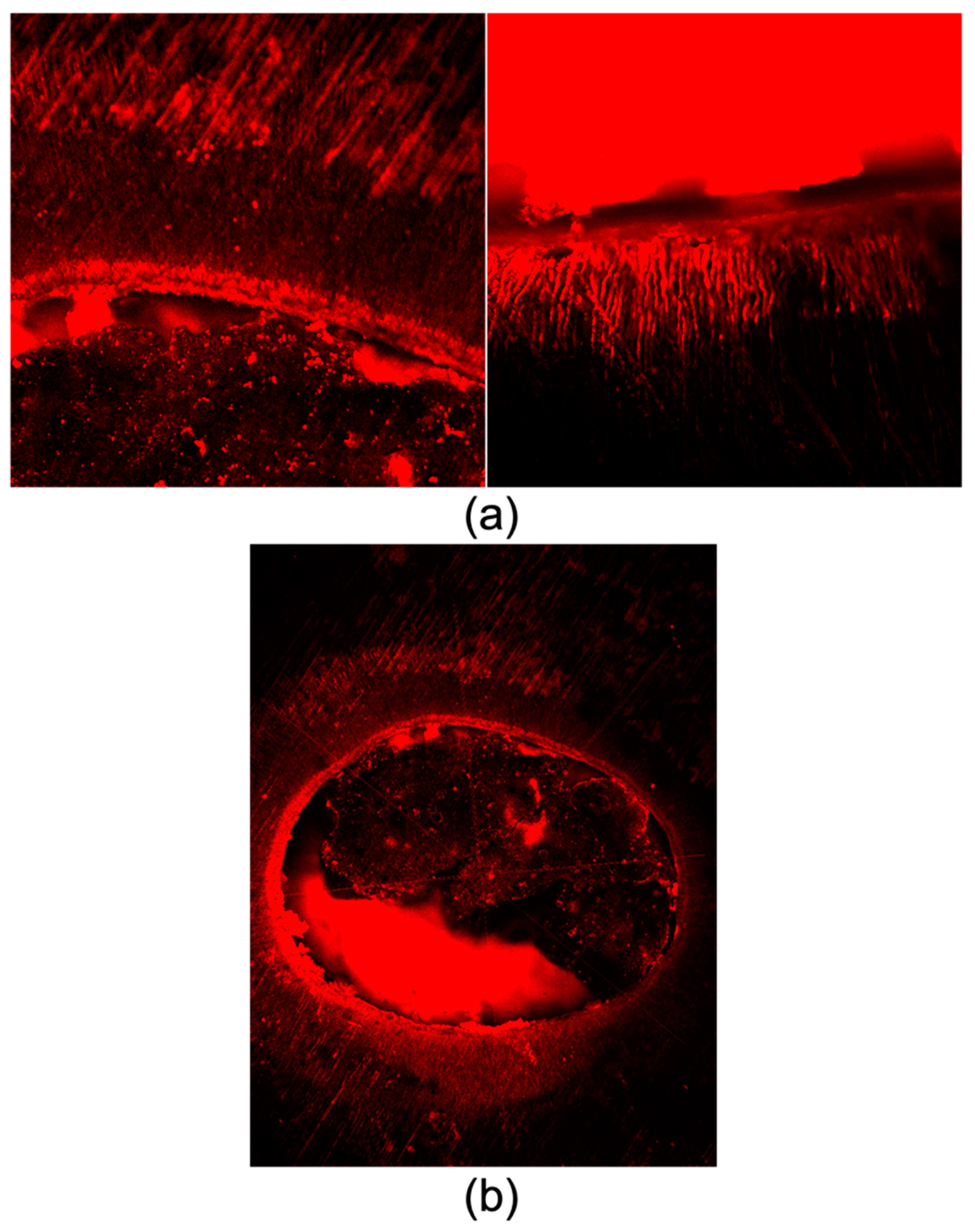
2.7. Confocal Laser Scanning Microscopy Analysis
2.8. Scanning Electron Microscope Observations
2.9. Flow Test
2.10. Statistical Analysis
3. Results
3.1. Micro-CT
3.2. Digital Microscopy
3.3. Confocal Laser Scanning Microscope
3.4. Scanning Electron Microscope (SEM vs. CLSM)
3.5. Flow Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esentürk, G.; Akkas, E.; Cubukcu, E.; Nagas, E.; Uyanik, O.; Cehreli, Z.C. A Micro-Computed Tomographic Assessment of Root Canal Preparation with Conventional and Different Rotary Files in Primary Teeth and Young Permanent Teeth. Int. J. Paediatr. Dent. 2020, 30, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hachem, C.E.; Nehme, W.; Kaloustian, M.K.; Ghosn, N.; Daou, M.; Zogheib, C.; Karam, M.; Mhanna, R.; Macaluso, V.; Kharouf, N.; et al. The Effectiveness of Different Irrigation Techniques on Debris and Smear Layer Removal in Primary Mandibular Second Molars: An In Vitro Study. J. Contemp. Dent. Pract. 2022, 23, 1173–1179. [Google Scholar] [CrossRef]
- Walia, T.; Ghanbari, A.H.; Mathew, S.; Ziadlou, A.H. An in Vitro Comparison of Three Delivery Techniques for Obturation of Root Canals in Primary Molars. Eur. Arch. Paediatr. Dent. 2017, 18, 17–23. [Google Scholar] [CrossRef]
- Silva Junior, M.F.; Wambier, L.M.; Gevert, M.V.; Chibinski, A.C.R. Effectiveness of Iodoform-Based Filling Materials in Root Canal Treatment of Deciduous Teeth: A Systematic Review and Meta-Analysis. Biomater. Investig. Dent. 2022, 9, 52–74. [Google Scholar] [CrossRef]
- Hachem, C.E.; Chedid, J.C.A.; Nehme, W.; Kaloustian, M.K.; Ghosn, N.; Sahnouni, H.; Mancino, D.; Haikel, Y.; Kharouf, N. Physicochemical and Antibacterial Properties of Conventional and Two Premixed Root Canal Filling Materials in Primary Teeth. J. Funct. Biomater. 2022, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Alamoudi, N.M.; El-Housseiny, A.A.; Al Tuwirqi, A.A.; Sabbagh, H.J. A Comparison of Calcium Hydroxide/Iodoform Paste and Zinc Oxide Eugenol as Root Filling Materials for Pulpectomy in Primary Teeth: A Systematic Review and Meta-Analysis. Clin. Exp. Dent. Res. 2019, 5, 294–310. [Google Scholar] [CrossRef]
- Aragão, A.C.; Pintor, A.V.B.; Marceliano-Alves, M.; Primo, L.G.; Silva, A.S.D.S.; Lopes, R.T.; Neves, A.D.A. Root Canal Obturation Materials and Filling Techniques for Primary Teeth: In Vitro Evaluation in Polymer-Based Prototyped Incisors. Int. J. Paediatr. Dent. 2020, 30, 381–389. [Google Scholar] [CrossRef]
- Barja-Fidalgo, F.; Moutinho-Ribeiro, M.; Oliveira, M.A.A.; de Oliveira, B.H. A Systematic Review of Root Canal Filling Materials for Deciduous Teeth: Is There an Alternative for Zinc Oxide-Eugenol? ISRN Dentistry 2011, 2011, 367318. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.C.B.; Conde Júnior, A.M.; Rizzo, M.S.; Moura, R.D.; Moura, M.S.; Lima, M.D.M.; Moura, L.F.A.D. Biocompatibility of Root Filling Pastes Used in Primary Teeth. Int. Endod. J. 2015, 48, 405–416. [Google Scholar] [CrossRef]
- Haapasalo, M.; Parhar, M.; Huang, X.; Wei, X.; Lin, J.; Shen, Y. Clinical Use of Bioceramic Materials. Endod. Top. 2015, 32, 97–117. [Google Scholar] [CrossRef]
- López-García, S.; Rodríguez-Lozano, F.J.; Sanz, J.L.; Forner, L.; Pecci-Lloret, M.P.; Lozano, A.; Murcia, L.; Sánchez-Bautista, S.; Oñate-Sánchez, R.E. Biological Properties of Ceraputty as a Retrograde Filling Material: An in Vitro Study on HPDLSCs. Clin. Oral. Investig. 2023, 1–11. [Google Scholar] [CrossRef]
- Kharouf, N.; Sauro, S.; Eid, A.; Zghal, J.; Jmal, H.; Seck, A.; Macaluso, V.; Addiego, F.; Inchingolo, F.; Affolter-Zbaraszczuk, C.; et al. Physicochemical and Mechanical Properties of Premixed Calcium Silicate and Resin Sealers. J. Funct. Biomater. 2022, 14, 9. [Google Scholar] [CrossRef]
- Çelik, B.N.; Mutluay, M.S.; Arıkan, V.; Sarı, Ş. The Evaluation of MTA and Biodentine as a Pulpotomy Materials for Carious Exposures in Primary Teeth. Clin. Oral. Investig. 2019, 23, 661–666. [Google Scholar] [CrossRef]
- Makhlouf, M.; Kaloustian, M.; Claire, E.H.; Habib, M.; Zogheib, C. Sealing Ability of Two Calcium Silicate-Based Materials in the Repair of Furcal Perforations: A Laboratory Comparative Study. J. Contemp. Dent. Pract. 2020, 21, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Kassab, P.; El Hachem, C.; Habib, M.; Nehme, W.; Zogheib, C.; Tonini, R.; Kaloustian, M.K. The Pushout Bond Strength of Three Calcium Silicate-Based Materials in Furcal Perforation Repair and the Effect of a Novel Irrigation Solution: A Comparative In Vitro Study. J. Contemp. Dent. Pract. 2022, 23, 289–294. [Google Scholar] [PubMed]
- Taha, N.A.; Abdulkhader, S.Z. Full Pulpotomy with Biodentine in Symptomatic Young Permanent Teeth with Carious Exposure. J. Endod. 2018, 44, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Romeiro, K.; Cassimiro, M.; Gominho, L.; Dantas, E.; Silva, S.; Albuquerque, D. Micro-CT Analysis of Dentinal Microcracks on Root Canals Filled with a Bioceramic Sealer and Retreated with Reciprocating Instruments. Sci. Rep. 2020, 10, 15264. [Google Scholar] [CrossRef]
- Barasuol, J.C.; Alcalde, M.P.; Bortoluzzi, E.A.; Duarte, M.a.H.; Cardoso, M.; Bolan, M. Shaping Ability of Hand, Rotary and Reciprocating Files in Primary Teeth: A Micro-CT Study in Vitro. Eur. Arch. Paediatr. Dent. 2021, 22, 195–201. [Google Scholar] [CrossRef]
- Hwang, J.H.; Chung, J.; Na, H.-S.; Park, E.; Kwak, S.; Kim, H.-C. Comparison of Bacterial Leakage Resistance of Various Root Canal Filling Materials and Methods: Confocal Laser-Scanning Microscope Study. Scanning 2015, 37, 422–428. [Google Scholar] [CrossRef]
- Mancino, D.; Kharouf, N.; Cabiddu, M.; Bukiet, F.; Haïkel, Y. Microscopic and Chemical Evaluation of the Filling Quality of Five Obturation Techniques in Oval-Shaped Root Canals. Clin. Oral. Investig. 2021, 25, 3757–3765. [Google Scholar] [CrossRef]
- Drukteinis, S.; Drukteiniene, A.; Drukteinis, L.; Martens, L.C.; Rajasekharan, S. Flowable Urethane Dimethacrylate-Based Filler for Root Canal Obturation in Primary Molars: A Pilot SEM and MicroCT Assessment. Children 2021, 8, 60. [Google Scholar] [CrossRef]
- Segato, R.A.B.; Pucinelli, C.M.; Ferreira, D.C.A.; Daldegan, A.D.R.; da Silva, R.S.; Nelson-Filho, P.; da Silva, L.A.B. Physicochemical Properties of Root Canal Filling Materials for Primary Teeth. Braz. Dent. J. 2016, 27, 196–201. [Google Scholar] [CrossRef]
- Pedrotti, D.; Bottezini, P.A.; Casagrande, L.; Braga, M.M.; Lenzi, T.L. Root Canal Filling Materials for Endodontic Treatment of Necrotic Primary Teeth: A Network Meta-Analysis. Eur. Arch. Paediatr. Dent. 2022, 24, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Irzooqee, A.F.; Al Haidar, A.H.M.J.; Abdul-Kareem, M. The Effect of Different Obturation Techniques in Primary Teeth on the Apical Microleakage Using Endoflas: A Comparative In Vitro Study. Int. J. Dent. 2023, 2023, 4982980. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.A.; Vargas, K.; Marghalani, A.A.; Chen, C.-Y.; AlShamali, S.; Dhar, V.; Crystal, Y.O. A Systematic Review and Meta-Analysis of Nonvital Pulp Therapy for Primary Teeth. Pediatr. Dent. 2020, 42, 256–461. [Google Scholar] [PubMed]
- Gharib, S.R.; Tordik, P.A.; Imamura, G.M.; Baginski, T.A.; Goodell, G.G. A Confocal Laser Scanning Microscope Investigation of the Epiphany Obturation System. J. Endod. 2007, 33, 957–961. [Google Scholar] [CrossRef]
- Kikinis, R.; Pieper, S.D.; Vosburgh, K.G. 3D Slicer: A Platform for Subject-Specific Image Analysis, Visualization, and Clinical Support. In Intraoperative Imaging and Image-Guided Therapy; Jolesz, F.A., Ed.; Springer: New York, NY, USA, 2014; pp. 277–289. ISBN 978-1-4614-7657-3. [Google Scholar]
- Kaloustian, M.K.; Hachem, C.E.; Zogheib, C.; Nehme, W.; Hardan, L.; Rached, P.; Kharouf, N.; Haikel, Y.; Mancino, D. Effectiveness of the REvision System and Sonic Irrigation in the Removal of Root Canal Filling Material from Oval Canals: An In Vitro Study. Bioengineering 2022, 9, 260. [Google Scholar] [CrossRef]
- Kharouf, N.; Eid, A.; Hardan, L.; Bourgi, R.; Arntz, Y.; Jmal, H.; Foschi, F.; Sauro, S.; Ball, V.; Haikel, Y.; et al. Antibacterial and Bonding Properties of Universal Adhesive Dental Polymers Doped with Pyrogallol. Polymers 2021, 13, 1538. [Google Scholar] [CrossRef]
- Kharouf, N.; Pedullà, E.; La Rosa, G.R.M.; Bukiet, F.; Sauro, S.; Haikel, Y.; Mancino, D. In Vitro Evaluation of Different Irrigation Protocols on Intracanal Smear Layer Removal in Teeth with or without Pre-Endodontic Proximal Wall Restoration. J. Clin. Med. 2020, 9, 3325. [Google Scholar] [CrossRef]
- Kharouf, N.; Arntz, Y.; Eid, A.; Zghal, J.; Sauro, S.; Haikel, Y.; Mancino, D. Physicochemical and Antibacterial Properties of Novel, Premixed Calcium Silicate-Based Sealer Compared to Powder–Liquid Bioceramic Sealer. J. Clin. Med. 2020, 9, 3096. [Google Scholar] [CrossRef]
- El Hachem, C.; Kaloustian, M.K.; Nehme, W.; Ghosn, N.; Abou Chedid, J.C. Three-Dimensional Modeling and Measurements of Root Canal Anatomy in Second Primary Mandibular Molars: A Case Series Micro CT Study. Eur. Arch. Paediatr. Dent. 2019, 20, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, U.; Küçük, M.; Versiani, M.A.; Orhan, K. Publication Trends in Micro-CT Endodontic Research: A Bibliometric Analysis over a 25-Year Period. Int. Endod. J. 2021, 54, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, R.A.; Duarte, M.a.H.; Vivan, R.R.; Alcalde, M.P.; Vasconcelos, B.C.; Bramante, C.M. Comparison of Three Retreatment Techniques with Ultrasonic Activation in Flattened Canals Using Micro-Computed Tomography and Scanning Electron Microscopy. Int. Endod. J. 2016, 49, 890–897. [Google Scholar] [CrossRef]
- Jung, M.; Lommel, D.; Klimek, J. The Imaging of Root Canal Obturation Using Micro-CT. Int. Endod. J. 2005, 38, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Celikten, B.; Uzuntas, C.F.; Orhan, A.I.; Orhan, K.; Tufenkci, P.; Kursun, S.; Demiralp, K.Ö. Evaluation of Root Canal Sealer Filling Quality Using a Single-Cone Technique in Oval Shaped Canals: An In Vitro Micro-CT Study. Scanning 2016, 38, 133–140. [Google Scholar] [CrossRef]
- Migliau, G.; Palaia, G.; Pergolini, D.; Guglielmelli, T.; Fascetti, R.; Sofan, A.; Del Vecchio, A.; Romeo, U. Comparison of Two Root Canal Filling Techniques: Obturation with Guttacore Carrier Based System and Obturation with Guttaflow2 Fluid Gutta-Percha. Dent. J. 2022, 10, 71. [Google Scholar] [CrossRef]
- Subramaniam, P.; Gilhotra, K. Endoflas, Zinc Oxide Eugenol and Metapex as Root Canal Filling Materials in Primary Molars—A Comparative Clinical Study. J. Clin. Pediatr. Dent. 2011, 35, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Orhan, A.I.; Tatli, E.C. Evaluation of Root Canal Obturation Quality in Deciduous Molars with Different Obturation Materials: An In Vitro Micro-Computed Tomography Study. Biomed. Res. Int. 2021, 2021, 6567161. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Favieri, A.; Gahyva, S.M.M.; Moraes, S.R.; Lima, K.C.; Lopes, H.P. Antimicrobial Activity and Flow Rate of Newer and Established Root Canal Sealers. J. Endod. 2000, 26, 274–277. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, B.-S.; Kim, Y.-M.; Lee, D.; Kim, S.-Y. The Penetration Ability of Calcium Silicate Root Canal Sealers into Dentinal Tubules Compared to Conventional Resin-Based Sealer: A Confocal Laser Scanning Microscopy Study. Materials 2019, 12, 531. [Google Scholar] [CrossRef]
- Caceres, C.; Larrain, M.R.; Monsalve, M.; Peña-Bengoa, F. Dentinal Tubule Penetration and Adaptation of Bio-C Sealer and AH-Plus: A Comparative SEM Evaluation. Eur. Endod. J. 2021, 6, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Furtado, T.C.; de Bem, I.A.; Machado, L.S.; Pereira, J.R.; Só, M.V.R.; da Rosa, R.A. Intratubular Penetration of Endodontic Sealers Depends on the Fluorophore Used for CLSM Assessment. Microsc. Res. Tech. 2021, 84, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluis, L.W.M.; Wu, M.-K.; Wesselink, P.R. The Efficacy of Ultrasonic Irrigation to Remove Artificially Placed Dentine Debris from Human Root Canals Prepared Using Instruments of Varying Taper. Int. Endod. J. 2005, 38, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.M.; Rached-Junior, F.J.A.; Versiani, M.A.; Souza-Gabriel, A.E.; Miranda, C.E.S.; Silva-Sousa, Y.T.C.; Sousa Neto, M.D. A Comparative Study of Physicochemical Properties of AH Plus, Epiphany, and Epiphany SE Root Canal Sealers. Int. Endod. J. 2009, 42, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Mirfendereski, M.; Roth, K.; Fan, B.; Dubrowski, A.; Carnahan, H.; Azarpazhooh, A.; Basrani, B.; Torneck, C.D.; Friedman, S. Technique Acquisition in the Use of Two Thermoplasticized Root Filling Methods by Inexperienced Dental Students: A Microcomputed Tomography Analysis. J. Endod. 2009, 35, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Cancio, V.; de Carvalho Ferreira, D.; Cavalcante, F.S.; Rosado, A.S.; Teixeira, L.M.; Braga Oliveira, Q.; Barcelos, R.; Gleiser, R.; Santos, H.F.; dos Santos, K.R.N.; et al. Can the Enterococcus Faecalis Identified in the Root Canals of Primary Teeth Be a Cause of Failure of Endodontic Treatment? Acta Odontol. Scand. 2017, 75, 423–428. [Google Scholar] [CrossRef]
- Huang, T.-H.; Hung, C.-J.; Chen, Y.-J.; Chien, H.-C.; Kao, C.-T. Cytologic Effects of Primary Tooth Endodontic Filling Materials. J. Dent. Sci. 2009, 4, 18–24. [Google Scholar] [CrossRef]
- Tannure, P.N.; Azevedo, C.P.; Barcelos, R.; Gleiser, R.; Primo, L.G. Long-Term Outcomes of Primary Tooth Pulpectomy with and without Smear Layer Removal: A Randomized Split-Mouth Clinical Trial. Pediatr. Dent. 2011, 33, 316–320. [Google Scholar]


| Coronal (%) | Middle (%) | Apical (%) | Statistical Analysis | |
|---|---|---|---|---|
| ZOE | 2.7 ± 1.3 | 2.3 ± 1.8 | 6.7 ± 4.9 | A > C, A > M |
| BC | 7.5 ± 4 | 8.9 ± 7.7 | 17.2 ± 14.8 | No |
| Statistical analysis | p < 0.001 | r = 0.002 | p = 0.049 |
| Coronal (%) | Middle (%) | Apical (%) | ||||
|---|---|---|---|---|---|---|
| Close | Open | Close | Open | Close | Open | |
| ZOE | 2.6 ± 2.1 | 0.2 ± 0.4 | 1.6 ± 1.8 | 0.3 ± 0.8 | 1.48 ± 1.77 | 1.7 ± 3.7 |
| BC | 4.4 ± 7.4 | 5 ± 7.7 | 2.2 ±1.6 | 4.8 ± 5 | 13.7 ± 32.7 | 9.4 ± 13.6 |
| Statistical analysis | p = 0.019 | p = 0.002 | p < 0.001 | |||
| Coronal (%) | Middle (%) | Apical (%) | Statistical Analysis | |
|---|---|---|---|---|
| ZOE | 122 ± 62 | 112 ± 55 | 102 ± 52 | C > A |
| BC | 277 ± 124 | 247 ± 118 | 218 ± 114 | C > A |
| Statistical analysis | p < 0.001 | p < 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hachem, C.; Chedid, J.C.A.; Nehme, W.; Kaloustian, M.K.; Ghosn, N.; Rabineau, M.; Kharouf, N.; Haikel, Y.; Mancino, D. The Contribution of Various In Vitro Methodologies to Comprehending the Filling Ability of Root Canal Pastes in Primary Teeth. Bioengineering 2023, 10, 818. https://doi.org/10.3390/bioengineering10070818
El Hachem C, Chedid JCA, Nehme W, Kaloustian MK, Ghosn N, Rabineau M, Kharouf N, Haikel Y, Mancino D. The Contribution of Various In Vitro Methodologies to Comprehending the Filling Ability of Root Canal Pastes in Primary Teeth. Bioengineering. 2023; 10(7):818. https://doi.org/10.3390/bioengineering10070818
Chicago/Turabian StyleEl Hachem, Claire, Jean Claude Abou Chedid, Walid Nehme, Marc Krikor Kaloustian, Nabil Ghosn, Morgane Rabineau, Naji Kharouf, Youssef Haikel, and Davide Mancino. 2023. "The Contribution of Various In Vitro Methodologies to Comprehending the Filling Ability of Root Canal Pastes in Primary Teeth" Bioengineering 10, no. 7: 818. https://doi.org/10.3390/bioengineering10070818
APA StyleEl Hachem, C., Chedid, J. C. A., Nehme, W., Kaloustian, M. K., Ghosn, N., Rabineau, M., Kharouf, N., Haikel, Y., & Mancino, D. (2023). The Contribution of Various In Vitro Methodologies to Comprehending the Filling Ability of Root Canal Pastes in Primary Teeth. Bioengineering, 10(7), 818. https://doi.org/10.3390/bioengineering10070818









