Medical 3D Printing Using Desktop Inverted Vat Photopolymerization: Background, Clinical Applications, and Challenges
Abstract
1. Introduction
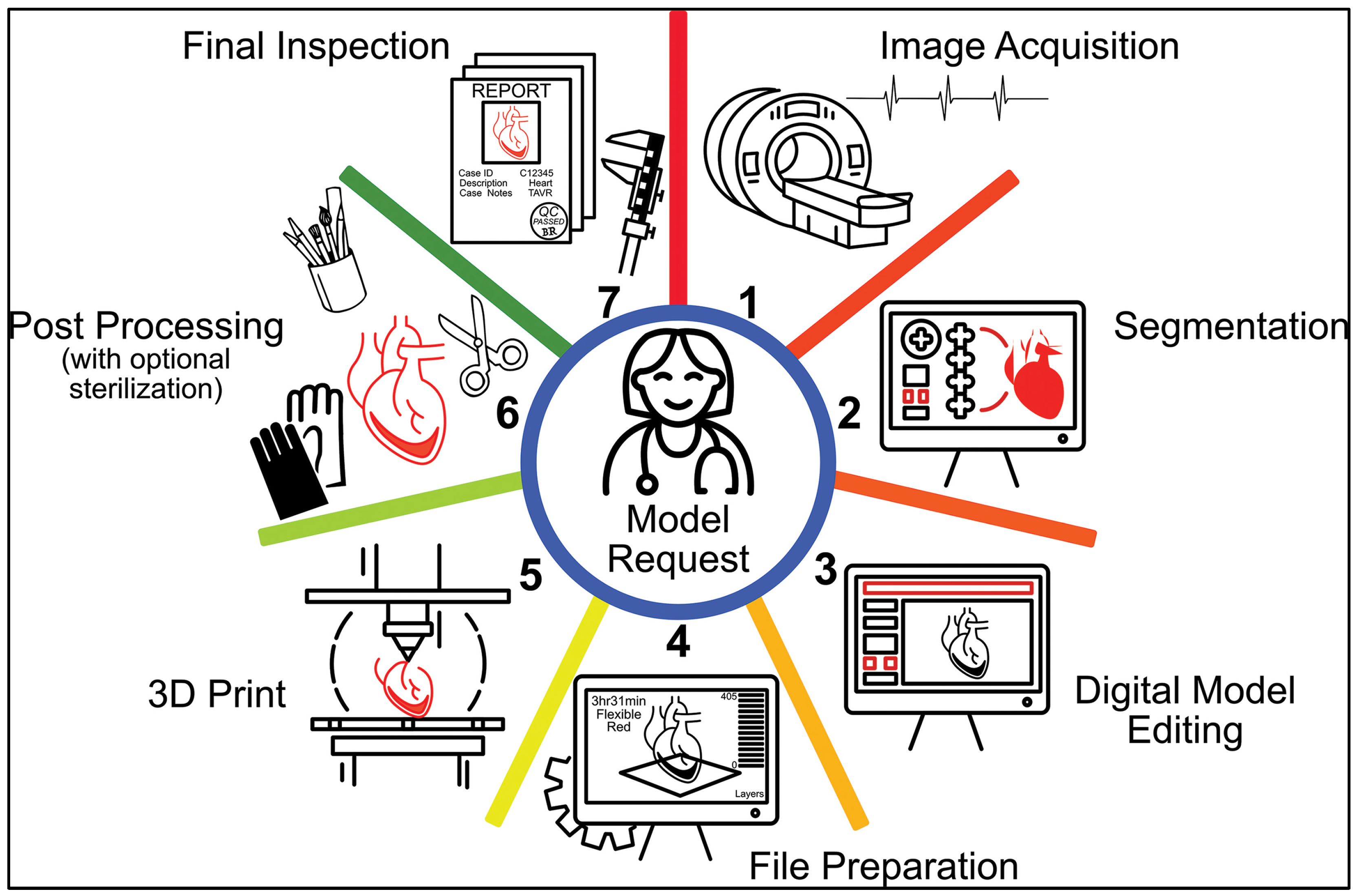
2. Medical 3D Printing Workflow
2.1. Image Acquisition
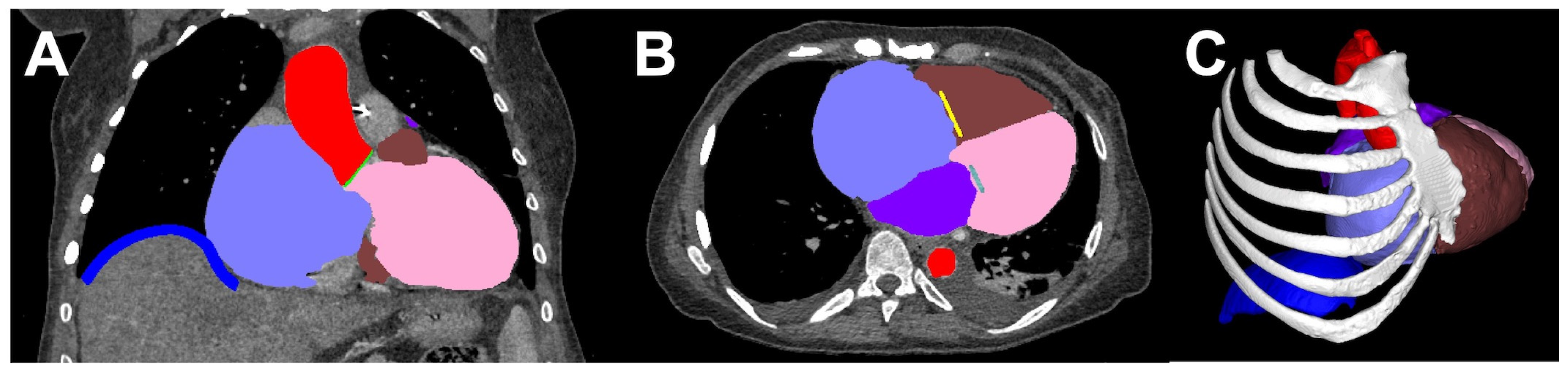
2.2. Segmentation
2.3. Computer-Aided Design (CAD) or Digital Model Editing
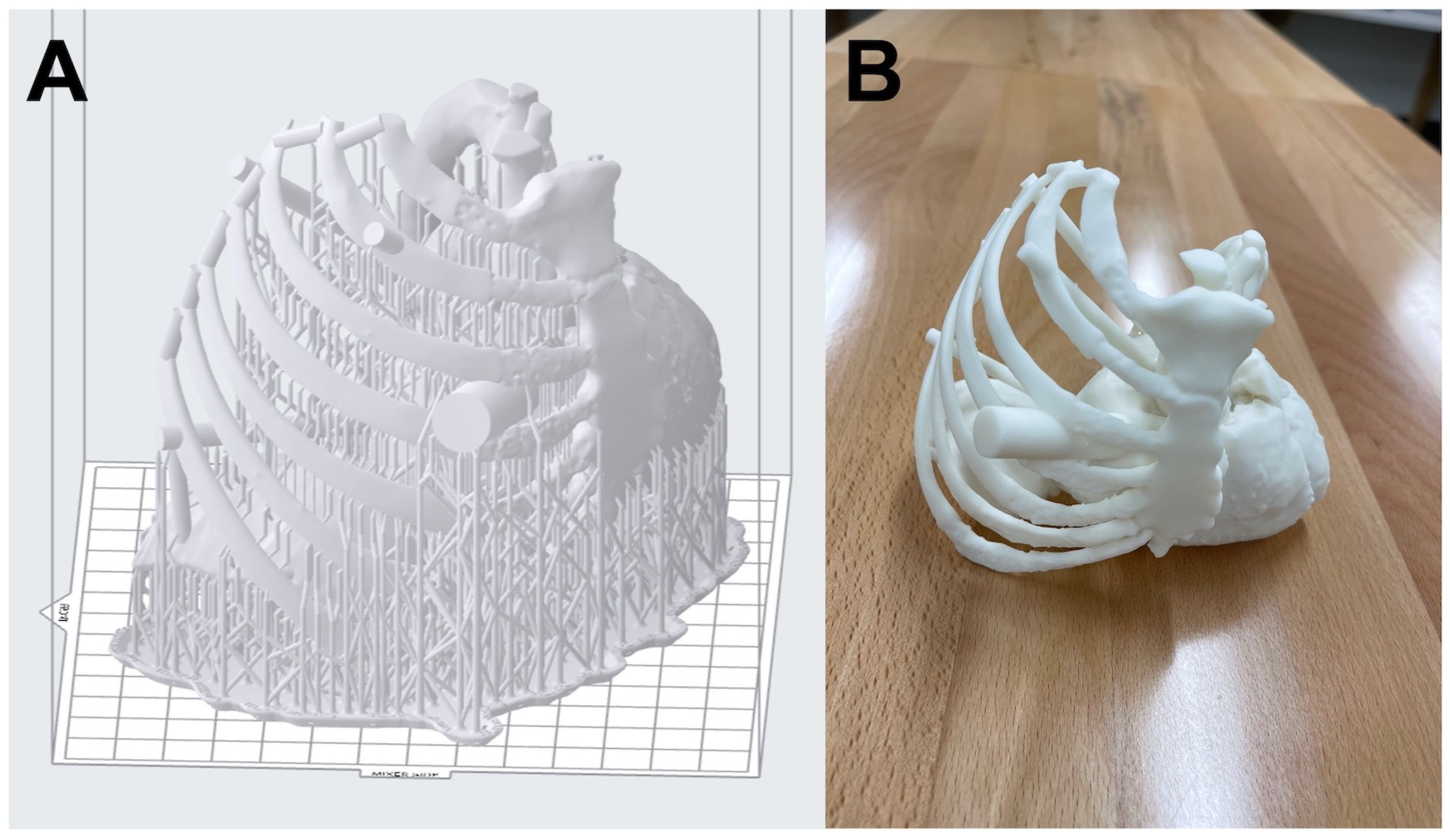
2.4. File Preparation
2.5. Three-Dimensional Printing
2.6. Post-Processing
2.7. Final Inspection
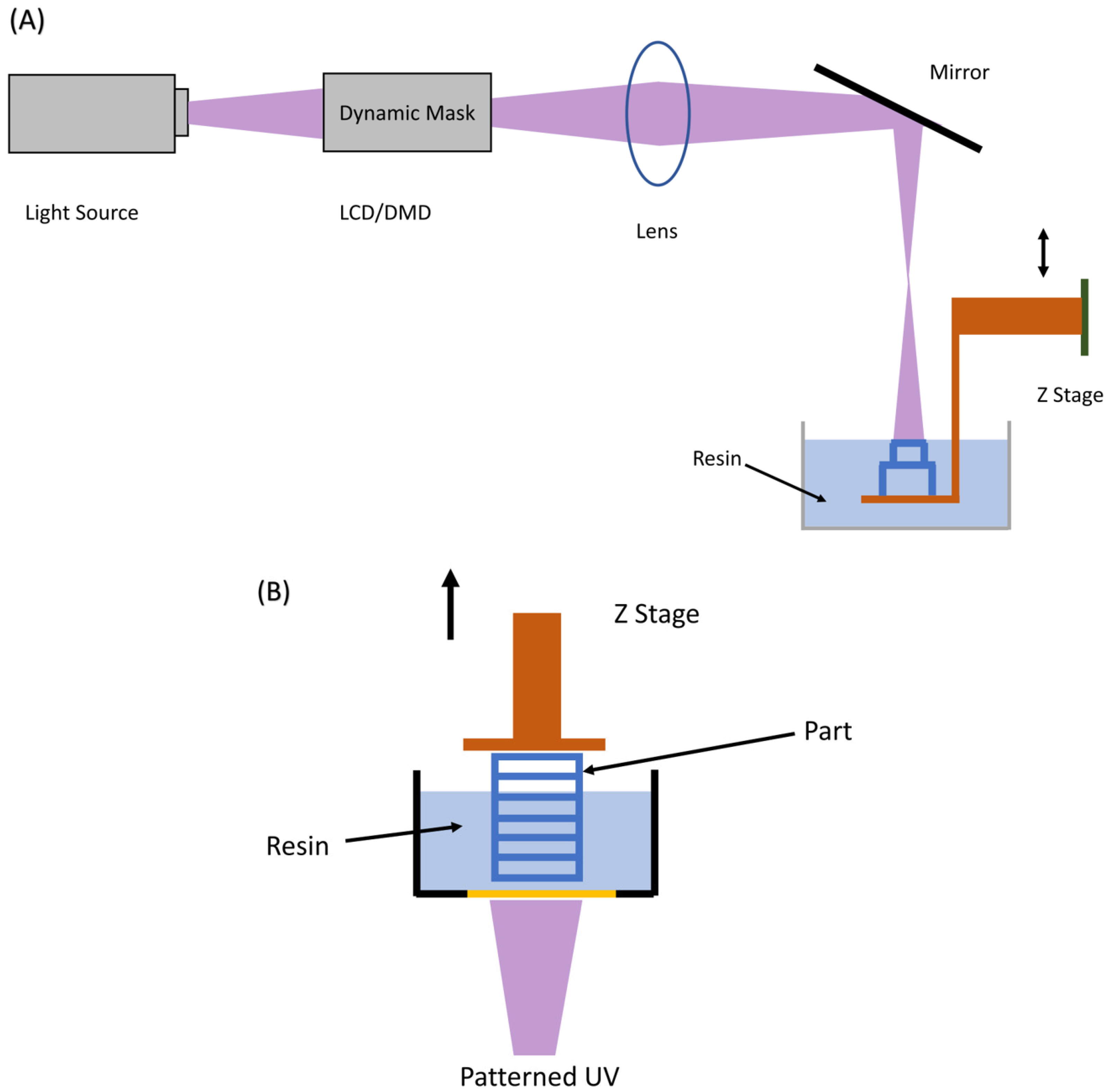
3. Principles of Desktop Inverted VP 3D Printing
3.1. Platform Motion Based
3.1.1. Top-Down Approach
3.1.2. Bottom-Up Approach
3.2. Laser Motion Based
3.3. Continuous Liquid Interface Production (CLIP)
4. Advantages of Desktop Inverted VP 3D Printing for Medicine
5. Medical Applications of Desktop Inverted VP 3D Printing
5.1. Anatomic Models
5.2. Surgical Guides and Surgical Planning
Accuracy of Surgical Guides
5.3. Prosthesis and Hearing Aids
5.4. Other Devices
6. Challenges of Desktop Inverted 3D Printing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitsouras, D.; Liacouras, P.; Imanzadeh, A.; Giannopoulos, A.A.; Cai, T.; Kumamaru, K.K.; George, E.; Wake, N.; Caterson, E.J.; Pomahac, B.; et al. Medical 3D Printing for the Radiologist. Radiographic 2015, 35, 1965–1988. [Google Scholar] [CrossRef] [PubMed]
- Mitsouras, D.; Liacouras, P.C.; Wake, N.; Rybicki, F.J. RadioGraphics Update: Medical 3D Printing for the Radiologist. Radiographics 2020, 40, E21–E23. [Google Scholar] [CrossRef] [PubMed]
- SME; MMI. Physicians as Manufacturers: The Rise of Point-of-care Manufacturing. Point Care Manuf. 2018, 20. [Google Scholar]
- United States Food and Drug Administration. Discussion Paper: 3D Printing Medical Devices at the Point of Care; United States Food and Drug Administration: Silver Spring, MA, USA, 2021.
- Ravi, P.; Burch, M.B.; Farahani, S.; Chepelev, L.L.; Yang, D.; Ali, A.; Joyce, J.R.; Lawera, N.; Stringer, J.; Morris, J.M.; et al. Utility and Costs During the Initial Year of 3D Printing in an Academic Hospital. J. Am. Coll. Radiol. 2022, 20, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.E.; Wake, N.; Chepelev, L.; Brantner, P.; Ryan, J.; Wang, K.C. A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print. Med. 2021, 7, 4–9. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Eshkalak, S.K.; Ghomi, E.R.; Dai, Y.; Choudhury, D.; Ramakrishna, S. The role of three-dimensional printing in healthcare and medicine. Mater. Des. 2020, 194, 108940. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications. Pharm. Res. 2018, 35, 1–22. [Google Scholar] [CrossRef]
- Anderson, J.; Wealleans, J.; Ray, J. Endodontic applications of 3D printing. Int. Endod. J. 2018, 51, 1005–1018. [Google Scholar] [CrossRef]
- Mills, D.K.; Tappa, K.; Jammalamadaka, U.; Mills, P.A.S.; Alexander, J.S.; Weisman, J.A. Medical Applications for 3D Printing. In Advances in Manufacturing and Processing of Materials and Structures; CRC Press: Boca Raton, FL, USA, 2018; pp. 163–186. [Google Scholar] [CrossRef]
- Dodziuk, H. Applications of 3D printing in healthcare. Pol. J. Cardio-Thoracic Surg. 2016, 3, 283–293. [Google Scholar] [CrossRef]
- Chae, M.P.; Rozen, W.M.; McMenamin, P.G.; Findlay, M.W.; Spychal, R.T.; Hunter-Smith, D.J. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front. Surg. 2015, 2, 25. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef]
- Rybicki, F.J. The impact of regulation, reimbursement, and research on the value of 3D printing and other 3D procedures in medicine. 3D Print. Med. 2022, 8, 6. [Google Scholar] [CrossRef]
- Bastawrous, S.; Wu, L.; Liacouras, P.C.; Levin, D.B.; Ahmed, M.T.; Strzelecki, B.; Amendola, M.F.; Lee, J.T.; Coburn, J.; Ripley, B. Establishing 3D Printing at the Point of Care: Basic Principles and Tools for Success. Radiographics 2022, 42, 451–468. [Google Scholar] [CrossRef]
- Chepelev, L.; Wake, N.; Ryan, J.; Althobaity, W.; Gupta, A.; Arribas, E.; Santiago, L.; Ballard, D.H.; Wang, K.C.; Weadock, W.; et al. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): Guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print. Med. 2018, 4, 11. [Google Scholar] [CrossRef]
- Winder, J.; Bibb, R. Medical Rapid Prototyping Technologies: State of the Art and Current Limitations for Application in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2005, 63, 1006–1015. [Google Scholar] [CrossRef]
- Matsumoto, J.S.; Morris, J.M.; Foley, T.A.; Williamson, E.E.; Leng, S.; McGee, K.P.; Kuhlmann, J.L.; Nesberg, L.E.; Vrtiska, T.J. Three-dimensional Physical Modeling: Applications and Experience at Mayo Clinic. RadioGraphics 2015, 35, 1989–2006. [Google Scholar] [CrossRef]
- Millar, W.S.; Klinghammer, O.; Durkin, M.S.; Tulipano, P.K.; Peitgen, H.-O.; Hahn, H.K. A Reliable and Efficient Method for Cerebral Ventricular Volumetry in Pediatric Neuroimaging. Methods Inf. Med. 2004, 43, 376–382. [Google Scholar] [CrossRef]
- Frakes, D.; Smith, M.; Parks, J.; Sharma, S.; Fogel, M.; Yoganathan, A. New Techniques for the Reconstruction of Complex Vascular Anatomies from MRI Images. J. Cardiovasc. Magn. Reson. 2005, 7, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Rane, R.; Mrinal, M.; Ganesan, V.; Taylor, R.; Jain, A. Characterization of the effect of in-process annealing using a novel print head assembly on the ultimate tensile strength & toughness of Fused Filament Fabrication (FFF) parts. Virtual Phys. Prototyp. 2022, 17, 989–1005. [Google Scholar] [CrossRef]
- Patel, P.T.; Ravi, P.; Shiakolas, P.S.; Welch, T.R.; Saini, T. Additive manufacturing of heterogeneous bio-resorbable constructs for soft tissue applications. In Materials Science and Technology 2018, MS and T 2018; Association for Iron and Steel Technology, AISTECH: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Patel, P. Additive Manufacturing Process Investigation for the Fabrication of Composite Scaffolds for Soft Tissue Application. 2018. Available online: https://www.proquest.com/docview/2314065319/previewPDF/8E22FE5010354BC3PQ/1?accountid=7117 (accessed on 4 March 2023).
- Shi, X.; Zhang, J.; Corrigan, N.A.; Boyer, C. Controlling mechanical properties of 3D printed polymer composites through photoinduced reversible addition–fragmentation chain transfer (RAFT) polymerization. Polym. Chem. 2021, 13, 44–57. [Google Scholar] [CrossRef]
- Zhu, Y.; Ramadani, E.; Egap, E. Thiol ligand capped quantum dot as an efficient and oxygen tolerance photoinitiator for aqueous phase radical polymerization and 3D printing under visible light. Polym. Chem. 2021, 12, 5106–5116. [Google Scholar] [CrossRef]
- Bastawrous, S.; Wu, L.; Strzelecki, B.; Levin, D.B.; Li, J.-S.; Coburn, J.; Ripley, B. Establishing Quality and Safety in Hospital-based 3D Printing Programs: Patient-first Approach. RadioGraphics 2021, 41, 1208–1229. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/ (accessed on 15 March 2023).
- Pruksakorn, D.; Chantarapanich, N.; Arpornchayanon, O.; Leerapun, T.; Sitthiseripratip, K.; Vatanapatimakul, N. Rapid-prototype endoprosthesis for palliative reconstruction of an upper extremity after resection of bone metastasis. Int. J. Comput. Assist. Radiol. Surg. 2014, 10, 343–350. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-dimensional Objects by Stereolithography. U.S. Patent Appl. No. 638905, 11 March 1984. [Google Scholar]
- Gillett, D.; Bashari, W.; Senanayake, R.; Marsden, D.; Koulouri, O.; MacFarlane, J.; van der Meulen, M.; Powlson, A.S.; Mendichovszky, I.A.; Cheow, H.; et al. Methods of 3D printing models of pituitary tumors. 3D Print. Med. 2021, 7, 24. [Google Scholar] [CrossRef]
- Ravi, P.; Chepelev, L.; Lawera, N.; Haque, K.M.A.; Chen, V.C.; Ali, A.; Rybicki, F.J. A systematic evaluation of medical 3D printing accuracy of multi-pathological anatomical models for surgical planning manufactured in elastic and rigid material using desktop inverted vat photopolymerization. Med. Phys. 2021, 48, 3223–3233. [Google Scholar] [CrossRef]
- Etemad-Shahidi, Y.; Qallandar, O.B.; Evenden, J.; Alifui-Segbaya, F.; Ahmed, K.E. Accuracy of 3-Dimensionally Printed Full-Arch Dental Models: A Systematic Review. J. Clin. Med. 2020, 9, 3357. [Google Scholar] [CrossRef]
- Sandrini, C.; Lombardi, C.; Shearn, A.I.U.; Ordonez, M.V.; Caputo, M.; Presti, F.; Luciani, G.B.; Rossetti, L.; Biglino, G. Three-Dimensional Printing of Fetal Models of Congenital Heart Disease Derived From Microfocus Computed Tomography: A Case Series. Front. Pediatr. 2020, 7, 567. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.T.; Stimec, B.V.; Edwin, B.; Kazaryan, A.M.; Maziarz, P.J.; Ignjatovic, D. Re-interpreting mesenteric vascular anatomy on 3D virtual and/or physical models: Positioning the middle colic artery bifurcation and its relevance to surgeons operating colon cancer. Surg. Endosc. 2021, 36, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.; Gupta, A.; Sun, Z. Clinical Value of Virtual Reality versus 3D Printing in Congenital Heart Disease. Biomolecules 2021, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Kellermann, S.; Faust, M.; Perrech, M.; Beutner, D.; Drzezga, A.; Zöller, J. Repair of Cerebrospinal Fluid Leakage Using a Transfrontal, Radial Adipofascial Flap: An Individual Approach Supported by Three-Dimensional Printing for Surgical Planning. World Neurosurg. 2018, 110, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Masalha, M.A.; VanKoevering, K.K.; Latif, O.S.; Powell, A.R.; Zhang, A.; Hod, K.H.; Prevedello, D.M.; Carrau, R.L. Simulation of Cerebrospinal Fluid Leak Repair Using a 3-Dimensional Printed Model. Am. J. Rhinol. Allergy 2021, 35, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.B.; Komáromy, A.M.; Sledge, D.G.; Smedley, R.; Coe, S.E.; Kim, S.Y. Transconjunctival excision of an orbital conjunctival cyst using computer-assisted 3-D surgical planning in a dog. Clin. Case Rep. 2021, 9, e04345. [Google Scholar] [CrossRef]
- Escobar, A.J.; Levi, D.S.; Van Arsdell, G.S.; Perens, G.S.; Mohan, U.R. Apical muscular ventricular septal defect closure via hybrid approach using a right ventricular stay suture. Catheter. Cardiovasc. Interv. 2020, 97, E514–E517. [Google Scholar] [CrossRef]
- Broeckx, C.-E.; Maal, T.J.; Vreeken, R.D.; Bos, R.R.; ter Laan, M. Single-Step Resection of an Intraosseous Meningioma and Cranial Reconstruction: Technical Note. World Neurosurg. 2017, 108, 225–229. [Google Scholar] [CrossRef]
- López, D.G.; Márquez, C.O.; Cicero, M.; Pereda, G.A.O. Use of cone beam computed tomography, a desktop 3D printer and freeware for manufacturing craniofacial bone prostheses: A pilot study. J. Oral Res. 2020, 9, 116–120. [Google Scholar] [CrossRef]
- Kitamura, G.; Albers, M.B.V.; Lesniak, B.P.; Rabuck, S.J.; Musahl, V.; Andrews, C.L.; Ghodadra, A.; Fu, F. 3-Dimensional Printed Models May Be a Useful Tool When Planning Revision Anterior Cruciate Ligament Reconstruction. Arthrosc. Sports Med. Rehabil. 2019, 1, e41–e46. [Google Scholar] [CrossRef]
- Sheu, A.Y.; Laidlaw, G.L.; Fell, J.C.; Triana, B.P.; Goettl, C.S.; Shah, R.P. Custom 3-Dimensional Printed Ultrasound-Compatible Vascular Access Models: Training Medical Students for Vascular Access. J. Vasc. Interv. Radiol. 2019, 30, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Nagassa, R.G.; McMenamin, P.G.; Adams, J.W.; Quayle, M.R.; Rosenfeld, J.V. Advanced 3D printed model of middle cerebral artery aneurysms for neurosurgery simulation. 3D Print. Med. 2019, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Coote, J.D.; Nguyen, T.; Tholen, K.; Stewart, C.; Verter, E.; McGee, J.; Celestre, P.; Sarkar, K. Three-Dimensional Printed Patient Models for Complex Pediatric Spinal Surgery. Ochsner J. 2019, 19, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Tserovski, S.; Georgieva, S.; Simeonov, R.; Bigdeli, A.; Röttinger, H.; Kinov, P. Advantages and disadvantages of 3D printing for pre-operative planning of revision hip surgery. J. Surg. Case Rep. 2019, 2019, rjz214. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Moharem-Elgamal, S.; Beckingham, R.; Hamilton, M.; Manghat, N.; Milano, E.G.; Bucciarelli-Ducci, C.; Caputo, M.; Biglino, G. Evaluating 3D-printed models of coronary anomalies: A survey among clinicians and researchers at a university hospital in the UK. BMJ Open 2019, 9, e025227. [Google Scholar] [CrossRef]
- Shearn, A.I.U.; Yeong, M.; Richard, M.; Ordoñez, M.V.; Pinchbeck, H.; Milano, E.G.; Hayes, A.; Caputo, M.; Biglino, G. Use of 3D Models in the Surgical Decision-Making Process in a Case of Double-Outlet Right Ventricle with Multiple Ventricular Septal Defects. Front. Pediatr. 2019, 7, 330. [Google Scholar] [CrossRef]
- Nawka, M.T.; Spallek, J.; Kuhl, J.; Krause, D.; Buhk, J.H.; Fiehler, J.; Frölich, A. Evaluation of a modular in vitro neurovascular procedure simulation for intracranial aneurysm embolization. J. NeuroInterv. Surg. 2019, 12, 214–219. [Google Scholar] [CrossRef]
- Hosny, A.; Shen, T.; Kuo, A.S.; Long, D.; Andrawes, M.N.; Dilley, J.D. Unlocking vendor-specific tags: Three-dimensional printing of echocardiographic data sets. J. Thorac. Cardiovasc. Surg. 2017, 155, 143–145.e1. [Google Scholar] [CrossRef]
- Ferrari, E.; Piazza, G.; Scoglio, M.; Berdajs, D.; Tozzi, P.; Maisano, F.; Von Segesser, L.K. Suitability of 3D-Printed Root Models for the Development of Transcatheter Aortic Root Repair Technologies. ASAIO J. 2019, 65, 874–881. [Google Scholar] [CrossRef]
- Zhao, K.; Kim, K.; Craig, J.; Palmer, J. Using 3D printed sinonasal models to visualize and optimize personalized sinonasal sinus irrigation strategies. Rhinology 2020, 58, 266–272. [Google Scholar] [CrossRef]
- Freiser, M.E.; Ghodadra, A.; McCall, A.A.; Shaffer, A.D.; Magnetta, M.; Jabbour, N. Operable, Low-Cost, High-Resolution, Patient-Specific 3D Printed Temporal Bones for Surgical Simulation and Evaluation. Ann. Otol. Rhinol. Laryngol. 2021, 130, 1044–1051. [Google Scholar] [CrossRef]
- Freiser, M.E.; Ghodadra, A.; Hart, L.; Griffith, C.; Jabbour, N. Safety of Drilling 3-Dimensional–Printed Temporal Bones. JAMA Otolaryngol. Neck Surg. 2018, 144, 797–801. [Google Scholar] [CrossRef]
- Ballard, D.H.; Trace, A.P.; Ali, S.; Hodgdon, T.; Zygmont, M.E.; DeBenedectis, C.M.; Smith, S.E.; Richardson, M.L.; Patel, M.J.; Decker, S.J.; et al. Clinical Applications of 3D Printing. Acad. Radiol. 2017, 25, 52–65. [Google Scholar] [CrossRef]
- Biglino, G.; Moharem-Elgamal, S.; Lee, M.; Tulloh, R.; Caputo, M. The Perception of a Three-Dimensional-Printed Heart Model from the Perspective of Different Stakeholders: A Complex Case of Truncus Arteriosus. Front. Pediatr. 2017, 5, 209. [Google Scholar] [CrossRef]
- Torres, I.; De Luccia, N. A simulator for training in endovascular aneurysm repair: The use of three dimensional printers. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 247–253. [Google Scholar] [CrossRef]
- Bastawrous, S. Utility and Costs Benchmarked in a New 3D Printing Service—Optimizing the Path Forward. J. Am. Coll. Radiol. 2022, 20, 205–206. [Google Scholar] [CrossRef]
- Vakharia, V.N.; Smith, L.; Tahir, Z.; Sparks, R.; Ourselin, S.; Tucker, S.; Thompson, D. Occipitocervical instrumented fixation utilising patient-specific C2 3D-printed spinal screw trajectory guides in complex paediatric skeletal dysplasia. Child’s Nerv. Syst. 2021, 37, 2643–2650. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, L.; Tan, M.; Yang, F.; Yi, P.; Tang, X. Accuracy assessment of atlantoaxial pedicle screws assisted by a novel drill guide template. Arch. Orthop. Trauma Surg. 2016, 136, 1483–1490. [Google Scholar] [CrossRef]
- Goldsmith, I.; Evans, P.L.; Goodrum, H.; Warbrick-Smith, J.; Bragg, T. Chest wall reconstruction with an anatomically designed 3-D printed titanium ribs and hemi-sternum implant. 3D Print. Med. 2020, 6, 26. [Google Scholar] [CrossRef]
- Weadock, W.J.; Heisel, C.J.; Kahana, A.; Kim, J. Use of 3D Printed Models to Create Molds for Shaping Implants for Surgical Repair of Orbital Fractures. Acad. Radiol. 2019, 27, 536–542. [Google Scholar] [CrossRef]
- Morales-Gómez, J.A.; Garcia-Estrada, E.; Leos-Bortoni, J.E.; Delgado-Brito, M.; Flores-Huerta, L.E.; De La Cruz-Arriaga, A.A.; Torres-Díaz, L.J.; de León, R.M.-P. Cranioplasty with a low-cost customized polymethylmethacrylate implant using a desktop 3D printer. J. Neurosurg. 2019, 130, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-S.; Chen, C.-F.; Luo, C.-A.; Chang, M.-C.; Lee, O.K.; Huang, Y.; Lin, S.-C. Clinical Experience Using a 3D-Printed Patient-Specific Instrument for Medial Opening Wedge High Tibial Osteotomy. BioMed Res. Int. 2018, 2018, 9246529. [Google Scholar] [CrossRef] [PubMed]
- Jalbert, F.; Boetto, S.; Nadon, F.; Lauwers, F.; Schmidt, E.; Lopez, R. One-step primary reconstruction for complex craniofacial resection with PEEK custom-made implants. J. Cranio-Maxillofac. Surg. 2014, 42, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Inoue, R.; Tsuchida, R.; Azuma, K.; Ino, K.; Konishi, M.; Hozumi, J.; Sumitani, M. Use of three-dimensional printing of a lumbar skeletal model for intrathecal administration of nusinersen: A brief technical report. Reg. Anesth. Pain Med. 2020, 45, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Sharkh, H.A.; Makhoul, N. In-House Surgeon-Led Virtual Surgical Planning for Maxillofacial Reconstruction. J. Oral Maxillofac. Surg. 2020, 78, 651–660. [Google Scholar] [CrossRef]
- Cabarcas, B.C.; Cvetanovich, G.L.; Orías, A.A.E.; Inoue, N.; Gowd, A.K.; Bernardoni, E.; Verma, N.N. Novel 3-dimensionally printed patient-specific guide improves accuracy compared with standard total shoulder arthroplasty guide: A cadaveric study. JSES Open Access 2019, 3, 83–92. [Google Scholar] [CrossRef]
- Lin, W.-S.; Harris, B.T.; Pellerito, J.; Morton, D. Fabrication of an interim complete removable dental prosthesis with an in-office digital light processing three-dimensional printer: A proof-of-concept technique. J. Prosthet. Dent. 2018, 120, 331–334. [Google Scholar] [CrossRef]
- Tredway, H.; Pasumarti, N.; Crystal, M.A.; Farooqi, K.M. 3D printing applications for percutaneous structural interventions in congenital heart disease. Mini-Invasive Surg. 2020, 4, 78. [Google Scholar] [CrossRef]
- Kessler, A.; Reichl, F.-X.; Folwaczny, M.; Högg, C. Monomer release from surgical guide resins manufactured with different 3D printing devices. Dent. Mater. 2020, 36, 1486–1492. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Biodegradable Polymers for Biomedical Additive Manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Roytman, G.R.; Ramji, A.F.; Beitler, B.; Yoo, B.; Leslie, M.P.; Baumgaertner, M.; Tommasini, S.; Wiznia, D.H. Accuracy of guide wire placement for femoral neck stabilization using 3D printed drill guides. 3D Print. Med. 2022, 8, 19. [Google Scholar] [CrossRef]
- Freiser, M.E.; Ghodadra, A.; Hirsch, B.E.; McCall, A.A. Evaluation of 3D Printed Temporal Bone Models in Preparation for Middle Cranial Fossa Surgery. Otol. Neurotol. 2019, 40, 246–253. [Google Scholar] [CrossRef]
- Panesar, S.S.; Abhinav, K.; Magnetta, M.; Mukherjee, D.R.; Gardner, P.; Fernandez-Miranda, J.C. Patient-Specific Three-Dimensionally Printed Models for Presurgical Planning, Training, and Patient Education in Skull Base Surgery. Neurosurg. Focus. 2019, 80, P060. [Google Scholar] [CrossRef]
- Salewski, C.; Spintzyk, S.; von Steuben, T.; Boburg, R.S.; Nemeth, A.; Schille, C.; Acharya, M.; Geis-Gerstorfer, J.; Wendel, H.-P.; Popov, A.-F.; et al. ECMO implantation training: Needle penetration in 3D printable materials and porcine aorta. Perfusion 2020, 36, 798–802. [Google Scholar] [CrossRef]
- Giannopoulos, A.A.; Steigner, M.L.; George, E.; Barile, M.; Hunsaker, A.R.; Rybicki, F.J.; Mitsouras, D. Cardiothoracic Applications of 3D Printing. J. Thorac. Imaging 2016, 31, 253. [Google Scholar] [CrossRef]
- Deeb, G.R.; Allen, R.K.; Hall, V.P.; Whitley, D.; Laskin, D.M.; Bencharit, S. How Accurate Are Implant Surgical Guides Produced with Desktop Stereolithographic 3-Dimentional Printers? J. Oral Maxillofac. Surg. 2017, 75, 2559.e1–2559.e8. [Google Scholar] [CrossRef]
- Altieri, F.; Iezzi, G.; Luzzi, V.; Di Giorgio, G.; Polimeni, A.; Cassetta, M. Computer-Guided Bone Biopsy: A Technical Note with the Description of a Clinical Case. Bioengineering 2021, 8, 214. [Google Scholar] [CrossRef]
- Park, G.-S.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y.; Seo, D.-G. Effects of Printing Parameters on the Fit of Implant-Supported 3D Printing Resin Prosthetics. Materials 2019, 12, 2533. [Google Scholar] [CrossRef]
- Hamilton-Bennett, S.E.; Oxley, B.; Behr, S. Accuracy of a patient-specific 3D printed drill guide for placement of cervical transpedicular screws. Veter Surg. 2017, 47, 236–242. [Google Scholar] [CrossRef]
- Marei, H.F.; Alshaia, A.; Alarifi, S.; Almasoud, N.; Abdelhady, A. Effect of Steam Heat Sterilization on the Accuracy of 3D Printed Surgical Guides. Implant. Dent. 2019, 28, 372–377. [Google Scholar] [CrossRef]
- Keßler, A.; Dosch, M.; Reymus, M.; Folwaczny, M. Influence of 3D-printing method, resin material, and sterilization on the accuracy of virtually designed surgical implant guides. J. Prosthet. Dent. 2021, 128, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ozan, O.; Turkyilmaz, I.; Ersoy, A.E.; McGlumphy, E.A.; Rosenstiel, S.F. Clinical Accuracy of 3 Different Types of Computed Tomography-Derived Stereolithographic Surgical Guides in Implant Placement. J. Oral Maxillofac. Surg. 2009, 67, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Aquino, E. Dimensional Accuracy of Tooth-Supported Implant Surgical Guides Printed at Different Arch and Angle Positions in a Desktop SLA 3-Dimensional Printer. Ph.D. Thesis, The University of Texas School of Dentistry at Houston, Houston, TX, USA, 2020. [Google Scholar]
- Rubayo, D.D.; Phasuk, K.; Vickery, J.M.; Morton, D.; Lin, W.-S. Influences of build angle on the accuracy, printing time, and material consumption of additively manufactured surgical templates. J. Prosthet. Dent. 2020, 126, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Philippe, B. Accuracy of position of cutting and drilling guide for sagittal split guided surgery: A proof of concept study. Br. J. Oral Maxillofac. Surg. 2020, 58, 940–946. [Google Scholar] [CrossRef]
- Juneja, M.; Thakur, N.; Kumar, D.; Gupta, A.; Bajwa, B.; Jindal, P. Accuracy in dental surgical guide fabrication using different 3-D printing techniques. Addit. Manuf. 2018, 22, 243–255. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.; Kim, G.B.; Hong, D.; Kwon, J.; Park, J.-W.; Kim, N. Accuracy of a simplified 3D-printed implant surgical guide. J. Prosthet. Dent. 2019, 124, 195–201.e2. [Google Scholar] [CrossRef]
- Revilla-León, M.; Sadeghpour, M.; Özcan, M. An update on applications of 3D printing technologies used for processing polymers used in implant dentistry. Odontology 2019, 108, 331–338. [Google Scholar] [CrossRef]
- L’Alzit, F.R.; Cade, R.; Naveau, A.; Babilotte, J.; Meglioli, M.; Catros, S. Accuracy of commercial 3D printers for the fabrication of surgical guides in dental implantology. J. Dent. 2022, 117, 103909. [Google Scholar] [CrossRef]
- Molnar, I.; Michal, D.; Simon, S.; Morovic, L.; Kostal, P. Design and manufacture of life size human model using material extrusion and vat photopolymerization additive processes. MATEC Web Conf. 2019, 299, 01010. [Google Scholar] [CrossRef]
- Vivero-Lopez, M.; Xu, X.; Muras, A.; Otero, A.; Concheiro, A.; Gaisford, S.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Anti-biofilm multi drug-loaded 3D printed hearing aids. Mater. Sci. Eng. C 2020, 119, 111606. [Google Scholar] [CrossRef]
- Hirsch, J.D.; Vincent, R.L.; Eisenman, D.J. Surgical reconstruction of the ossicular chain with custom 3D printed ossicular prosthesis. 3D Print. Med. 2017, 3, 4–11. [Google Scholar] [CrossRef]
- Sandström, C.G. The non-disruptive emergence of an ecosystem for 3D Printing—Insights from the hearing aid industry’s transition 1989–2008. Technol. Forecast. Soc. Chang. 2016, 102, 160–168. [Google Scholar] [CrossRef]
- Sandström, C. Adopting 3D Printing for manufacturing—The case of the hearing aid industry. Ratio Work. Pap. 2015, 262, 20. [Google Scholar]
- Jin, Y.-A.; Plott, J.; Chen, R.; Wensman, J.; Shih, A. Additive Manufacturing of Custom Orthoses and Prostheses—A Review. Procedia CIRP 2015, 36, 199–204. [Google Scholar] [CrossRef]
- Capobussi, M.; Moja, L. An open-access and inexpensive 3D printed otoscope for low-resource settings and health crises. 3D Print. Med. 2021, 7, 36. [Google Scholar] [CrossRef]
- Yilmaz, B.; Kara, B.Y. Mathematical surface function-based design and 3D printing of airway stents. 3D Print. Med. 2022, 8, 24. [Google Scholar] [CrossRef]
- Lin, H.; Shi, L.; Wang, D. A rapid and intelligent designing technique for patient-specific and 3D-printed orthopedic cast. 3D Print. Med. 2016, 2, 4. [Google Scholar] [CrossRef]
- Pham, Y.L.; Beauchamp, J.; Clement, A.; Wiegandt, F.; Holz, O. 3D-printed mouthpiece adapter for sampling exhaled breath in medical applications. 3D Print. Med. 2022, 8, 27. [Google Scholar] [CrossRef]
- Ford, J.; Goldstein, T.; Trahan, S.; Neuwirth, A.; Tatoris, K.; Decker, S. A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print. Med. 2020, 6, 21. [Google Scholar] [CrossRef]
- Xun, H.; Shallal, C.; Unger, J.; Tao, R.; Torres, A.; Vladimirov, M.; Frye, J.; Singhala, M.; Horne, B.; Kim, B.S.; et al. Translational design for limited resource settings as demonstrated by Vent-Lock, a 3D-printed ventilator multiplexer. 3D Print. Med. 2022, 8, 29. [Google Scholar] [CrossRef]
- Duke, D.J.; Clarke, A.L.; Stephens, A.L.; Djumas, L.; Gregory, S.D. A computational fluid dynamics assessment of 3D printed ventilator splitters and restrictors for differential multi-patient ventilation. 3D Print. Med. 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Kumat, S.S.; Shiakolas, P.S. Design, inverted vat photopolymerization 3D printing, and initial characterization of a miniature force sensor for localized in vivo tissue measurements. 3D Print. Med. 2022, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Adejokun, S.A.; Kumat, S.S.; Shiakolas, P.S. A Microrobot with an Attached Microforce Sensor for Transurethral Access to the Bladder Interior Wall. J. Eng. Sci. Med. Diagn. Ther. 2023, 6, 031001. [Google Scholar] [CrossRef]
- Adejokun, S.; Kumat, S.; Shiakolas, P.S. A Microrobot with an Attached Micro-Force Sensor for Natural Orifice Access to the Bladder Interior Wall. In ASME International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Rischall, M.A.; Alexander, A.E.; Dickens, H.J.; Lanzino, G.; Morris, J.M. Intracranial vasculature 3D printing: Review of techniques and manufacturing processes to inform clinical practice. 3D Print. Med. 2020, 6, 18. [Google Scholar] [CrossRef]
- Sharma, P.K.; Choudhury, D.; Yadav, V.; Murty, U.S.N.; Banerjee, S. 3D printing of nanocomposite pills through desktop vat photopolymerization (stereolithography) for drug delivery reasons. 3D Print. Med. 2022, 8, 3. [Google Scholar] [CrossRef]
- Wilke, C.T.; Zaid, M.; Chung, C.; Fuller, C.D.; Mohamed, A.S.R.; Skinner, H.; Phan, J.; Gunn, G.B.; Morrison, W.H.; Garden, A.S.; et al. Design and fabrication of a 3D–printed oral stent for head and neck radiotherapy from routine diagnostic imaging. 3D Print. Med. 2017, 3, 12. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; Xu, X.; Seijo-Rabina, A.; Ong, J.J.; Alvarez-Lorenzo, C.; Rial, C.; Nieto, D.; Gaisford, S.; Basit, A.W.; Goyanes, A. Volumetric 3D printing for rapid production of medicines. Addit. Manuf. 2022, 52, 102673. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, e1904209. [Google Scholar] [CrossRef]
- Oldhoff, M.; Mirzaali, M.; Tümer, N.; Zhou, J.; Zadpoor, A. Comparison in clinical performance of surgical guides for mandibular surgery and temporomandibular joint implants fabricated by additive manufacturing techniques. J. Mech. Behav. Biomed. Mater. 2021, 119, 104512. [Google Scholar] [CrossRef]
- Sarabi, M.R.; Bediz, B.; Falo, L.D.; Korkmaz, E.; Tasoglu, S. 3D printing of microneedle arrays: Challenges towards clinical translation. J. 3D Print. Med. 2021, 5, 65–70. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2020, 329, 743–757. [Google Scholar] [CrossRef]
- Bertolini, M.; Rossoni, M.; Colombo, G. Operative Workflow from CT to 3D Printing of the Heart: Opportunities and Challenges. Bioengineering 2021, 8, 130. [Google Scholar] [CrossRef]
- Abudayyeh, I.; Gordon, B.; Ansari, M.M.; Jutzy, K.; Stoletniy, L.; Hilliard, A. A practical guide to cardiovascular 3D printing in clinical practice: Overview and examples. J. Interv. Cardiol. 2017, 31, 375–383. [Google Scholar] [CrossRef]




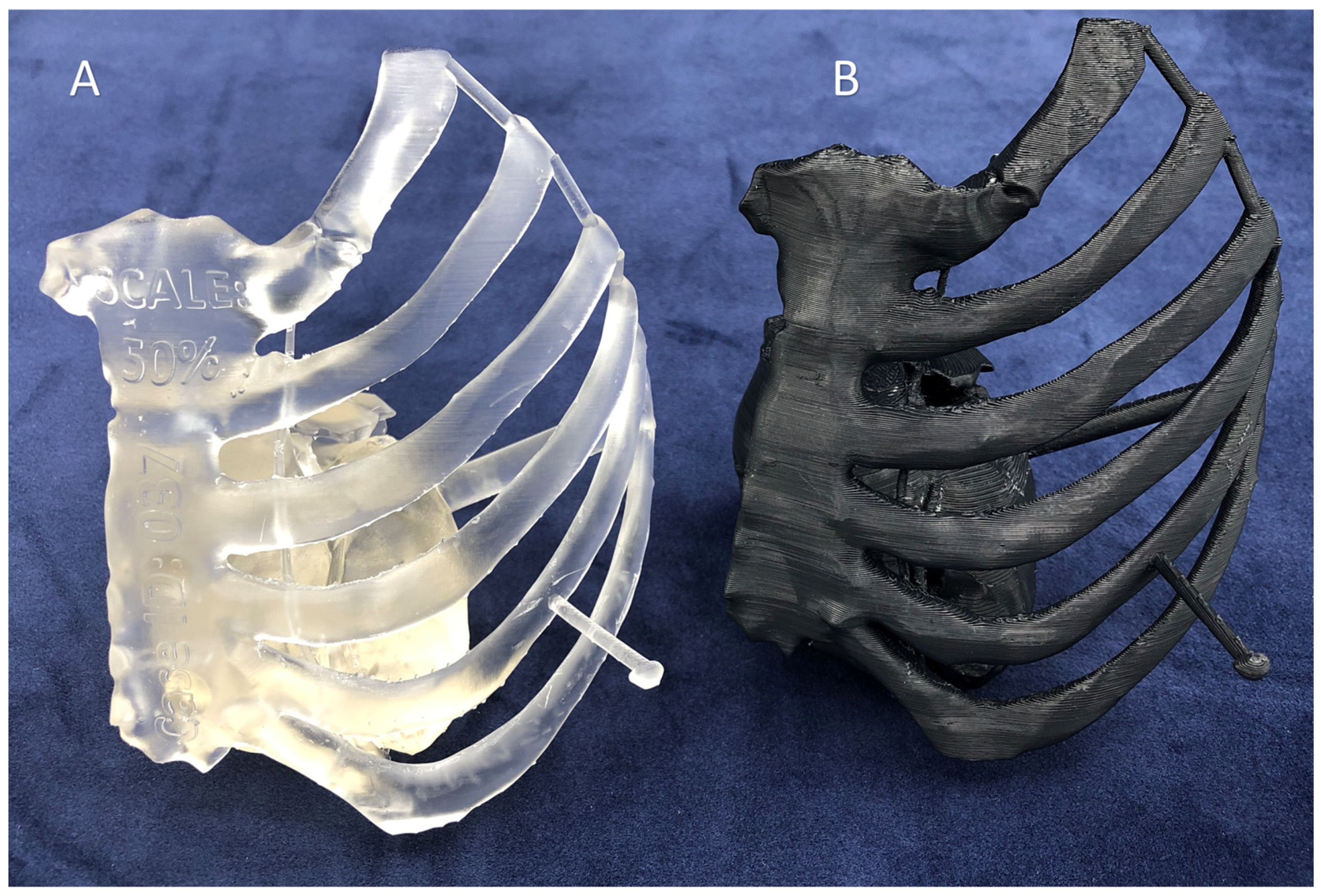

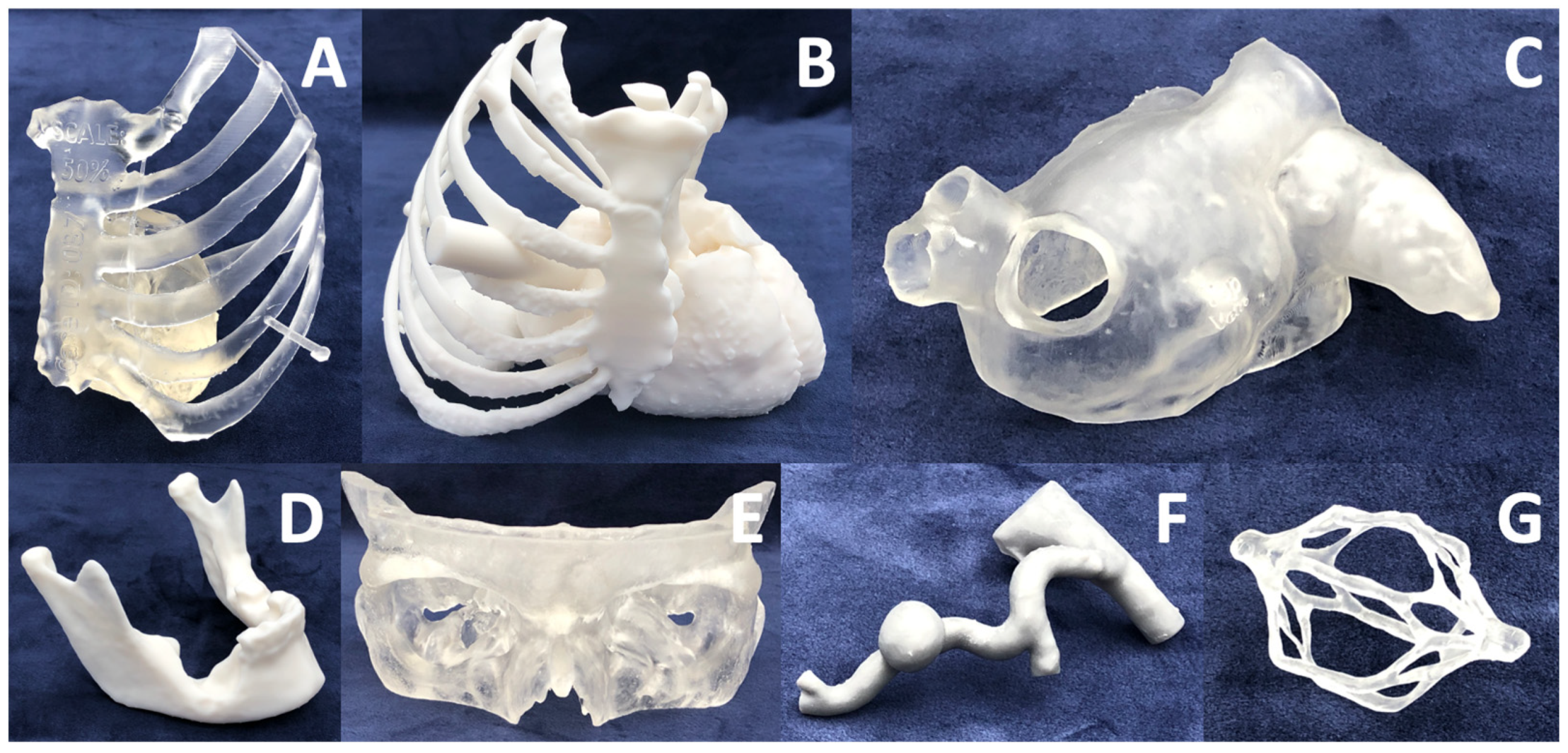
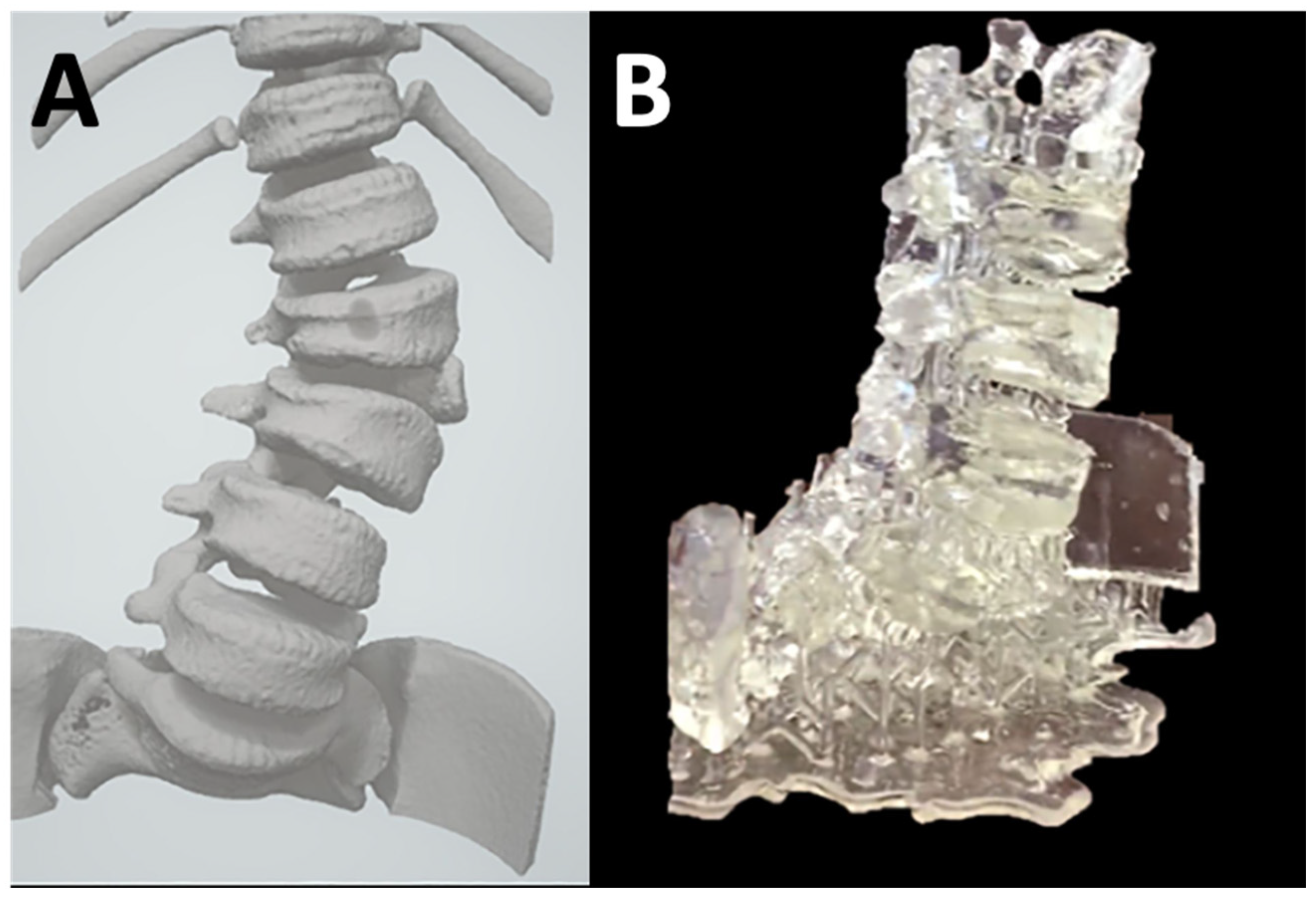


| Characteristic | Inverted VP | MJT | PBF | MEX | BJT |
|---|---|---|---|---|---|
| Surface Quality | High | High | Medium | Low | Low |
| Accuracy | High | High | High | Low | Low |
| Geometric Complexity | Medium | Medium | High | Low | High |
| Cost | Low | High | High | Low | High |
| Material Versatility | High | Low | Low | Medium | Low |
| Surgical Guide/Anatomical Area/Description | 3D Printer and Resin | Accuracy Results | Ref. |
|---|---|---|---|
| Measure distance and angular deviations in faciolingual and mesiodistal locations of the implants | Form 2; Dental SG Resin | Placed and planned implants in mesiodistal location had a mean difference of 0.28mm while the faciolingual direction had 0.49mm, and the angulation deviations were 0.84° and 3.37°. | [82] |
| Effect of process parameters on the internal gap and marginal fit of model for dental implants | DLP printer D2-120; Polymethyl methacrylate (PMMA) resin | 45° and 60° build orientations gave clinically acceptable models in line with milling and cast restoration processes while the layer height of 100 µm and 50 µm had similar marginal fit. | [83] |
| Compared milled surgical guides with 3D printed guides or printing device, resin material, and preoperative sterilization | Rapidshape D20II and Form2; NextDent SG resin | The location of the implant was influenced by both the type of printer used and the resin material at both the crest and apex. Despite these variables affecting implant position, the 3D printed guides were comparable to the milled ones and the displacement of the printed implants fell within an acceptable range for safety. | [87] |
| A drill guide for posterior atlantoaxial pedicle screw fixation | Form 1+; Acrylic resin | No difference between the actual and planned trajectories of axis and atlas of those screws | [64] |
| Surgical guides for cervical pedicle (CPS) screws in breed dogs | Form 2; Dental SG resin | Results showed 29 of 32 CPS were placed without any vertebral canal breach | [85] |
| Effect of sterilization on the stability and the accuracy of surgical guides | Form 2 and Simplant; Poly methyl methacrylate (PMMA) resin | No significant difference between pre-sterilized and post sterilized guides | [86] |
| Tooth, bone, and mucosa-supported surgical guides for linear and angular deviation | Custom VP machine; Stereocol resin | Surgical guides supported by tooth were more accurate as compared to other two (bone and mucosa) based on angular deviation of 2.91° ± 1.3°, 4.63° ± 2.6°, and 4.51° ± 2.1° (tooth, bone, and mucosa). | [88] |
| Evaluating accuracy of tooth supported SGs which were printed for various angle and arch. | Form 2; Dental SG | The build orientation angle had no significant effect on the guides | [89] |
| Impact of build angle on the material usage, accuracy, and speed | Form 2; Dental SG | The accuracy of the surgical template varied depending on the build angle, with 0-degree and 45-degree angles producing the most accurate templates while 90-degree angles produced the least accurate. The 0-degree build angle had the fastest printing speed, while the speed decreased with an increase in build angle, with 90-degree angles taking the longest time. However, the increased speed was at the expense of using more material, with the 0-degree angle using the most material and the 90-degree angle using the least amount of material. | [90] |
| Analyzed the precision of guides by the inserting reference screws mandibular models | 3D Systems Viper; DSM Somos’ RX opaque resin | Reference screws can be positioned accurately using guides during guided bilateral sagittal split osteotomy | [91] |
| Human Anatomy or Application | Specific Applications | 3D Printer and Resin | Title 4 Technology (SLA/DLP/CLIP) | Ref. |
|---|---|---|---|---|
| Spine | 3D printed guide for spinal screw on C2 vertebra during operation | Form 3B, Surgical Guide resin | SLA | [63] |
| A patient-specific model of congenital scoliosis secondary to an L3 hemivertebra (spine) was created for surgical planning | Form 2, Clear resin | SLA | [49] | |
| A drill guide for atlantoaxial pedicle screw positioning | Form1+, Acrylate resin (Somos 14120) | SLA | [64] | |
| Skull | 3D model was printed to understand the leakage of cerebrospinal fluid and surgical planning | Form 2, Grey resin | SLA | [40] |
| A mold was 3D printed to give shape to the implant for orbital blow-out fracture | Form 2, Yellow resin | SLA | [66] | |
| Printed a mold using VP which was later used to create PMMA implant for cranioplasty | Form 2, Unknown | SLA | [67] | |
| Patient-specific models were printed to improve resident training and patient education for delicate carinal nerve structures | Form 2, Acrylic resin | SLA | [79] | |
| Anatomical model for resection of a tumor and cranioplasty | Form 2, Flexible resin | SLA | [44] | |
| Cranial bone prosthesis was 3D printed for feasibility study | Form 2, Gray v4 resin | SLA | [45] | |
| 3D printed models of the middle cerebral artery aneurysms for creating wax casts | Form 2, White resin | SLA | [48] | |
| Patient-specific temporal bones were 3D printed | Form 2, White resin | SLA | [57] | |
| Colon | 3D printed model helped identify the bifurcation position for colic artery for colon cancer procedure | Form 1+ , White resin | SLA | [38] |
| Heart | Models helped with surgical planning in Multiple Ventricular Septal Defects and their relationship with aortas | Form 2, White resin | SLA | [52] |
| Transcatheter aortic root repairs model was developed to replicate the coronary flow | Visijet M3, Crystal resin; Form 2, Flexible resin; Heart print, Clear resin | SLA, DLP | [55] | |
| 3D printed heart models helped to enhance the understanding of coronary abnormalities. | Form 2, White resin | SLA | [51] | |
| Virtual Reality model was compared with VP model for congenital heart disease | Form 2, Flexible resin | SLA | [39] | |
| Model printed in planning of an apical muscular ventricular septal defect closure | Form 3, Flexible resin | SLA | [43] | |
| Models of an aortic valve, left atrial appendage, and normal/diseased mitral valve were printed | Form 2, Unknown | SLA | [54] | |
| Airway | 3D printing of patient-specific airway stents | Anycubic Photon Mono; Soybean-based biodegradable photopolymer resin | SLA | [103] |
| Aorta | Low-cost models for training endovascular aneurysm repair | Form 1+, Flexible resin | SLA | [61] |
| An aortic root model for training simulation for transcatheter aortic valve replacement | Form 2, Grey resin | SLA | [59] | |
| Hip | A model of the femoral head and acetabulum for revision hip surgery | Form 2, Unknown | SLA | [50] |
| Femoral neck stabilization surgery | Form 3 using Grey V4 resin | SLA | [77] | |
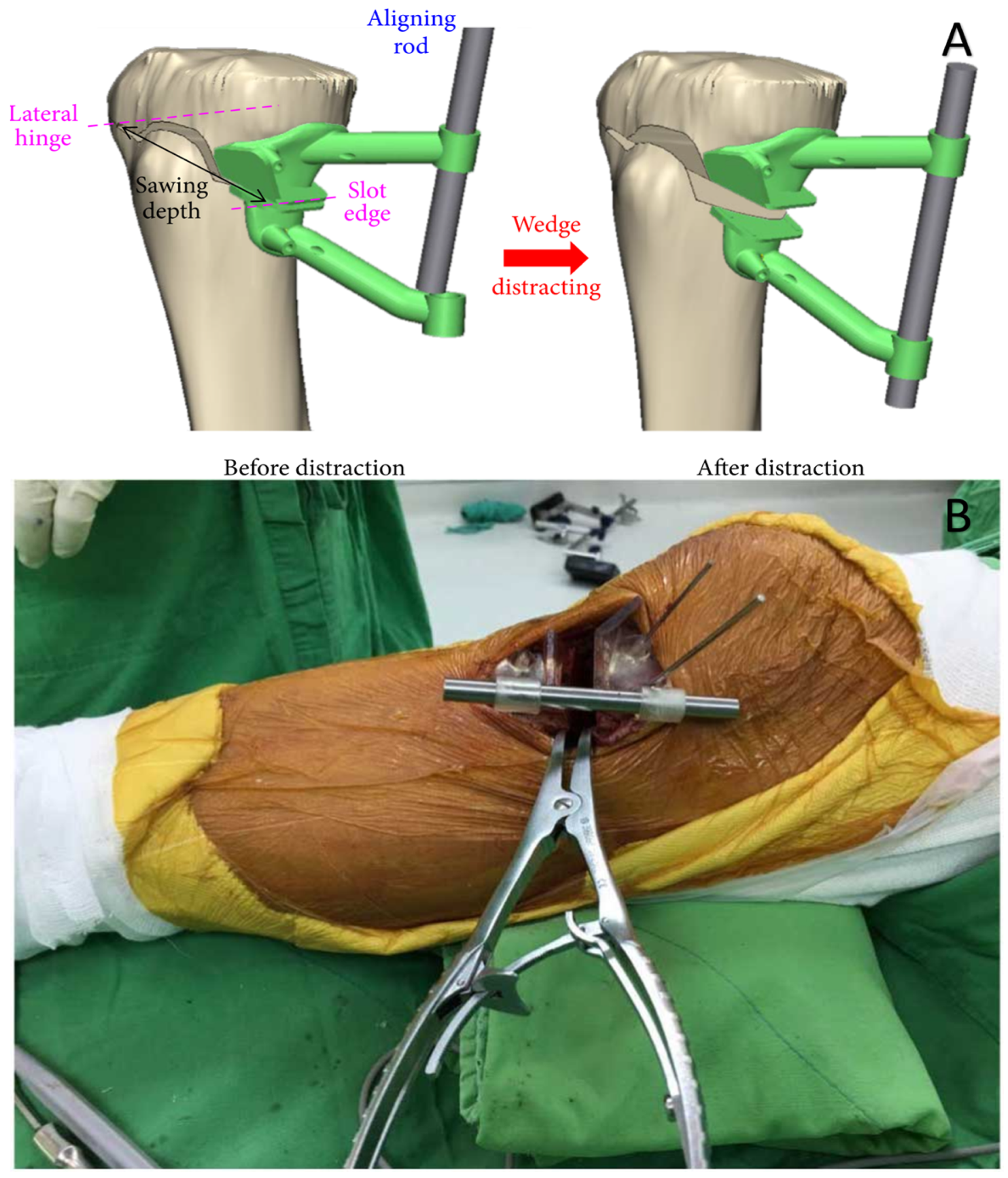
| Knee and femur | A patient-specific intraoperative guide was 3D printed for precise creation and distraction of high tibial osteotomy wedge | Form 2, Dental SG | SLA | [68] |
| Physicians and surgeons evaluated whether the current femoral, as well as tibial tunnles, were adequate in revision anterior cruciate ligament reconstruction with and without the 3D models | Form 2, Acrylic resin | SLA | [46] | |
| 3D printed the anatomy comprising the femoral artery, vein, and pelvis for training medical students | Form 2, Grey resin | SLA | [47] | |
| Maxillofacial | When combined with virtual surgical planning, surgical guides for maxillofacial reconstruction improved the accuracy of bony reconstruction. | Form 2, Dental SG | SLA | [71] |
| Middle cranial fossa filled with an internal auditory canal was printed to simulate realistic drilling process. | Form 2, white acrylic resin | SLA | [78] | |
| Tooth, bone, and mucosa-supported SG enhanced the accuracy of implants | Custom VP machine; Stereocol resin | DLP | [88] | |
| VP guides used to precisely insert screws on mandibular models | 3D Systems Viper; RX opaque resin | SLA | [91] | |
| Teeth | Tooth-relying VP guides improved the accuracy of implants. | Form 2; Dental SG Resin | SLA | [82] |
| Nose | Fabricated models with internal nasal anatomy to plan the repair/simulate a cerebrospinal fluid leak | Form 2, Grey acrylic resin | SLA | [40,41] |
| 3D print patient-specific nasal replicas for personalized irrigation strategies | Form 2, Acrylic resin | SLA | [56] | |
| 3D printing of Nasal Swabs for COVID testing | Form 2 and Form 3B, standard FDA-approved resin | SLA | [106] | |
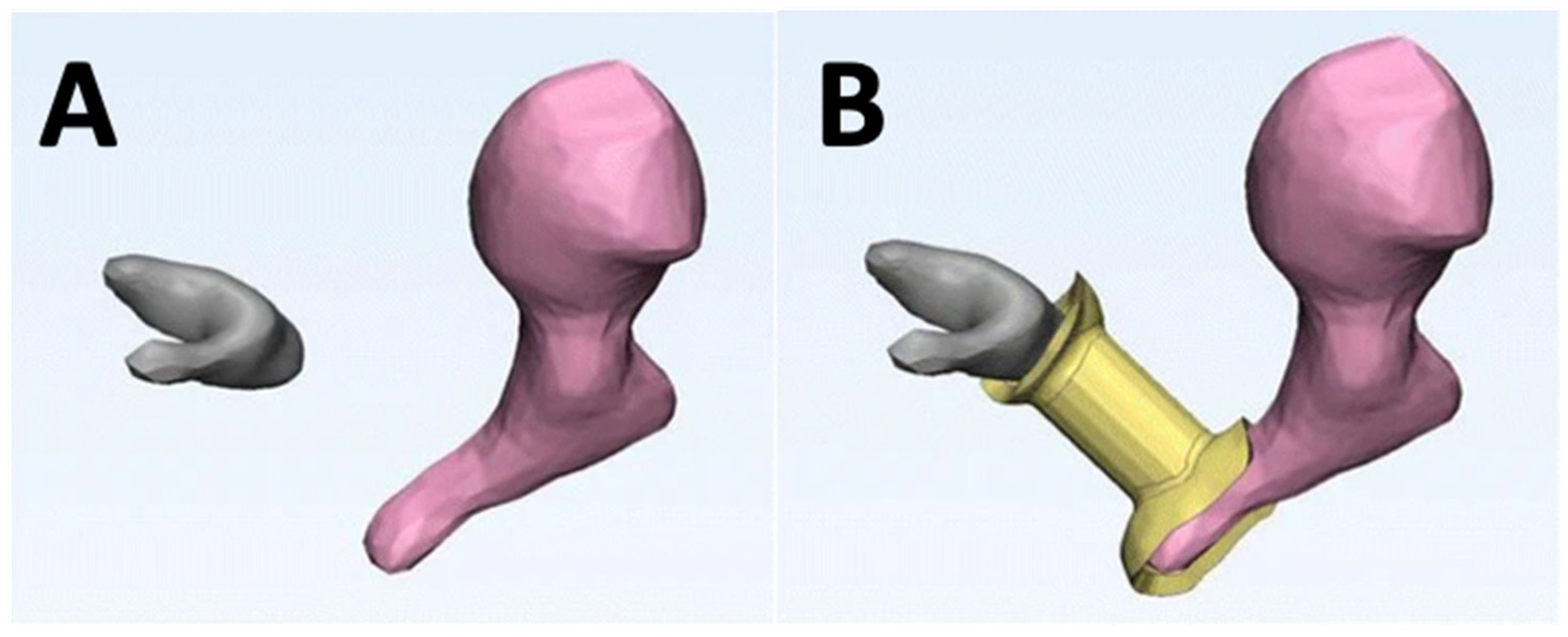
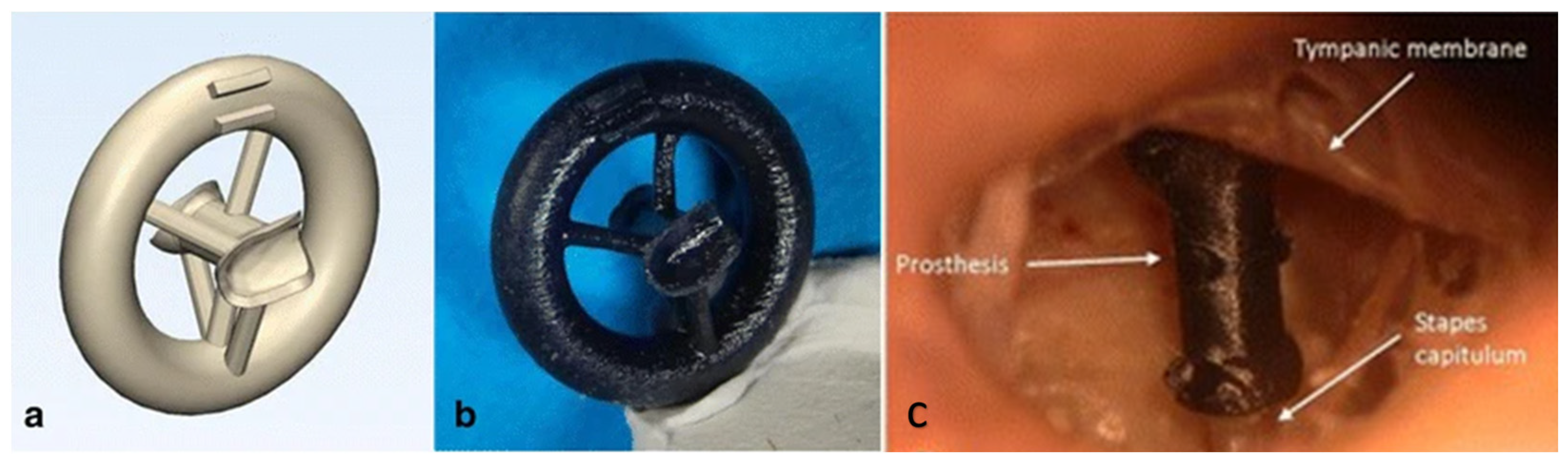
| Ear | Printed prosthesis incuses of temporal bone to identify if VP can produce unique shapes to reconfigure ossicular continuity | Form 2, Black resin | SLA | [98] |
| Drug-loaded hearing aid | Kudo3D Titan 2 HR 3D printer, Kudo3D 3DSR Flexible resin and hard resin mixed with drug | DLP | [97] | |
| Otoscope (a medical device used to examine the ear canal and eardrum) | Prusa i3 Pro-B with ABS Filament Mars Pro with Elegoo transparent resin | MEX/SLA | [102] | |
| Pituitary gland | 3D Models of pituitary tumors for surgical planning | EOS100 with Nylon PA2200, Stratasys J750, Prusa Research (Prague, Czech Republic) MK3 with PETG filaments, Prusa Research SL1 with transparent Resin | PBF/MJ/ MEX/VP | [34] |
| Mouth | Mouthpiece adapter to sample breath | Formlabs Form 3B, with Surgical Guide, Tough v5, and BioMed Clear resins | SLA | [105] |
| Customized oral stent for head and neck radiotherapy | Form 2, standard clear resin | SLA | [114] | |
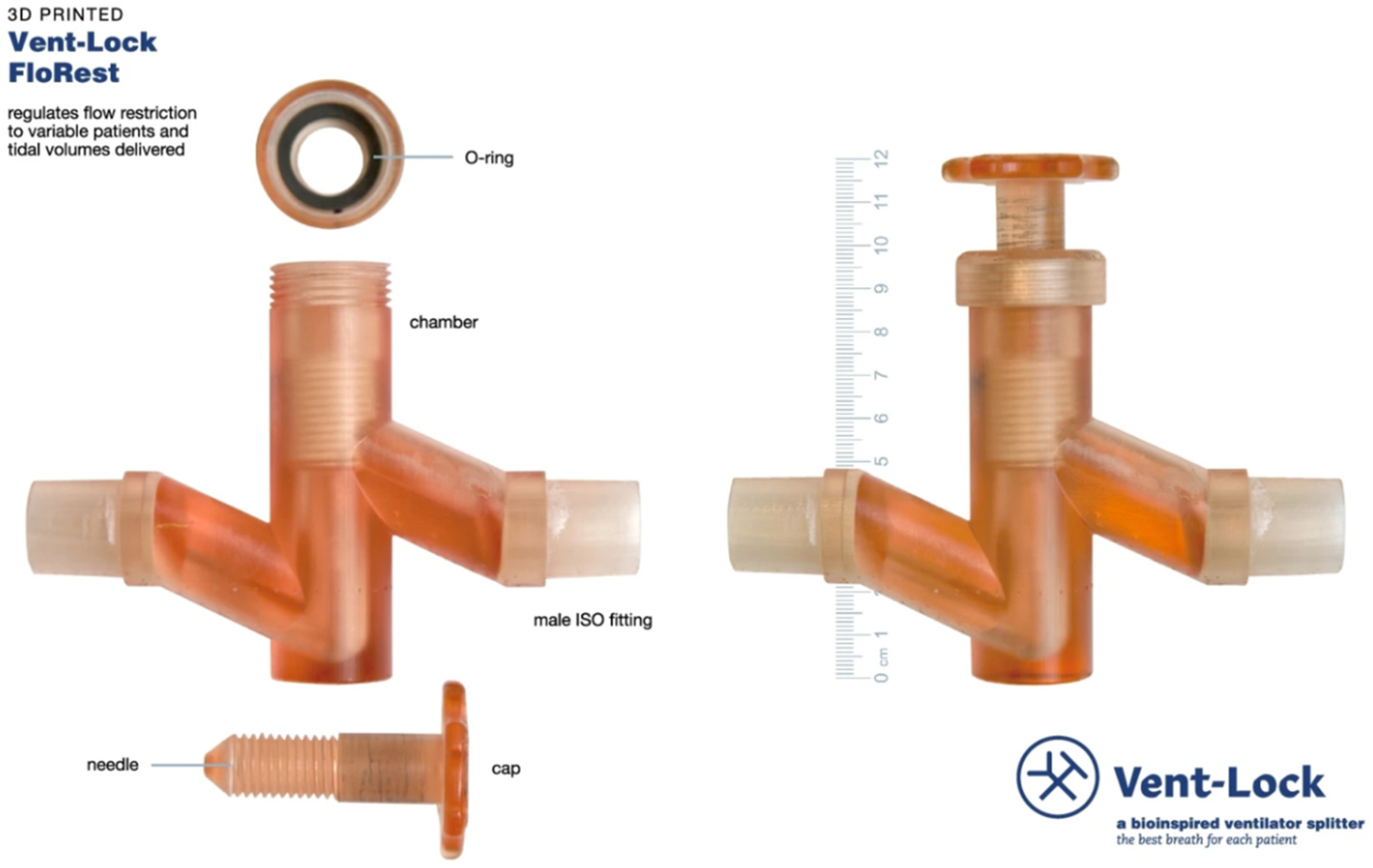
| Other Device | A 3D printed ventilator multiplexer that allows multiple patients to be ventilated using a single ventilator | Form 2, Form 3, or Form 3B; SG resin | SLA | [107] |
| Bladder | Designing and producing a non-invasive miniature force sensor | Form3; Grey resin | Low Force Stereo- lithography (LFS) | [109] |
| Drug Delivery | Nanocomposite pills for drug delivery applications | Form 2; Resin made out of PEGDA, PEO, and the photoinitiator (TPO) | SLA | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P.; Dhal, K.; Gupta, R.; Tappa, K.; Rybicki, F.J.; Ravi, P. Medical 3D Printing Using Desktop Inverted Vat Photopolymerization: Background, Clinical Applications, and Challenges. Bioengineering 2023, 10, 782. https://doi.org/10.3390/bioengineering10070782
Patel P, Dhal K, Gupta R, Tappa K, Rybicki FJ, Ravi P. Medical 3D Printing Using Desktop Inverted Vat Photopolymerization: Background, Clinical Applications, and Challenges. Bioengineering. 2023; 10(7):782. https://doi.org/10.3390/bioengineering10070782
Chicago/Turabian StylePatel, Parimal, Kashish Dhal, Rajul Gupta, Karthik Tappa, Frank J. Rybicki, and Prashanth Ravi. 2023. "Medical 3D Printing Using Desktop Inverted Vat Photopolymerization: Background, Clinical Applications, and Challenges" Bioengineering 10, no. 7: 782. https://doi.org/10.3390/bioengineering10070782
APA StylePatel, P., Dhal, K., Gupta, R., Tappa, K., Rybicki, F. J., & Ravi, P. (2023). Medical 3D Printing Using Desktop Inverted Vat Photopolymerization: Background, Clinical Applications, and Challenges. Bioengineering, 10(7), 782. https://doi.org/10.3390/bioengineering10070782







