“Could Patient Age and Gender, along with Mass Size, Be Predictive Factors for Benign Kidney Tumors?”: A Retrospective Analysis of 307 Consecutive Single Renal Masses Treated with Partial or Radical Nephrectomy
Abstract
1. Introduction
2. Study Sample
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Hollingsworth, J.M.; Miller, D.C.; Daignault, S.; Hollenbeck, B.K. Rising Incidence of Small Renal Masses: A Need to Reassess Treatment Effect. Gynecol. Oncol. 2006, 98, 1331–1334. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Mallin, K.; Ritchey, J.; Villalta, J.D.; Carroll, P.R.; Kane, C.J. Decreasing Size at Diagnosis of Stage 1 Renal Cell Carcinoma: Analysis From the National Cancer Data Base, 1993 to 2004. J. Urol. 2008, 179, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.E.; Bach, A.; Kattan, M.W.; Raj, G.V.; Reuter, V.E.; Russo, P. Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in radiological diameter: Influence of sex. J. Urol. 2006, 176, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.G.; Lee, S.R.; Kim, K.H.; Oh, Y.T.; Cho, N.H.; Rha, K.H.; Yang, S.C.; Han, W.K. Benign lesions after partial nephrectomy for presumed renal cell carcinoma in masses 4 cm or less: Prevalence and predictors in Korean patients. Urology 2010, 76, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, B.; Gudeloglu, A.; Inci, K.; Gunay, L.M.; Koni, A.; Ozen, H. Prevalence and predictors of benign lesions in renal masses smaller than 7 cm presumed to be renal cell carcinoma. Clin. Genitourin. Cancer 2012, 10, 121–125. [Google Scholar] [CrossRef]
- Crispen, P.L.; Viterbo, R.; Fox, E.B.; Greenberg, R.E.; Chen, D.Y.T.; Uzzo, R.G. Delayed intervention of sporadic renal masses undergoing active surveillance. Cancer 2008, 112, 1051. [Google Scholar] [CrossRef]
- Marszalek, M.; Ponholzer, A.; Brössner, C.; Wachter, J.; Maier, U.; Madersbacher, S. Elective open nephron-sparing surgery for renal masses: Single-center experience with 129 consecutive patients. Urology 2004, 64, 38–42. [Google Scholar] [CrossRef]
- Uzzo, R.G.; Novick, A.C. Nephron sparing surgery for renal tumors: Indications, techniques and outcomes. J. Urol. 2001, 166, 6–18. [Google Scholar] [CrossRef]
- Ferda, J.; Hora, M.; Hes, O.; Reischig, T.; Kreuzberg, B.; Mirka, H.; Ferdova, E.; Ohlidalova, K.; Baxa, J.; Urge, T. Computed tomography of renal cell carcinoma in patients with terminal renal impairment. Eur. J. Radiol. 2007, 63, 295–301. [Google Scholar] [CrossRef]
- Wu, Y.H.; Song, B.; Gong, Q.Y.; Wu, B.; Chen, W.X.; Liu, R.B.; Wu, B.; Li, Z.L. Renal and non-renal tumors within the perirenal space in infants and children: Multi-detector row CT characteristics. Sichuan Da Xue Xue Bao Yi Xue Ban 2010, 41, 288–291. [Google Scholar]
- Del Vecchio, S.J.; Urquhart, A.J.; Dong, X.; Ellis, R.J.; Ng, K.L.; Samaratunga, H.; Gustafson, S.; Galloway, G.J.; Gobe, G.C.; Wood, S.; et al. Two-dimensional correlated spectroscopy distinguishes clear cell renal cell carcinoma from other kidney neoplasms and non-cancer kidney. Transl. Androl. Urol. 2022, 11, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Remzi, M.; Katzenbeisser, D.; Waldert, M.; Klingler, H.C.; Susani, M.; Memarsadeghi, M.; Heinz Peer, G.; Haitel, A.; Herwig, R.; Marberger, M. Renal tumour size measured radiologically before surgery is an unreliable variable for predicting histopathological features: Benign tumours are not necessarily small. BJU Int. 2007, 99, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Black, W.C. Overdiagnosis in cancer. J. Natl. Cancer Inst. 2010, 102, 605. [Google Scholar] [CrossRef]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Guideline for Management of the Clinical T1 Renal Mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef]
- Hafez, K.S.; Fergany, A.F.; Novick, A.C. Nephron sparing surgery for localized renal cell carcinoma: Impact of tumor size on patient survival, tumor recurrence and TNM staging. J. Urol. 1999, 162, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, J.; Yossepowitch, O.; Kattan, M.W.; Simmons, R.; Motzer, R.J.; Reuter, V.E.; Russo, P. Partial nephrectomy for renal cortical tumors: Pathologic findings and impact on outcome. Urology 2002, 60, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Pahernik, S.; Roos, F.; Hampel, C.; Gillitzer, R.; Melchior, S.W.; Thüroff, J.W. Nephron Sparing Surgery for Renal Cell Carcinoma With Normal Contralateral Kidney: 25 Years of Experience. J. Urol. 2006, 175, 2027–2031. [Google Scholar] [CrossRef]
- Patard, J.J.; Shvarts, O.; Lam, J.S.; Pantuck, A.; Kim, H.; Ficarra, V.; Cindolo, L.; Han, K.-R.; De La Taille, A.; Tostain, J.; et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multi center experience. J. Urol. 2004, 171, 2181–2185. [Google Scholar] [CrossRef]
- Baio, R.; Molisso, G.; Caruana, C.; Di Mauro, U.; Intilla, O.; Pane, U.; D’Angelo, C.; Campitelli, A.; Pentimalli, F.; Sanseverino, R. “To Be or Not to Be Benign” at Partial Nephrectomy for Presumed RCC Renal Masses: Single-Center Experience with 195 Consecutive Patients. Diseases 2023, 11, 27. [Google Scholar] [CrossRef]
- Filipas, D.; Fichtner, J.; Spix, C.; Black, P.; Carus, W.; Hohenfellner, R.; Thüroff, J.W. Nephron sparing surgery of renal cell carcinoma with a normal opposite kidney: Long term outcome in 180 patients. Urology 2000, 56, 387–392. [Google Scholar] [CrossRef]
- Baio, R.; Molisso, G.; Caruana, C.; Intilla, O.; Di Mauro, U.; Pane, U.; Campitelli, A.; Pentimalli, F.; Sanseverino, R. Incidence rate and management of diaphragmatic injury during laparoscopic nephrectomies: Single-center experience. J. Surg. Case Rep. 2022, 2022, rjac127. [Google Scholar] [CrossRef]
- Silverman, S.G.; Gan, Y.U.; Mortele, K.J.; Tuncali, K.; Cibas, E.S. Renal masses in the adult patient: The role of percutaneous biopsy. Radiology 2006, 240, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.N.; Crispen, P.L.; Hanlon, A.L.; Greenberg, R.E.; Chen, D.Y.; Uzzo, R.G. The natural history of observed enhancing renal masses: Meta-analysis and review of the world literature. J. Urol. 2006, 175, 425. [Google Scholar] [CrossRef] [PubMed]
- Israel, G.M.; Bosniak, M.A. An update of the Bosniak renal cyst classification system. Urology 2005, 66, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Johnson, M.H.; Pierorazio, P.M.; Sozio, S.; Sharma, R.; Iyoha, E.; Bass, E.; Allaf, M.E. Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. J. Urol. 2016, 195, 1340–1347. [Google Scholar] [CrossRef]
- Violette, P.; Abourbih, S.; Szymanski, K.M.; Tanguay, S.; Aprikian, A.; Matthews, K.; Brimo, F.; Kassouf, W. Solitary solid renal mass: Can we predict malignancy? BJU Int. 2012, 110 Pt B, E548–E552. [Google Scholar] [CrossRef]
- Zisman, A.; Patard, J.-J.; Raz, O.; Klatte, T.; Haifler, M.; Mendlovic, S.; Sandbank, J.; Belldegrun, A.S.; Lindner, A.; Leibovici, D.; et al. Sex, age, and surgeon decision on nephron-sparing surgery are independent predictors of renal masses with benign histologic findings—A multicenter survey. Urology 2010, 76, 541–546. [Google Scholar] [CrossRef]
- DeRoche, T.; Walker, E.; Magi-Galluzzi, C.; Zhou, M. Pathologic characteristics of solitary small renal masses: Can they be predicted by preoperative clinical parameters? Am. J. Clin. Pathol. 2008, 130, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Komai, Y.; Saito, K.; Iimura, Y.; Yonese, J.; Kawakami, S.; Ishikawa, Y.; Kumagai, J.; Kihara, K.; Fukui, I. Incidence of benign pathologic lesions at partial nephrectomy for presumed RCC renal masses: Japanese dual-center experience with 176 consecutive patients. Urology 2008, 72, 598–602. [Google Scholar] [CrossRef]
- Murphy, A.M.; Buck, A.M.; Benson, M.C.; McKiernan, J.M. Increasing detection rate of benign renal tumors: Evaluation of factors predicting for benign tumor histologic features during past two decades. Urology 2009, 73, 1293–1297. [Google Scholar] [CrossRef]
- Frank, I.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Weaver, A.L.; Zincke, H. Solid renal tumors: An analysis of pathological features related to tumor size. J. Urol. 2003, 170, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Remzi, M.; Özsoy, M.; Klingler, H.-C.; Susani, M.; Waldert, M.; Seitz, C.; Schmidbauer, J.; Marberger, M. Are Small Renal Tumors Harmless? Analysis of Histopathological Features According to Tumors 4 Cm or Less in Diameter. J. Urol. 2006, 176, 896–899. [Google Scholar] [CrossRef]
- Leveridge, M.J.; Finelli, A.; Kachura, J.R.; Evans, A.; Chung, H.; Shiff, D.A.; Fernandes, K.; Jewett, M.A. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur. Urol. 2011, 60, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Kachura, J.R.; Geddie, W.R.; Evans, A.J.; Gharajeh, A.; Saravanan, A.; Jewett, M.A. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J. Urol. 2007, 178, 379–386. [Google Scholar] [CrossRef]
- Osawa, T.; Hafez, K.S.; Miller, D.C.; Montgomery, J.S.; Morgan, T.M.; Palapattu, G.S.; Weizer, A.Z.; Caoili, E.M.; Ellis, J.H.; Kunju, L.P.; et al. Age, Gender and R.E.N.A.L. Nephrometry Score do not Improve the Accuracy of a Risk Stratification Algorithm Based on Biopsy and Mass Size for Assigning Surveillance versus Treatment of Renal Tumors. J. Urol. 2016, 195, 574–580. [Google Scholar] [CrossRef]
- Blumenfeld, A.J.; Guru, K.; Fuchs, G.J.; Kim, H.L. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology 2010, 76, 610. [Google Scholar] [CrossRef]
- Shah, R.B.; Bakshi, N.; Hafez, K.S.; Wood, D.P.; Kunju, L.P. Image-guided biopsy in the evaluation of renal mass lesions in contemporary urological practice: Indications, adequacy, clinical impact, and limitations of the pathological diagnosis. Hum. Pathol. 2005, 36, 1309. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.W.; Bezerra, S.M.; Gorin, M.A.; Cowan, M.; Pavlovich, C.P.; Pierorazio, P.M.; Netto, G.J.; Allaf, M.E. Grade heterogeneity in small renal masses: Potential implications for renal mass biopsy. J. Urol. 2015, 193, 36. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, Y.; Gan, H.; Wang, H.; Shi, G.; Zhang, H.; Dai, B.; Wang, C.; Ye, D. Use of RENAL nephrometry scores for predicting tumor upgrading between core biopsies and surgical specimens: A prospective ex vivo study. Medicine 2015, 94, e581. [Google Scholar] [CrossRef]
- Volpe, A.; Finelli, A.; Gill, I.S.; Jewett, M.A.; Martignoni, G.; Polascik, T.J.; Remzi, M.; Uzzo, R.G. Rationale for 710 percutaneous biopsy and histologic characterisation of renal tumours. Eur. Urol. 2012, 62, 491. [Google Scholar] [CrossRef]
- Kassouf, W.; Aprikian, A.G.; Laplante, M.; Tanguay, S. Natural history of renal masses followed expectantly. J. Urol. 2004, 171, 111–113; discussion 113. [Google Scholar] [CrossRef]
- Abou Youssif, T.; Kassouf, W.; Steinberg, J.; Aprikian, A.G.; Laplante, M.P.; Tanguay, S. Active surveillance for selected patients with renal masses: Updated results with long-term follow-up. Cancer 2007, 110, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Jewett, M.A.; Mattar, K.; Basiuk, J.; Morash, C.G.; Pautler, S.E.; Siemens, D.R.; Tanguay, S.; Rendon, R.A.; Gleave, M.E.; Drachenberg, D.E.; et al. Active surveillance of small renal masses: Progression patterns of early stage kidney cancer. Eur. Urol. 2011, 60, 39–44. [Google Scholar] [CrossRef]
- Aron, M.; Gill, I.S. Minimally invasive nephron sparing surgery (MINSS) for renal tumours. Part II: Probe ablative therapy. Eur. Urol. 2007, 51, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; Novick, A.C. Nephron sparing surgery. BJU Int. 2007, 99, 1245–1250. [Google Scholar] [CrossRef]
- Piasentin, A.; Claps, F.; Silvestri, T.; Rebez, G.; Traunero, F.; Mir, M.C.; Rizzo, M.; Celia, A.; Cicero, C.; Urbani, M.; et al. Assessing Trifecta Achievement after Percutaneous Cryoablation of Small Renal Masses: Results from a Multi-Institutional Collaboration. Medicina 2022, 58, 1041. [Google Scholar] [CrossRef] [PubMed]
- Rich, B.J.; Noy, M.A.; Dal Pra, A. Stereotactic Body Radiotherapy for Localized Kidney Cancer. Curr. Urol. Rep. 2022, 23, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; Samplaski, M.K.; Herts, B.R.; Zhou, M.; Novick, A.C.; Campbell, S.C. Renal mass biopsy—A renaissance? J. Urol. 2008, 179, 20–27. [Google Scholar] [CrossRef]
- Schmidbauer, J.; Remzi, M.; Memarsadeghi, M.; Haitel, A.; Klingler, H.C.; Katzenbeisser, D.; Wiener, H.; Marberger, M. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur. Urol. 2008, 53, 1003–1011. [Google Scholar] [CrossRef]
- Halverson, S.J.; Kunju, L.P.; Bhalla, R.; Gadzinski, A.J.; Alderman, M.; Miller, D.C.; Montgomery, J.S.; Weizer, A.Z.; Wu, A.; Hafez, K.S.; et al. Accuracy of determining small renal mass management with risk stratified biopsies: Confirmation by final pathology. J. Urol. 2013, 189, 441–446. [Google Scholar] [CrossRef]
- Hsu, P.K.; Huang, H.C.; Hsieh, C.C.; Hsu, H.S.; Wu, Y.C.; Huang, M.H.; Hsu, W.H. Effect of formalin fixation on tumor size determination in stage I non-small cell lung cancer. Ann. Thorac. Surg. 2007, 84, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
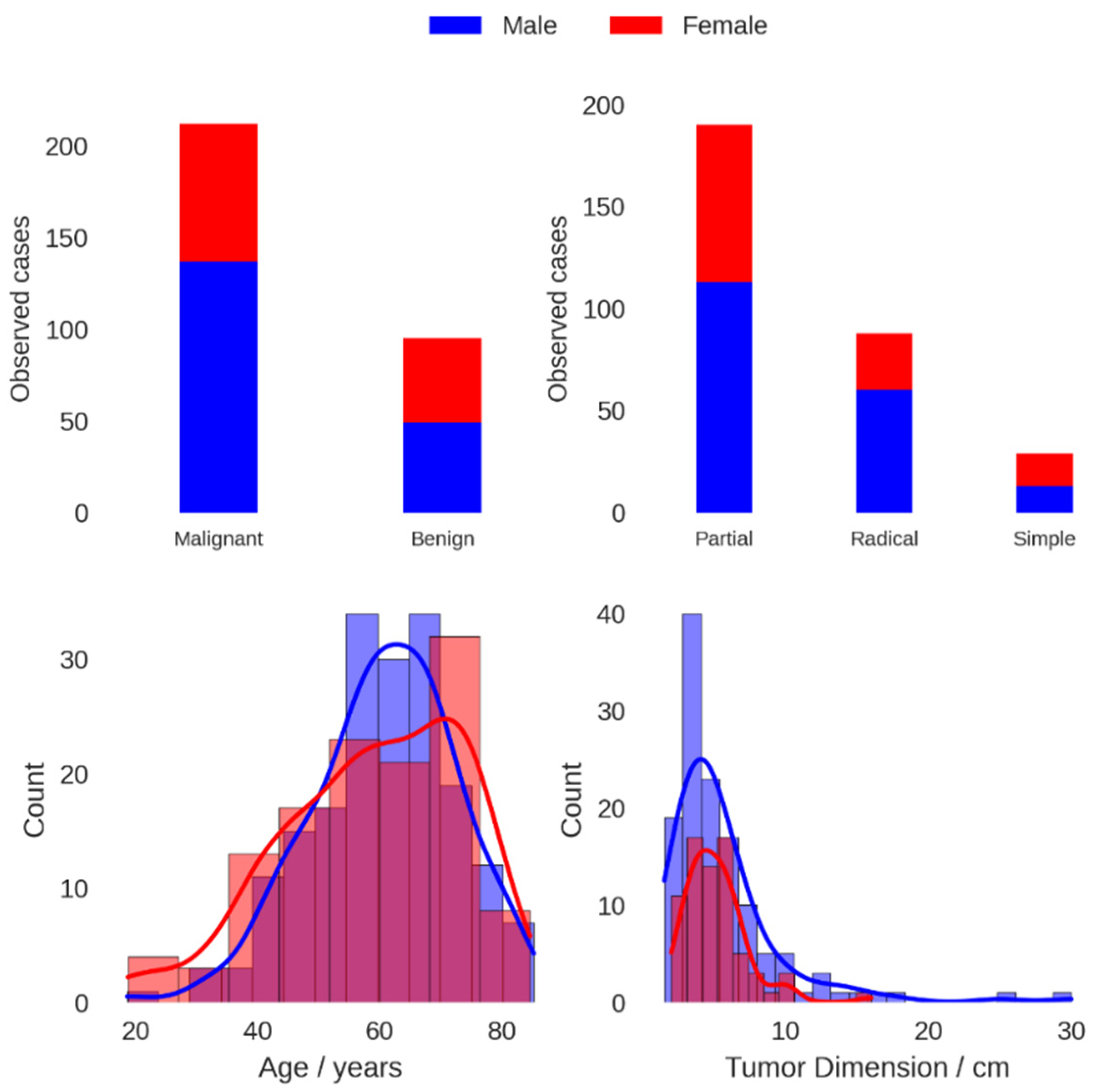

| Population Characteristics | |
|---|---|
| Gender, n (%) | |
| Female | 121 (39.4) |
| Male | 186 (60.6) |
| Age, mean (SD), years | 60 (13) |
| Histological type: malignant tumors, n (%) | |
| RCC | 154 (72.6) |
| Papillary | 26 (12.3) |
| Chromophobe | 16 (7.5) |
| Others | 16 (7.5) |
| Histological type: benign conditions, n (%) | |
| Oncocytoma | 34 (35.8) |
| Pyelonephritis | 21 (22.1) |
| Angiomyolipoma | 11 (11.6) |
| Hydronephrosis | 10 (10.5) |
| Others | 19 (20.0) |
| Intervention type: malignant tumors, n (%) | |
| Laparo | 189 (89.2) |
| Open | 20 (9.4) |
| Removal type: malignant tumors, n (%) | |
| Partial | 135 (63.7) |
| Radical | 76 (35.8) |
| Simple | 1 (0.5) |
| Tumor size malignant, mean (SD), cm | 5.7 (3.8) |
| Tumor size benign, mean (SD), cm | 4.4 (2.1) |
| Univariate Logistic Regression Analysis | |||
|---|---|---|---|
| Variable Name | n (%) | OR (95% CI) | p |
| Tumor size | 201 | 1.12 (1.03–1.42) | 0.032 * |
| ≤3 cm | 40 (19.9) | 1—Reference | NA |
| 3–4 cm | 47 (23.4) | 1.25 (0.48–3.23) | 0.642 |
| 4–5 cm | 36 (17.9) | 1.29 (0.47–3.62) | 0.629 |
| >5 cm | 78 (38.8) | 2.61 (1.03–6.71) | 0.043 * |
| Age | 307 | 1.04 (1.02–1.06) | <0.001 ** |
| <50 years | 69 (22.5) | 0.36 (0.18–0.72) | 0.004 ** |
| 50–60 years | 77 (25.1) | 1.13 (0.55–2.32) | 0.74 |
| 60–70 years | 87 (28.3) | 1.51 (0.74–3.16) | 0.258 |
| ≥70 years | 74 (24.1) | 1—Reference | NA |
| Male | 186 (60.6) | 1.71 (1.05–2.81) | 0.031 * |
| Right side | 146 (47.6) | 0.89 (0.55–1.45) | 0.653 |
| Year | 307 | 1.07 (1.01–1.14) | 0.022 * |
| Multivariate Logistic Regression Analysis | ||||
|---|---|---|---|---|
| Variable Name | Continuous | Categorized | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Tumor size | 1.22 (1.05–1.47) | 0.023 * | NA | NA |
| ≤3 cm | NA | NA | 1–Reference | NA |
| 3–4 cm | NA | NA | 1.27 (0.48–3.40) | 0.628 |
| 4–5 cm | NA | NA | 1.48 (0.52–4.40) | 0.465 |
| >5 cm | NA | NA | 3.21 (1.22–8.68) | 0.019 * |
| Age | 1.03 (1.00–1.06) | 0.043 * | NA | NA |
| <50 years | NA | NA | 0.46 (0.16–1.26) | 0.131 |
| 50–60 years | NA | NA | 0.72 (0.27–1.87) | 0.499 |
| 60–70 years | NA | NA | 1.64 (0.58–4.83) | 0.354 |
| ≥70 years | NA | NA | 1–Reference | NA |
| Male | 1.72 (0.85–3.49) | 0.133 | 1.78 (0.86–3.69) | 0.120 |
| Right side | 0.76 (0.38–1.52) | 0.438 | 0.83 (0.40–1.69) | 0.604 |
| Year | 0.97 (0.88–1.07) | 0.596 | 0.98 (0.89–1.08) | 0.693 |
| Sex | Age | Tumor Dimension (cm) | Kidney | Malignancy Risk (%) | Risk Ratio |
|---|---|---|---|---|---|
| Female | 51.75 | 4.6 | Right | 63.0 | 0.91 |
| Female | 61.2 | 4.6 | Right | 69.1 | 1.00 |
| Female | 69.75 | 4.6 | Right | 74.1 | 1.07 |
| Female | 61.2 | 3.5 | Right | 64.2 | 0.93 |
| Female | 61.2 | 6 | Right | 74.7 | 1.08 |
| Male | 51.75 | 4.6 | Right | 74.5 | 1.08 |
| Male | 61.2 | 4.6 | Right | 79.3 | 1.15 |
| Male | 69.75 | 4.6 | Right | 83.1 | 1.20 |
| Male | 61.2 | 3.5 | Right | 75.5 | 1.09 |
| Male | 61.2 | 6 | Right | 83.5 | 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baio, R.; Molisso, G.; Caruana, C.; Di Mauro, U.; Intilla, O.; Pane, U.; D’Angelo, C.; Campitelli, A.; Pentimalli, F.; Sanseverino, R. “Could Patient Age and Gender, along with Mass Size, Be Predictive Factors for Benign Kidney Tumors?”: A Retrospective Analysis of 307 Consecutive Single Renal Masses Treated with Partial or Radical Nephrectomy. Bioengineering 2023, 10, 794. https://doi.org/10.3390/bioengineering10070794
Baio R, Molisso G, Caruana C, Di Mauro U, Intilla O, Pane U, D’Angelo C, Campitelli A, Pentimalli F, Sanseverino R. “Could Patient Age and Gender, along with Mass Size, Be Predictive Factors for Benign Kidney Tumors?”: A Retrospective Analysis of 307 Consecutive Single Renal Masses Treated with Partial or Radical Nephrectomy. Bioengineering. 2023; 10(7):794. https://doi.org/10.3390/bioengineering10070794
Chicago/Turabian StyleBaio, Raffaele, Giovanni Molisso, Christian Caruana, Umberto Di Mauro, Olivier Intilla, Umberto Pane, Costantino D’Angelo, Antonio Campitelli, Francesca Pentimalli, and Roberto Sanseverino. 2023. "“Could Patient Age and Gender, along with Mass Size, Be Predictive Factors for Benign Kidney Tumors?”: A Retrospective Analysis of 307 Consecutive Single Renal Masses Treated with Partial or Radical Nephrectomy" Bioengineering 10, no. 7: 794. https://doi.org/10.3390/bioengineering10070794
APA StyleBaio, R., Molisso, G., Caruana, C., Di Mauro, U., Intilla, O., Pane, U., D’Angelo, C., Campitelli, A., Pentimalli, F., & Sanseverino, R. (2023). “Could Patient Age and Gender, along with Mass Size, Be Predictive Factors for Benign Kidney Tumors?”: A Retrospective Analysis of 307 Consecutive Single Renal Masses Treated with Partial or Radical Nephrectomy. Bioengineering, 10(7), 794. https://doi.org/10.3390/bioengineering10070794







