Impact of Aspiration Percutaneous Vertebroplasty in Reducing Bone Cement Leakage and Enhancing Distribution—An Ex Vivo Study in Goat Vertebrae
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of Ex Vivo Bone Models with Defects
2.1.1. Preparation of Specimens
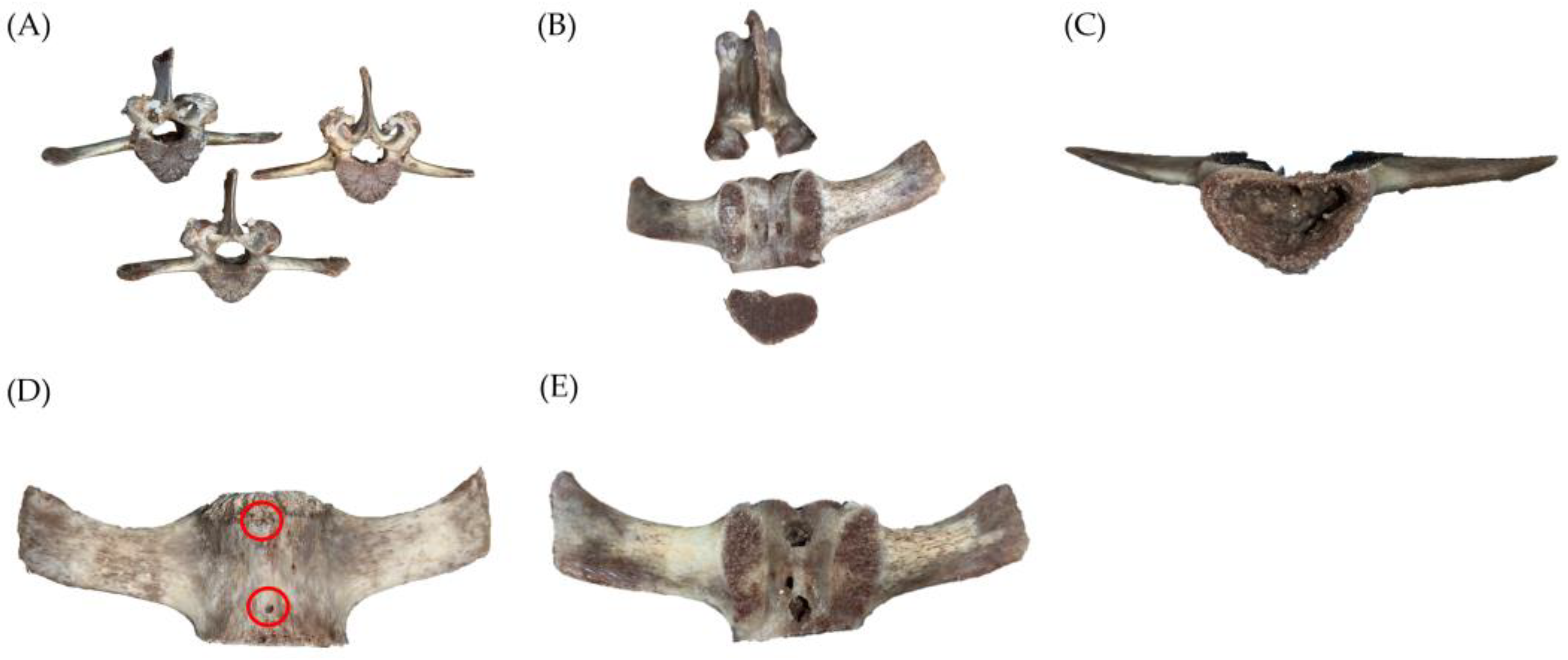
2.1.2. Vertebral Bone Defect Creation
2.2. Equipment Setup and Pressure Pump Testing
2.3. Bone Cement Preparation/Infusion/Cooling
- (1)
- Volume fraction percentage of VB:
- (2)
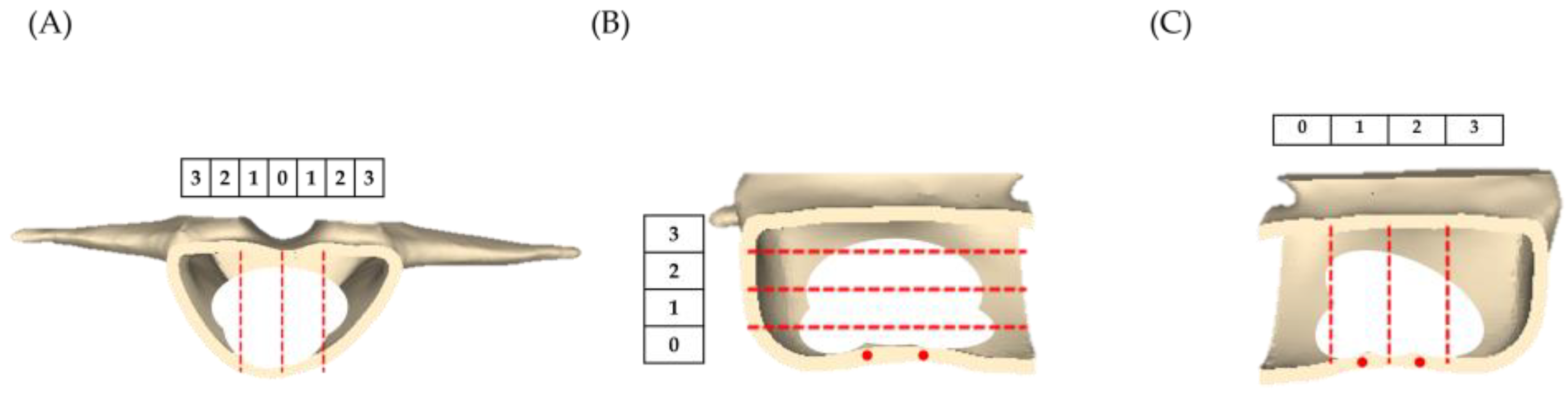
- Distribution of bone cement: The 9-score method is an objective scoring system used to assess the distribution of cement within the vertebra [29,30,31]. We calculate the scores of the vertical distribution in the posterior view, horizontal distribution on the right side, and vertical distribution on the left side of the vertebra. If the bone cement distribution was within 0% to 25% of the vertebra, it scores 0 points; within 25% to 50% scores 1 point; within 50% to 75% scores 2 points; and over 75% scores 3 points, as shown in Figure 4.
- (3)
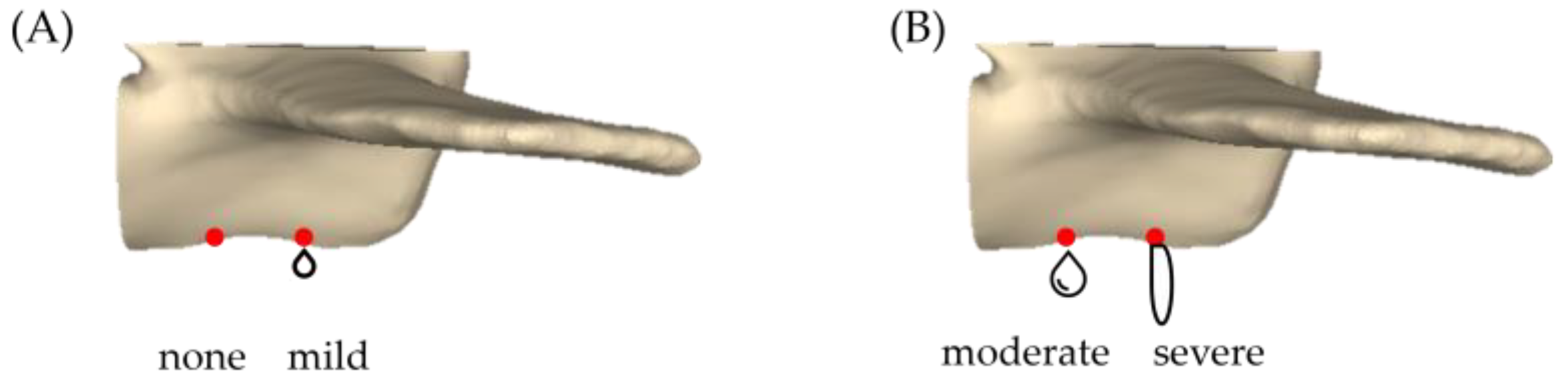
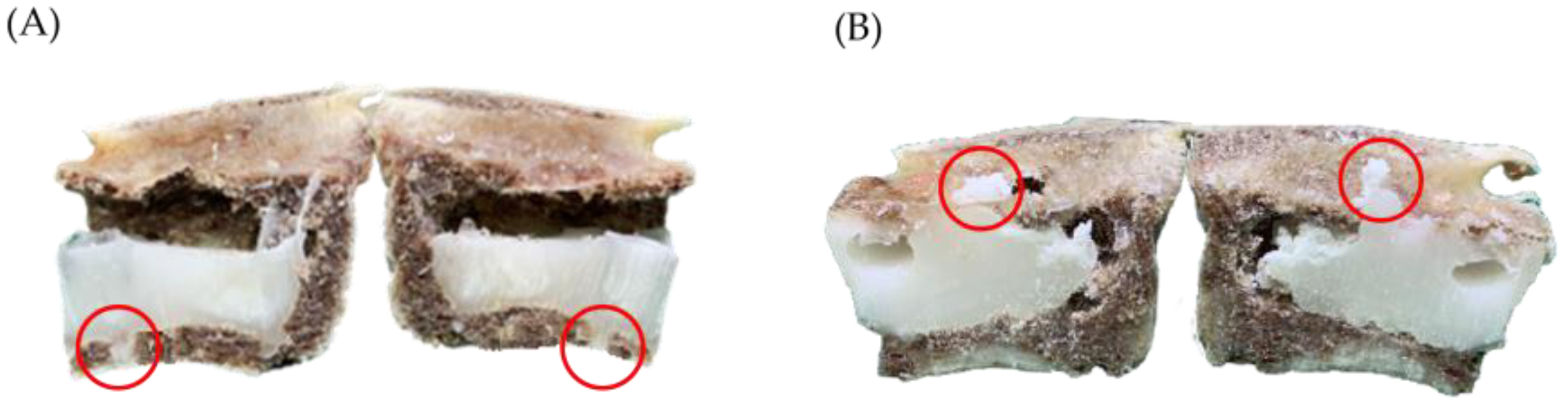
- Leakage rate of bone cement: Based on Churojana’s research, the degree of leakage has been adjusted [32]. The study measured the CL rate by examining artificially drilled holes created beneath the vertebra. The degree of CL was classified into four categories: no leakage, mild leakage, moderate leakage, and severe leakage, as depicted in Figure 5.
2.4. Statistical Analysis
3. Results
3.1. Volume Fraction Percentage
3.2. Cement Distribution
3.3. Leakage Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990-2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Q.; Yang, Y.; Feng, H.; Zhao, Y.; Guo, P.; Li, D.; Du, X.; Song, Q. Feasibility Study of 3D FACT and IVIM Sequences in the Evaluation of Female Osteoporosis. Bioengineering 2023, 10, 710. [Google Scholar] [CrossRef]
- Gao, H.; Huang, J.; Wei, Q.; He, C. Advances in Animal Models for Studying Bone Fracture Healing. Bioengineering 2023, 10, 201. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46. [Google Scholar] [CrossRef]
- Long, Y.; Yi, W.; Yang, D. Advances in Vertebral Augmentation Systems for Osteoporotic Vertebral Compression Fractures. Pain Res. Manag. 2020, 2020, 3947368. [Google Scholar] [CrossRef] [PubMed]
- Prost, S.; Pesenti, S.; Fuentes, S.; Tropiano, P.; Blondel, B. Treatment of osteoporotic vertebral fractures. Orthop. Traumatol. Surg. Res. 2021, 107, 102779. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.K.; Chen, W.J.; Lee, M.S.; Lin, S.Y.; Liu, M.Y.; Lee, D.M.; Tai, C.L. Biomechanical Evaluation of a Novel Expandable Vertebral Augmentation System Using Human Cadaveric Vertebrae. Appl. Sci. 2022, 12, 10165. [Google Scholar] [CrossRef]
- Liao, J.C.; Chen, M.J.W.; Lin, T.Y.; Chen, W.P. Biomechanical comparison of vertebroplasty, kyphoplasty, vertebrae stent for osteoporotic vertebral compression fractures—A finite element analysis. Appl. Sci. 2021, 11, 5764. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Z.; Wang, H.; Yao, R.; Li, F.; Yang, Y.; Sun, F. Treatment of Elderly Patients with Acute Symptomatic OVCF: A Study of Comparison of Conservative Treatment and Percutaneous Kyphoplasty. Front. Surg. 2022, 9, 942195. [Google Scholar] [CrossRef]
- Zhang, J.N.; He, X.; Fan, Y.; Du, J.P.; Hao, D.J. Risk factors for conservative treatment failure in acute osteoporotic vertebral compression fractures (OVCFs). Arch. Osteoporos. 2019, 14, 24. [Google Scholar] [CrossRef]
- Castro, A.P.G. Computational challenges in tissue engineering for the spine. Bioengineering 2021, 8, 25. [Google Scholar] [CrossRef]
- Schmidt-Rohlfing, B.; Reilmann, H.; Pfeifer, R.; Kobbe, P.; Pape, H.C. Kyphoplastie und Vertebroplastie. Der Unfallchirurg 2011, 5, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Fiani, B.; Newhouse, A.; Sarhadi, K.J.; Arshad, M.; Soula, M.; Cathel, A. Special Considerations to Improve Clinical Outcomes in Patients with Osteoporosis Undergoing Spine Surgery. Int. J. Spine Surg. 2021, 15, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, C.P.; Song, L.X.; Zhu, L. Balloon kyphoplasty versus percutaneous vertebroplasty for osteoporotic vertebral compression fracture: A meta-analysis and systematic review. J. Orthop. Surg. Res. 2018, 13, 264. [Google Scholar] [CrossRef]
- Semaan, H.; Obri, T.; Bazerbashi, M.; Paull, D.; Liu, X.; Sarrouj, M.; Elgafy, H. Clinical outcome and subsequent sequelae of cement extravasation after percutaneous kyphoplasty and vertebroplasty: A comparative review. Acta Radiol. 2018, 59, 861–868. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, J.; Liao, H.; Tan, H.; Yang, K. Risk factors for cement leakage after vertebroplasty or kyphoplasty: A meta-analysis of published evidence. World Neurosurg. 2017, 101, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, K.; Chang, X.; Cai, S.; Gao, J.; Wang, Y. Cement leakage following percutaneous kyphoplasty in a patient after a posterior lumbar fusion: A case report. BMC Surg. 2020, 20, 74. [Google Scholar] [CrossRef]
- Hsieh, M.K.; Kao, F.C.; Chiu, P.Y.; Chen, L.H.; Yu, C.W.; Niu, C.C.; Lai, P.L.; Tsai, T.T. Risk factors of neurological deficit and pulmonary cement embolism after percutaneous vertebroplasty. J. Orthop. Surg. Res. 2019, 14, 406. [Google Scholar] [CrossRef]
- Kong, M.; Xu, X.; Shen, J.; Liu, Q.; Wang, G. Clinical characteristics and management of cardiac and/or pulmonary cement embolus after percutaneous vertebroplasty: A single center experience. Ann. Transl. Med. 2019, 7, 372. [Google Scholar] [CrossRef]
- Chu, W.; Tsuei, Y.C.; Liao, P.H.; Lin, J.H.; Chou, W.H.; Chu, W.C.; Young, S.T. Decompressed percutaneous vertebroplasty: A secured bone cement delivery procedure for vertebral augmentation in osteoporotic compression fractures. Injury 2013, 44, 813–818. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Lv, N.; Wang, S.; Wang, Y.; Wu, S.; He, F.; Chen, A.; Qian, Z.; Chen, J. The effect of bone cement distribution on clinical efficacy after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Medicine 2019, 98, e18217. [Google Scholar] [CrossRef]
- Sun, H.B.; Shan, J.L.; Tang, H. Percutaneous vertebral augmentation for osteoporotic vertebral compression fractures will increase the number of subsequent fractures at adjacent vertebral levels: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5176–5188. [Google Scholar] [PubMed]
- Yu, W.; Xiao, X.; Zhang, J.; Li, Z.; Wang, X.; Tang, F.; Jiang, X.; Zhong, Y. Cement distribution patterns in osteoporotic vertebral compression fractures with intravertebral cleft: Effect on therapeutic efficacy. World Neurosurg. 2019, 123, e408–e415. [Google Scholar] [CrossRef] [PubMed]
- Banstola, A.; Reynolds, J.N. The sheep as a large animal model for the investigation and treatment of human disorders. Biology 2022, 11, 1251. [Google Scholar] [CrossRef]
- Zhu, X.S.; Zhang, Z.M.; Mao, H.Q.; Geng, D.C.; Zou, J.; Wang, G.L.; Zhang, Z.G.; Wang, J.H.; Chen, L.; Yang, H.L. A novel sheep vertebral bone defect model for injectable bioactive vertebral augmentation materials. J. Mater. Sci. Mater. Med. 2011, 22, 159–164. [Google Scholar] [CrossRef]
- Benneker, L.M.; Gisep, A.; Krebs, J.; Boger, A.; Heini, P.F.; Boner, V. Development of an in vivo experimental model for percutaneous vertebroplasty in sheep. Vet. Comp. Orthop. Traumatol. 2012, 25, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Wang, T.; Pelletier, M.H.; Walsh, W.R. Effects of cement augmentation on the mechanical stability of multilevel spine after vertebral compression fracture. J. Spine Surg. 2016, 2, 111. [Google Scholar] [CrossRef]
- Pflugmacher, R.; Taylor, R.; Agarwal, A.; Melcher, I.; Disch, A.; Haas, N.P.; Klostermann, C. Balloon kyphoplasty in the treatment of metastatic disease of the spine: A 2-year prospective evaluation. Eur. Spine J. 2008, 17, 1042–1048. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Liu, H.; Gu, Z.; Zhang, Y.; Yu, S. A novel and convenient method to evaluate bone cement distribution following percutaneous vertebral augmentation. Sci. Rep. 2020, 10, 16320. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.B.; Jing, X.S.; Liu, Y.Z.; Qi, M.; Wang, X.K.; Hai, Y. The optimal volume fraction in percutaneous vertebroplasty evaluated by pain relief, cement dispersion, and cement leakage: A prospective cohort study of 130 patients with painful osteoporotic vertebral compression fracture in the thoracolumbar vertebra. World Neurosurg. 2018, 114, e677–e688. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Zhang, Y.; Gu, Z.C.; Yu, S.H. Percutaneous vertebral augmentation for osteoporotic vertebral compression fracture in the midthoracic vertebrae (T5-8): A retrospective study of 101 patients with 111 fractured segments. World Neurosurg. 2019, 122, e1381–e1387. [Google Scholar] [CrossRef]
- Churojana, A.; Songsaeng, D.; Khumtong, R.; Suwanbundit, A.; Saliou, G. Is intervertebral cement leakage a risk factor for new adjacent vertebral collapse? Interv. Neuroradiol. 2014, 20, 637–645. [Google Scholar] [CrossRef]
- Liao, P.H.; Tsuei, Y.C.; Chu, W. Application of Machine Learning in Developing Decision-Making Support Models for Decompressed Vertebroplasty. Healthcare 2022, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Lee, H.M.; Shih, J.T.; Hung, S.T. Combined extraforaminal and intradiscal cement leakage following percutaneous vertebroplasty. Spine 2007, 32, E358–E362. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Wu, Z.; Teng, Y.; Huang, Y.; Liu, T.; Yang, H. A Comparison of Percutaneous Kyphoplasty with High-Viscosity and Low-Viscosity Bone Cement for Treatment of Osteoporotic Vertebral Compression Fractures: A Retrospective Study. Geriatr. Orthop. Surg. Rehabil. 2022, 13, 21514593221119625. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Zhang, R.; Zhang, H.; Zhang, D.; Hu, N.; Wang, Y. Systematic Retrospective Analysis of Risk Factors and Preventive Measures of Bone Cement Leakage in Percutaneous Kyphoplasty. World Neurosurg. 2023, 171, e828–e836. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Feng, H.; Luo, D.; Zhang, W.; Yang, K.; Ji, C.; Liu, J.; Xu, H. Efficacy and safety of high-viscosity cement in percutaneous vertebroplasty for treatment of Osteoporotic vertebral compression fractures: A retrospective cohort study. Medicine 2020, 99, e20515. [Google Scholar] [CrossRef]
- Chen, W.C.; Tsai, S.H.L.; Goyal, A.; Fu, T.S.; Lin, T.Y.; Bydon, M. Comparison between vertebroplasty with high or low viscosity cement augmentation or kyphoplasty in cement leakage rate for patients with vertebral compression fracture: A systematic review and network meta-analysis. Eur. Spine J. 2021, 30, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Takizawa, K.; Ogawa, Y.; Fujikawa, A.; Yoshimatsu, M.; Nakajima, Y. Percutaneous vertebroplasty for vertebral compression fractures with intravertebral cleft: Cement injection under vacuum aspiration. J. Vasc. Interv. Radiol. 2011, 22, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Jin, P.; Jia, P.; Liu, X.W.; Tang, H.; Sun, G. Percutaneous vertebral augmentation using drill rotation for osteoporotic vertebral compression fractures with intravertebral vacuum cleft. Skelet. Radiol. 2020, 49, 1459–1465. [Google Scholar] [CrossRef]
- Albers, C.E.; Schott, P.M.; Ahmad, S.S.; Benneker, L.M.; Nieuwkamp, N.; Hoppe, S. Vertebral body lavage reduces hemodynamic response to vertebral body augmentation with PMMA. Glob. Spine J. 2019, 9, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, E.I.; Isalberti, M.; Pileggi, M.; Distefano, D.; Hirsch, J.A.; Cianfoni, A. Mechanical cavity creation with curettage and vacuum suction (Q-VAC) in lytic vertebral body lesions with posterior wall dehiscence and epidural mass before cement augmentation. Medicina 2019, 55, 633. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, Q.; Zheng, Y.; Liu, Z.; Liu, X.; Guo, X.; Liu, P.; Chen, P.; Yuan, S.; Tian, Y.; et al. Effect of unilateral pulsed jet lavage prior to vertebroplasty on the intravertebral pressure and cement distribution. J. Orthop. Surg. Res. 2020, 15, 259. [Google Scholar] [CrossRef]
- Li, Q.; Long, X.; Wang, Y.; Guan, T.; Fang, X.; Guo, D.; Lv, J.; Hu, X.; Jiang, X.; Cai, L. Clinical observation of two bone cement distribution modes after percutaneous vertebroplasty for osteoporotic vertebral compression fractures. BMC Musculoskelet. Disord. 2021, 22, 577. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Xie, D.; Yang, X.; Zheng, Z. Comparison of unipedicular and bipedicular kyphoplasty on the stiffness and biomechanical balance of compression fractured vertebrae. Eur. Spine J. 2011, 20, 1272–1280. [Google Scholar] [CrossRef] [PubMed]

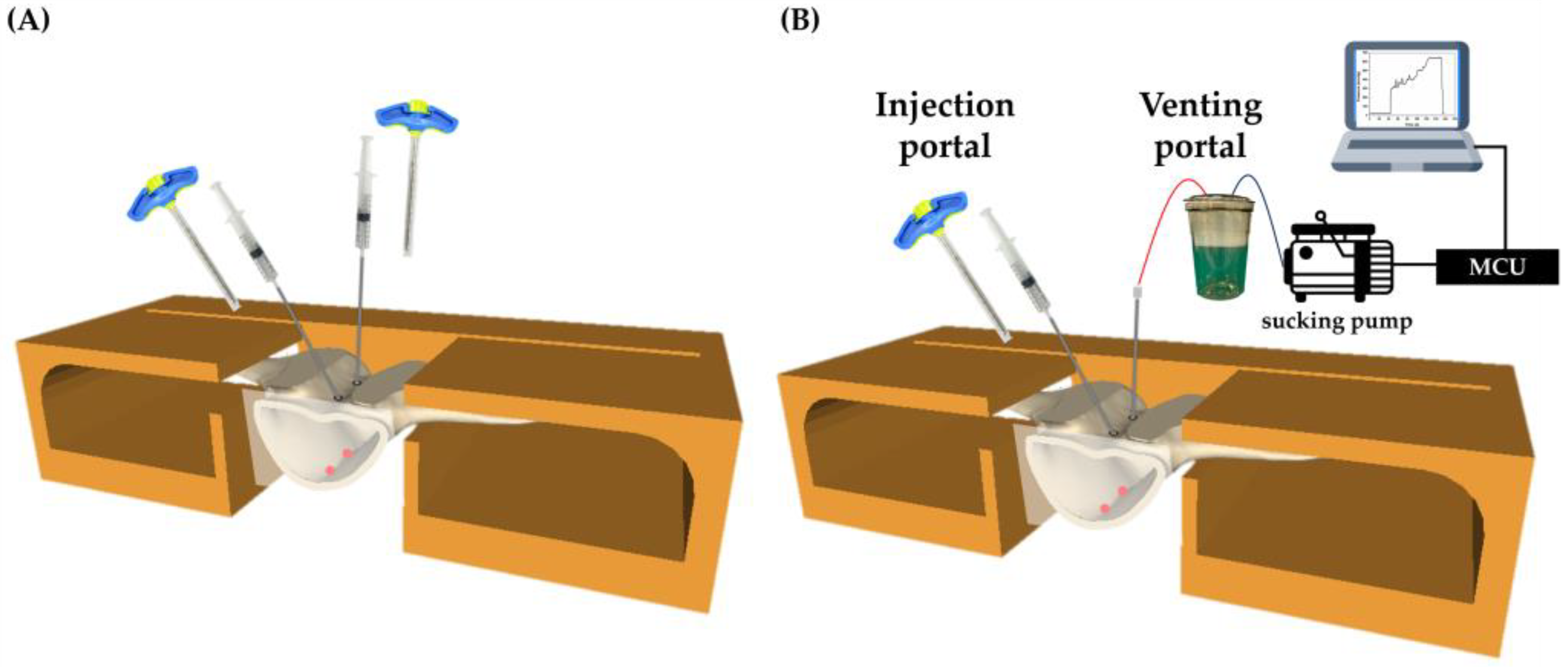



| PVP (Control Group) | APV (Experimental Group) | |
|---|---|---|
| Injection method | About 1.2 mL to 1.5 mL of cement was injected into each side. | One side is injected with cement, while the other side is connected to the MCU to read the pressure values. |
| Termination timing + | Continue the injection until the estimated volume is met. | Keep the injection until the pressure value reaches the 600~700 mmHg, and last for 10 to 20 s. |
| PVP Group | APV Group | p-Value | |
|---|---|---|---|
| Estimated cavity volume (mL) | 2.70 ± 0.34 | 2.40 ± 0.42 | 0.22 |
| Bone cement volume within the cavity (mL) | 1.20 ± 0.27 | 1.45 ± 0.49 | 0.035 * |
| Weight of bone cement within the cavity (g) | 1.80 ± 0.39 | 1.90 ± 0.65 | 0.015 * |
| Volume filling percentage of bone cement (%) | 45 ± 13 | 61 ± 16 | 0.2 |
| Average score using 9-score method | 4 ± 1.78 | 7 ± 1.30 | <0.01 ** |
| Leakage rate (%) | 53 | 13 | - |
| Remark | Bone cement is filled from bottom to top | Bone cement is filled layer by layer | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.-T.; Lin, J.-Y.; Tsuei, Y.-C.; Hsu, Y.-F.; Chen, C.-Y.; Cheng, S.-H.; Chu, W.; Li, C.; Chu, W.-C. Impact of Aspiration Percutaneous Vertebroplasty in Reducing Bone Cement Leakage and Enhancing Distribution—An Ex Vivo Study in Goat Vertebrae. Bioengineering 2023, 10, 795. https://doi.org/10.3390/bioengineering10070795
Lu H-T, Lin J-Y, Tsuei Y-C, Hsu Y-F, Chen C-Y, Cheng S-H, Chu W, Li C, Chu W-C. Impact of Aspiration Percutaneous Vertebroplasty in Reducing Bone Cement Leakage and Enhancing Distribution—An Ex Vivo Study in Goat Vertebrae. Bioengineering. 2023; 10(7):795. https://doi.org/10.3390/bioengineering10070795
Chicago/Turabian StyleLu, Hsin-Tzu, Jia-Yi Lin, Yu-Chuan Tsuei, Yung-Fu Hsu, Chung-Yi Chen, Shih-Hao Cheng, William Chu, Chuan Li, and Woei-Chyn Chu. 2023. "Impact of Aspiration Percutaneous Vertebroplasty in Reducing Bone Cement Leakage and Enhancing Distribution—An Ex Vivo Study in Goat Vertebrae" Bioengineering 10, no. 7: 795. https://doi.org/10.3390/bioengineering10070795
APA StyleLu, H.-T., Lin, J.-Y., Tsuei, Y.-C., Hsu, Y.-F., Chen, C.-Y., Cheng, S.-H., Chu, W., Li, C., & Chu, W.-C. (2023). Impact of Aspiration Percutaneous Vertebroplasty in Reducing Bone Cement Leakage and Enhancing Distribution—An Ex Vivo Study in Goat Vertebrae. Bioengineering, 10(7), 795. https://doi.org/10.3390/bioengineering10070795









