Long-Term Bilateral Neuromuscular Function and Knee Osteoarthritis after Anterior Cruciate Ligament Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Study Protocol
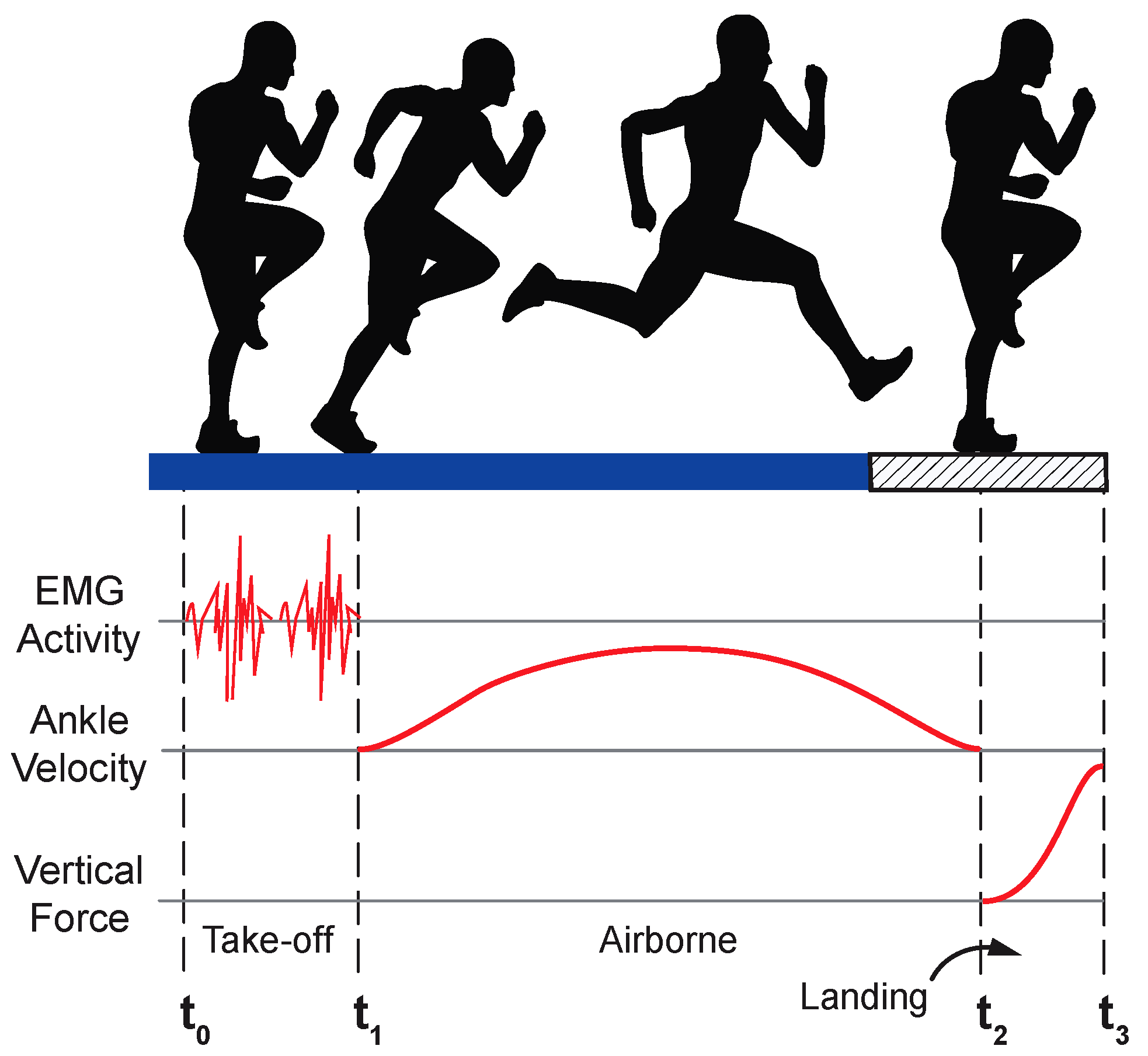
2.3. EMG Post-Processing
2.3.1. Signal Preparation
2.3.2. Signal Conditioning and Generation of Wavelets
2.3.3. Wavelet Normalization and Visualization
2.3.4. Machine Learning Classification
2.3.5. EMG Statistical Approach
2.4. Muscle Girth, Clinical, Functional, and Patient-Reported Outcomes
2.5. Knee PTOA Score
2.6. Correlation and Regression Analyses
3. Results
3.1. Muscle Activity Pattern Classification
3.2. Muscle Girth, Clinical, Functional, and Structural Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sequence |
|---|
| Sagittal T1-weighted water-excitation three-dimensional (3D) fast low-angle shot (3D FLASH): 20/7.6 [TR msec/ TE msec]; 12° [flip angle]; 160 mm [field of view, FOV]; 1.5 mm/0 [slice thickness/interslice gap]; 80 slices per slab; 130 hz/pixel [bandwidth, BW]; 512 × 512 [matrix]; right/left [phase encoding axis]; one average of two excitations. |
| Coronal Intermediate-weighted turbo-spin echo (TSE): 3850/29; 7 [echo train length, ETL]; 140 mm; 3 mm/0 mm; 41 slices; 352 hz/pixel; 307 × 384; right/left; one average. |
| Sagittal † T2*-weighted WE-3D double echo steady state (WE-3D DESS): 16.3/4.7; 25°; 140 mm; 0.7 mm/0 mm; 185 hz/pixel; 307 × 384; anterior/posterior; one average. |
| Sagittal Intermediate-weighted TSE with fat-saturation: 3460/36; 5 ETL; 160 mm; 3 mm/0 mm; 248 hz/pixel; 314 × 448; superior/inferior; one average. |
References
- Herzog, M.M.; Marshall, S.W.; Lund, J.L.; Pate, V.; Mack, C.D.; Spang, J.T. Trends in Incidence of ACL Reconstruction and Concomitant Procedures Among Commercially Insured Individuals in the United States, 2002-2014. Sport. Health 2018, 10, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Larson, D.R.; Dahm, D.L.; Levy, B.A.; Stuart, M.J.; Krych, A.J. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am. J. Sport. Med. 2016, 44, 1502–1507. [Google Scholar] [CrossRef]
- Siegel, L.; Vandenakker-Albanese, C.; Siegel, D. Anterior cruciate ligament injuries: Anatomy, physiology, biomechanics, and management. Clin. J. Sport Med. 2012, 22, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Feller, J.A.; Leigh, W.B.; Richmond, A.K. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am. J. Sport. Med. 2014, 42, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Luc, B.; Gribble, P.A.; Pietrosimone, B.G. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: A systematic review and numbers-needed-to-treat analysis. J. Athl. Train. 2014, 49, 806–819. [Google Scholar] [CrossRef]
- Risberg, M.A.; Oiestad, B.E.; Gunderson, R.; Aune, A.K.; Engebretsen, L.; Culvenor, A.; Holm, I. Changes in Knee Osteoarthritis, Symptoms, and Function After Anterior Cruciate Ligament Reconstruction: A 20-Year Prospective Follow-up Study. Am. J. Sport. Med. 2016, 44, 1215–1224. [Google Scholar] [CrossRef]
- Di Stasi, S.; Myer, G.D.; Hewett, T.E. Neuromuscular training to target deficits associated with second anterior cruciate ligament injury. J. Orthop. Sport. Phys. Ther. 2013, 43, 766–851. [Google Scholar] [CrossRef]
- Hewett, T.E.; Di Stasi, S.L.; Myer, G.D. Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am. J. Sport. Med. 2013, 41, 216–224. [Google Scholar] [CrossRef]
- Nagelli, C.; Wordeman, S.; Di Stasi, S.; Hoffman, J.; Marulli, T.; Hewett, T.E. Biomechanical Deficits at the Hip in Athletes With ACL Reconstruction Are Ameliorated With Neuromuscular Training. Am. J. Sport. Med. 2018, 46, 2772–2779. [Google Scholar] [CrossRef]
- Paterno, M.V.; Schmitt, L.C.; Ford, K.R.; Rauh, M.J.; Myer, G.D.; Huang, B.; Hewett, T.E. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am. J. Sport. Med. 2010, 38, 1968–1978. [Google Scholar] [CrossRef]
- Perraton, L.; Clark, R.; Crossley, K.; Pua, Y.H.; Whitehead, T.; Morris, H.; Telianidis, S.; Bryant, A. Impaired voluntary quadriceps force control following anterior cruciate ligament reconstruction: Relationship with knee function. Knee Surg. Sport. Traumatol. Arthrosc. 2017, 25, 1424–1431. [Google Scholar] [CrossRef]
- Rice, D.; Lewis, G.; McNair, P. Impaired Regulation of Submaximal Force after ACL Reconstruction: Role of Muscle Spindles. Int. J. Sport. Med. 2021, 42, 550–558. [Google Scholar] [CrossRef]
- Ageberg, E.; Thomee, R.; Neeter, C.; Silbernagel, K.G.; Roos, E.M. Muscle strength and functional performance in patients with anterior cruciate ligament injury treated with training and surgical reconstruction or training only: A two to five-year followup. Arthritis Rheum. 2008, 59, 1773–1779. [Google Scholar] [CrossRef]
- Palmieri-Smith, R.M.; Thomas, A.C. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc. Sport Sci. Rev. 2009, 37, 147–153. [Google Scholar] [CrossRef]
- Baumeister, J.; Reinecke, K.; Schubert, M.; Weiss, M. Altered electrocortical brain activity after ACL reconstruction during force control. J. Orthop. Res. 2011, 29, 1383–1389. [Google Scholar] [CrossRef]
- Grooms, D.R.; Page, S.J.; Nichols-Larsen, D.S.; Chaudhari, A.M.; White, S.E.; Onate, J.A. Neuroplasticity Associated With Anterior Cruciate Ligament Reconstruction. J. Orthop. Sport. Phys. Ther. 2017, 47, 180–189. [Google Scholar] [CrossRef]
- Kapreli, E.; Athanasopoulos, S.; Gliatis, J.; Papathanasiou, M.; Peeters, R.; Strimpakos, N.; Van Hecke, P.; Gouliamos, A.; Sunaert, S. Anterior cruciate ligament deficiency causes brain plasticity: A functional MRI study. Am. J. Sport. Med. 2009, 37, 2419–2426. [Google Scholar] [CrossRef]
- Needle, A.R.; Lepley, A.S.; Grooms, D.R. Central Nervous System Adaptation After Ligamentous Injury: A Summary of Theories, Evidence, and Clinical Interpretation. Sport. Med. 2017, 47, 1271–1288. [Google Scholar] [CrossRef]
- Ericsson, Y.B.; Roos, E.M.; Owman, H.; Dahlberg, L.E. Association between thigh muscle strength four years after partial meniscectomy and radiographic features of osteoarthritis 11 years later. BMC Musculoskelet. Disord. 2019, 20, 512. [Google Scholar] [CrossRef]
- Sherman, D.A.; Glaviano, N.R.; Norte, G.E. Hamstrings Neuromuscular Function After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Sport. Med. 2021, 51, 1751–1769. [Google Scholar] [CrossRef]
- Behnke, A.L.; Parola, L.R.; Karamchedu, N.P.; Badger, G.J.; Fleming, B.C.; Beveridge, J.E. Neuromuscular function in anterior cruciate ligament reconstructed patients at long-term follow-up. Clin. Biomech. 2021, 81, 105231. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.R.; van den Bogert, J.; Myer, G.D.; Shapiro, R.; Hewett, T.E. The effects of age and skill level on knee musculature co-contraction during functional activities: A systematic review. Br. J. Sport. Med. 2008, 42, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.S.; Axe, M.J.; Buchanan, T.S.; Scholz, J.P.; Snyder-Mackler, L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg. Sport. Traumatol. Arthrosc. 2001, 9, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Akelman, M.R.; Fadale, P.D.; Hulstyn, M.J.; Shalvoy, R.M.; Garcia, A.; Chin, K.E.; Duryea, J.; Badger, G.J.; Tung, G.A.; Fleming, B.C. Effect of Matching or Overconstraining Knee Laxity During Anterior Cruciate Ligament Reconstruction on Knee Osteoarthritis and Clinical Outcomes: A Randomized Controlled Trial With 84-Month Follow-up. Am. J. Sport. Med. 2016, 44, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.Q.; Badger, G.J.; Chrostek, C.A.; Carvalho, O.D.; Faiola, S.L.; Fadale, P.D.; Hulstyn, M.J.; Gil, H.C.; Shalvoy, R.M.; Fleming, B.C. Effects of Initial Graft Tension and Patient Sex on Knee Osteoarthritis Outcomes After ACL Reconstruction: A Randomized Controlled Clinical Trial With 10- to 12-Year Follow-up. Am. J. Sport. Med. 2022, 50, 3510–3521. [Google Scholar] [CrossRef]
- Fleming, B.C.; Fadale, P.D.; Hulstyn, M.J.; Shalvoy, R.M.; Oksendahl, H.L.; Badger, G.J.; Tung, G.A. The effect of initial graft tension after anterior cruciate ligament reconstruction: A randomized clinical trial with 36-month follow-up. Am. J. Sport. Med. 2013, 41, 25–34. [Google Scholar] [CrossRef]
- von Tscharner, V. Intensity analysis in time-frequency space of surface myoelectric signals by wavelets of specified resolution. J. Electromyogr. Kinesiol. 2000, 10, 433–445. [Google Scholar] [CrossRef]
- Zandiyeh, P.; Parola, L.R.; Fleming, B.C.; Beveridge, J.E. Wavelet analysis reveals differential lower limb muscle activity patterns long after anterior cruciate ligament reconstruction. J. Biomech. 2022, 133, 110957. [Google Scholar] [CrossRef]
- Oiestad, B.E.; Juhl, C.B.; Eitzen, I.; Thorlund, J.B. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 171–177. [Google Scholar] [CrossRef]
- Spindler, K.P.; Huston, L.J.; Chagin, K.M.; Kattan, M.W.; Reinke, E.K.; Amendola, A.; Andrish, J.T.; Brophy, R.H.; Cox, C.L.; Dunn, W.R.; et al. Ten-Year Outcomes and Risk Factors After Anterior Cruciate Ligament Reconstruction: A MOON Longitudinal Prospective Cohort Study. Am. J. Sport. Med. 2018, 46, 815–825. [Google Scholar] [CrossRef]
- Hopkins, J.T.; Ingersoll, C.D. Arthrogenic Muscle inhibition: A Limiting Factor in Joint Rehabilitation. J. Sport Rehabil. 2000, 9, 135–159. [Google Scholar] [CrossRef]
- Rudolph, K.S.; Axe, M.J.; Snyder-Mackler, L. Dynamic stability after ACL injury: Who can hop? Knee Surg. Sport. Traumatol. Arthrosc. 2000, 8, 262–269. [Google Scholar] [CrossRef]
- Xergia, S.A.; Pappas, E.; Georgoulis, A.D. Association of the Single-Limb Hop Test With Isokinetic, Kinematic, and Kinetic Asymmetries in Patients After Anterior Cruciate Ligament Reconstruction. Sport. Health 2015, 7, 217–223. [Google Scholar] [CrossRef]
- Barber, S.D.; Noyes, F.R.; Mangine, R.E.; McCloskey, J.W.; Hartman, W. Quantitative assessment of functional limitations in normal and anterior cruciate ligament-deficient knees. Clin. Orthop. Relat. Res. 1990, 255, 204–214. [Google Scholar] [CrossRef]
- Noyes, F.R.; Barber, S.D.; Mangine, R.E. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am. J. Sport. Med. 1991, 19, 513–518. [Google Scholar] [CrossRef]
- Coats-Thomas, M.S.; Miranda, D.L.; Badger, G.J.; Fleming, B.C. Effects of ACL reconstruction surgery on muscle activity of the lower limb during a jump-cut maneuver in males and females. J. Orthop. Res. 2013, 31, 1890–1896. [Google Scholar] [CrossRef]
- Miranda, D.L.; Fadale, P.D.; Hulstyn, M.J.; Shalvoy, R.M.; Machan, J.T.; Fleming, B.C. Knee biomechanics during a jump-cut maneuver: Effects of sex and ACL surgery. Med. Sci. Sport. Exerc. 2013, 45, 942–951. [Google Scholar] [CrossRef]
- Conforto, S.; D’Alessio, T.; Pignatelli, S. Optimal rejection of movement artefacts from myoelectric signals by means of a wavelet filtering procedure. J. Electromyogr. Kinesiol. 1999, 9, 47–57. [Google Scholar] [CrossRef]
- Noirhomme, Q.; Lesenfants, D.; Gomez, F.; Soddu, A.; Schrouff, J.; Garraux, G.; Luxen, A.; Phillips, C.; Laureys, S. Biased binomial assessment of cross-validated estimation of classification accuracies illustrated in diagnosis predictions. Neuroimage Clin. 2014, 4, 687–694. [Google Scholar] [CrossRef]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef]
- Capin, J.J.; Zarzycki, R.; Ito, N.; Khandha, A.; Dix, C.; Manal, K.; Buchanan, T.S.; Snyder-Mackler, L. Gait Mechanics in Women of the ACL-SPORTS Randomized Control Trial: Interlimb Symmetry Improves Over Time Regardless of Treatment Group. J. Orthop. Res. 2019, 37, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.M.; Pietrosimone, B.; Hertel, J.; Ingersoll, C.D. Quadriceps activation following knee injuries: A systematic review. J. Athl. Train. 2010, 45, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; McNair, P.J.; Rice, D.A.; Ochiai, S.; Hagino, T. Stretch reflex changes in ACL-deficient individuals and healthy controls during normal and surprise landings. Scand. J. Med. Sci. Sport. 2020, 30, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Nyland, J.; Wera, J.; Klein, S.; Caborn, D.N. Lower extremity neuromuscular compensations during instrumented single leg hop testing 2-10 years following ACL reconstruction. Knee 2014, 21, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.G.; Lepley, A.S.; Ericksen, H.M.; Clements, A.; Sohn, D.H.; Gribble, P.A. Neural Excitability Alterations After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2015, 50, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Urbach, D.; Nebelung, W.; Becker, R.; Awiszus, F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris. J. Bone Jt. Surg. Br. 2001, 83, 1104–1110. [Google Scholar] [CrossRef]
- Tayfur, B.; Charuphongsa, C.; Morrissey, D.; Miller, S.C. Neuromuscular Function of the Knee Joint Following Knee Injuries: Does It Ever Get Back to Normal? A Systematic Review with Meta-Analyses. Sport. Med. 2021, 51, 321–338. [Google Scholar] [CrossRef]
- Kuenze, C.M.; Hertel, J.; Weltman, A.; Diduch, D.; Saliba, S.A.; Hart, J.M. Persistent neuromuscular and corticomotor quadriceps asymmetry after anterior cruciate ligament reconstruction. J. Athl. Train. 2015, 50, 303–312. [Google Scholar] [CrossRef]
- Konradsen, L. Sensori-motor control of the uninjured and injured human ankle. J. Electromyogr. Kinesiol. 2002, 12, 199–203. [Google Scholar] [CrossRef]
- Draganich, L.F.; Vahey, J.W. An In Vitro Study of Anterior Cruciate Ligament Strain Induced by Quadriceps and Hamstrings Forces. J. Orthop. Res. 1990, 8, 57–63. [Google Scholar] [CrossRef]
- Houck, J.R.; De Haven, K.E.; Maloney, M. Influence of anticipation on movement patterns in subjects with ACL deficiency classified as noncopers. J. Orthop. Sport. Phys. Ther. 2007, 37, 56–64. [Google Scholar] [CrossRef]
- Houck, J.R.; Wilding, G.E.; Gupta, R.; De Haven, K.E.; Maloney, M. Analysis of EMG patterns of control subjects and subjects with ACL deficiency during an unanticipated walking cut task. Gait Posture 2007, 25, 628–638. [Google Scholar] [CrossRef]
- Thorstensson, A.; Petersson, I.F.; Jacobsson, L.T.H.; Boegard, T.L.; Roos, E.M. Reduced fucntional performance in the lower extremity predicted radiographic knee osteoarthritis five years later. Ann. Rheum. Dis. 2004, 63, 402–407. [Google Scholar] [CrossRef]
- Shrier, I. Muscle dysfunction versus wear and tear as a cause of exercise related osteoarthritis: An epidemiological update. Br. J. Sport. Med. 2004, 38, 526–535. [Google Scholar] [CrossRef]
- Khella, C.M.; Asgarian, R.; Horvath, J.M.; Rolauffs, B.; Hart, M.L. An Evidence-Based Systematic Review of Human Knee Post-Traumatic Osteoarthritis (PTOA): Timeline of Clinical Presentation and Disease Markers, Comparison of Knee Joint PTOA Models and Early Disease Implications. Int. J. Mol. Sci. 2021, 22, 1996. [Google Scholar] [CrossRef]
- Williams, J.R.; Neal, K.; Alfayyadh, A.; Lennon, K.; Capin, J.J.; Khandha, A.; Manal, K.; Potter, H.G.; Snyder-Mackler, L.; Buchanan, T.S. Knee cartilage T(2) relaxation times 3 months after ACL reconstruction are associated with knee gait variables linked to knee osteoarthritis. J. Orthop. Res. 2022, 40, 252–259. [Google Scholar] [CrossRef]
- Cavanaugh, M.T.; Aboodarda, S.J.; Behm, D.G. Intrasession and Intersession Reliability of Quadriceps’ and Hamstrings’ Electromyography During a Standardized Hurdle Jump Test With Single Leg Landing. J. Strength Cond. Res. 2017, 31, 1601–1609. [Google Scholar] [CrossRef]


| Sex | Subject Group | Age | Index Limb | BMI | Follow-Up Year | Tegner Score | KT-1000 a | Hop Distance b |
|---|---|---|---|---|---|---|---|---|
| Male | ACLR | 27 | L | 25 | 12 | 6 | −1 | 94 |
| Male | ACLR | 29 | L | 33 | 12 | 3 | 3 | 85 |
| Male | ACLR | 30 | L | 29 | 12 | 9 | −2 | 93 |
| Male | ACLR | 30 | R | 26 | 12 | 6 | 0 | 84 |
| Male | ACLR | 27 | R | 27 | 10 | 5 | 1 | 113 |
| Female | ACLR | 31 | L | 22 | 15 | 6 | 1 | 100 |
| Female | ACLR | 39 | R | 29 | 12 | 6 | 0 | 97 |
| Female | ACLR | 29 | R | 32 | 12 | 5 | −2 | 85 |
| Female | ACLR | 36 | R | 20 | 12 | 7 | 0 | 104 |
| Female | ACLR | 60 | L | 26 | 12 | 4 | −14 | 108 |
| Female | ACLR | 44 | L | 28 | 10 | 6 | 3 | 95 |
| Male | Control | 33 | R | 27 | 12 | 5 | 0 | 108 |
| Male | Control | 34 | L | 27 | 12 | 4 | 0 | 95 |
| Male | Control | 41 | L | 26 | 12 | 5 | 1 | 100 |
| Male | Control | 35 | L | 26 | 12 | 7 | −1 | 95 |
| Male | Control | 31 | R | 20 | 10 | 6 | −1 | 101 |
| Male | Control | 47 | L | 24 | 12 | 7 | 0 | 94 |
| Male | Control | 31 | L | 31 | 12 | 3 | −1 | 111 |
| Female | Control | 43 | L | 21 | 12 | 6 | 1 | 96 |
| Female | Control | 38 | R | 26 | 10 | 6 | 0 | 106 |
| Female | Control | 49 | R | 23 | 15 | 6 | 0 | 91 |
| Female | Control | 45 | R | 21 | 12 | 6 | −1 | 100 |
| Female | Control | 26 | L | 22 | N/A | 4 | 0 | 91 |
| Comparison | Variable Names |
|---|---|
| ControlIdx vs. ControlContra |
| ACLRSx vs. ACLRContra |
| ACLRContra vs. ControlIdx |
| (1) ControlIdx vs. ControlContra | (2) ACLRSx vs. ACLRContra | (3) ACLRContra vs. ControlIdx | ||
| Classification (%) | Classification (%) | Classification (%) | ||
| Muscle | GM | 34.8% | 69.4% | 77.8% |
| GL | 55.1% | 69.4% | 82.5% | |
| TA | 44.9% | 75.8% | 90.5% | |
| VM | 49.3% | 66.1% | 85.7% | |
| RF | 56.5% | 69.4% | 77.8% | |
| ST | 40.6% | 69.4% | 88.9% | |
| BF | 47.8% | 71.0% | 93.7% |
| (a) | ACLRSx vs. ACLRContra | ||
| Take-off | Airborne | Landing | |
| GM | 64.5% | 58.1% | 46.8% |
| GL | 67.7% | 64.5% | 56.5% |
| TA | 72.6% | 72.6% | 58.1% |
| VM | 75.8% | 62.9% | 51.6% |
| RF | 77.4% | 62.9% | 46.8% |
| BF | 67.7% | 62.9% | 62.9% |
| ST | 61.3% | 67.7% | 58.1% |
| (b) | ACLRContra vs. ControlIdx | ||
| Take-off | Airborne | Landing | |
| GM | 63.5% | 63.5% | 58.7% |
| GL | 82.5% | 66.7% | 55.6% |
| TA | 69.8% | 66.7% | 81.0% |
| VM | 68.3% | 73.0% | 50.8% |
| RF | 63.5% | 63.5% | 46.0% |
| BF | 58.7% | 82.5% | 68.3% |
| ST | 71.4% | 77.8% | 69.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zandiyeh, P.; Parola, L.R.; Costa, M.Q.; Hague, M.J.; Molino, J.; Fleming, B.C.; Beveridge, J.E. Long-Term Bilateral Neuromuscular Function and Knee Osteoarthritis after Anterior Cruciate Ligament Reconstruction. Bioengineering 2023, 10, 812. https://doi.org/10.3390/bioengineering10070812
Zandiyeh P, Parola LR, Costa MQ, Hague MJ, Molino J, Fleming BC, Beveridge JE. Long-Term Bilateral Neuromuscular Function and Knee Osteoarthritis after Anterior Cruciate Ligament Reconstruction. Bioengineering. 2023; 10(7):812. https://doi.org/10.3390/bioengineering10070812
Chicago/Turabian StyleZandiyeh, Payam, Lauren R. Parola, Meggin Q. Costa, Madalyn J. Hague, Janine Molino, Braden C. Fleming, and Jillian E. Beveridge. 2023. "Long-Term Bilateral Neuromuscular Function and Knee Osteoarthritis after Anterior Cruciate Ligament Reconstruction" Bioengineering 10, no. 7: 812. https://doi.org/10.3390/bioengineering10070812
APA StyleZandiyeh, P., Parola, L. R., Costa, M. Q., Hague, M. J., Molino, J., Fleming, B. C., & Beveridge, J. E. (2023). Long-Term Bilateral Neuromuscular Function and Knee Osteoarthritis after Anterior Cruciate Ligament Reconstruction. Bioengineering, 10(7), 812. https://doi.org/10.3390/bioengineering10070812






