Abstract
To develop medical-grade stainless-steel 316L implants that are biocompatible, non-toxic and antibacterial, such implants need to be coated with biomaterials to meet the current demanding properties of biomedical materials. Hydroxyapatite (HA) is commonly used as a bone implant coating due to its excellent biocompatible properties. Zinc oxide (ZnO) nanoparticles are added to HA to increase its antibacterial and cohesion properties. The specimens were made of a stainless-steel grade 316 substrate coated with HA-ZnO using the electrophoretic deposition technique (EPD), and were subsequently characterized using scanning electron microscopy (SEM), energy dispersive X-ray (EDX), stylus profilometry, electrochemical corrosion testing and Fourier transform infrared (FTIR) spectroscopy. Additionally, cross-hatch tests, cell viability assays, antibacterial assessment and in vitro activity tests in simulated body fluid (SBF) were performed. The results showed that the HA-ZnO coating was uniform and resistant to corrosion in an acceptable range. FTIR confirmed the presence of HA-ZnO compositions, and the in vitro response and adhesion were in accordance with standard requirements for biomedical materials. Cell viability confirmed the viability of cells in an acceptable range (>70%). In addition, the antibacterial activity of ZnO was confirmed on Staphylococcus aureus. Thus, the HA-ZnO samples are recommended for biomedical applications.
1. Introduction
Bone implants have been important in the treatment of damaged and fractured bones for several decades. Several materials such as titanium and its alloys, stainless steel, cobalt and magnesium are used as bone implants [1].
Organic and inorganic coatings on implants are used to achieve biocompatibility, inflammation resistance and to exhibit antibacterial behavior. Out of the vast range of available coatings, hydroxyapatite (HA) is one of the preferred inorganic coatings due to its excellent biomedical properties, including its bioactivity and biocompatibility [2]. It further helps in regenerative purposes and enhances bone healing [3]. Among several methods of depositing HA coatings on bone implants, the plasma spray coating technique was used to deposit particles of different particle sizes [4]. It was observed that the morphology and crystallinity of the HA film on a substrate depends on the particle size of HA powders [5]. A more crystalline structure of HA films was observed using amorphous powders through combustion flame spraying [6]. S.W.K. Kweh [7] observed that the fracture toughness and the elastic modulus of the HA coatings is enhanced through plasma spraying [8]. The particle size of the HA used as feedstock has a significant effect on the mechanical properties of HA films The electrophoretic deposition technique is capable of producing coatings that possess a range of functionalities, including bioactivity, antibacterial action and biocompatibility, thus making them multifunctional [9]. M. Sabzi et al. coated HA-ZnO on a NiTi shape memory alloy of 135 µm thickness via EPD and concluded that the coating was uniform, crack-free, biologically active and highly resistant to corrosion [10]. A. Kand et al. deposited HA on AZ31 via EPD and observed that the degradation of the AZ31 alloy became passive due to the HA coating and biological apatite sediments formed upon immersion in SBF [11].
To further enhance the anti-bacterial, bone regeneration and cohesion properties of bone implants, zinc oxide (ZnO) nanopowder is an excellent choice for implant coatings [12]. The United States Federal Drug Administration (21CFR182.8991) has classified ZnO as generally recognized as safe (GRAS) [13]. ZnO powder is often mixed with other coating materials to enhance their anti-inflammatory, antibacterial and cohesive properties. However, limited research studies are available on the characterization of such ZnO-reinforced coatings. Puneet Bansal et al. [14] used the plasma spray technique to characterize HA-ZnO coatings on an AZ31 Mg alloy. They observed that the addition of ZnO improved the surface properties and corrosion resistance of the coatings. Jingan Li et al. investigated that the alloy of Mg-xLi-Zn is highly recommended for bone implants due to the development of mechanical properties.
The addition of ZnO in HA was reported to improve cell viability and antibacterial properties, and no cytotoxic effect on the structural organization of the cells was observed [15].
Kummara Madhusudana Rao et al. [16] conducted an investigation on the preparation of hyaluronic acid–zinc oxide hydrogels using a one-pot method. These hydrogels exhibited excellent antibacterial properties and were found to be effective hemostatic agents. Based on these findings, the researchers suggest that hydrogels are potential candidates for their use as wound dressing materials. According to Baikere Maimaiti [17], a stable nanocoating of ZnO-doped hydroxyapatite on titanium can be beneficial for both anti-infection and osteogenic purposes.
Puneet Bansal [18] investigated Ti13Nb13Zr titanium alloy with plasma-sprayed HA-ZnO coatings for biomedical applications. They observed that the addition of ZnO in HA improved hardness and antibacterial properties. Jie Zhou et al. [19] coated nano-HA-ZnO on biodegradable Mg-Zn-Ca via the hydrothermal method, and the results showed that the coated implant is more biologically active than the traditional metals. Jianghong Luo et al. [19] coated HA doped with multi-metal ions on a Ti substrate via the pulse electrochemical method and observed that the coated substrate was highly antibacterial and biologically friendly.
SS 316L [20,21] bone implants are more vulnerable to cytotoxicity compared to other implant types [22]. ZnO is specifically used to enhance the cohesion characteristics of the HA coatings applied to SS implants [23]. Moreover, HA-based zinc oxide nanocomposites possess similar mechanical properties to bones [24]. There are several reliable techniques that offer HA coatings, including radio frequency (RF) magnetron sputtering [25,26,27], plasma spraying [28,29,30], high-velocity oxy-fuel plasma (HVOF) coating [23], the sol–gel process [31] and the cold spray technique [32]. One major drawback of using these methods is the cost and maintenance of the equipment required and the lack of precise control over the coating process, which makes it very difficult to utilize RF sputtering [33], sol–gel [34] or HVOF [35] spraying techniques for intricate shapes such as dental implants. The electrophoretic deposition (EPD) method is a highly effective technique for coating metals, polymers, ceramics and composites with complex shapes and structures. In addition, this method enables the deposition of polymers, proteins and peptides at room temperature, without compromising their chemical composition [36]. Moreover, EPD has the ability to create multifunctional coatings that possess bioactive, antibacterial and biocompatible properties. EPD uses a simple setup that includes two electrodes, and it can be powered by either a direct current (DC) or alternating current (AC) [9] since, with the help of suspension, any sort of uniform coating can be achieved on the opposite electrode [37]. It has been observed that the deposition of HA-ZnO composite coatings on SS 316L via the EPD technique has not been carried out to date, as per the author’s knowledge.
The objective of this work is to investigate the effect of ZnO addition on HA coatings deposited on a SS 316L substrate via the EPD process and to analyze its resultant surface and anti-bacterial properties in depth via different characterization techniques and conduct in vitro testing in SBF medium.
2. Materials and Methods
2.1. Materials
ZnO powder (CAS 1314-13-2) with an average size of 600 nm and HA powder with an average particle size of 0.69 µm (Sigma Aldrich, St. Louis, MO, USA) were used, and coatings were deposited on SS 316 L substrate.
2.2. Suspension
The HA-ZnO coating on SS substrate using EPD technique was performed by making a suspension of the powders in ethanol. For the HA-ZnO composite powder suspension, 1.5 g of HA and 1 g of ZnO were added in 50 mL of pure ethanol. The suspension was sonicated for 8 h. These suspensions were mixed properly and placed on a 78HW-I thermostatic magnetic stirrer for 5 h before first use.
2.3. EPD Coating
The parameters such as time and voltage for the experiments were optimized for the EPD process, as per previous reported literature [38]. A schematic diagram of suspension preparation and the EPD technique are shown in Figure 1a,b. To achieve coatings with good adhesion and surface properties, further EPD process parameters such as concentration, voltage and time were varied and optimized [39]. Next, 3 × 2 cm samples of stainless steel were hammered from edges and then cleaned with distilled water. The adhesion EPD coatings are sensitive to dust and debris, and special care was taken to protect them. EPD was set up by placing a stainless-steel anode and cathode in the suspensions one by one. SS substrates were coated by maintaining the following parameters 5 min, 20 V and 10 mm distance between electrodes. These suspensions were magnetically mixed after each coating in order to achieve excellent and uniform adhesion. pH was maintained for each suspension, i.e., 3.86 pH at 21 °C for HA-ZnO; pH was checked using a HANNA HI 2211 bench pH meter. The EPD setup and prepared samples are shown in Figures S1 and S2, respectively.
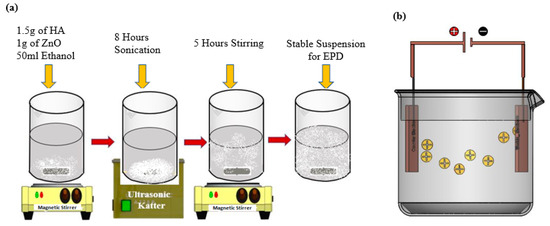
Figure 1.
Schematic illustration of development of HA-ZnO-based coatings: (a) suspension preparation, (b) electrophoretic deposition (EPD) technique.
2.4. SEM Analysis
The morphology of the coating powder and coatings was examined using a TESCAN field emission scanning electron microscope (FESEM) (Brno, Czech Republic), with a resolution of 5 nm. Coated samples of size 1 × 1 cm were used to analyze the structure and morphology. The applied voltage during SEM was 20 kV. The elemental analysis was performed using an energy dispersive X-ray spectroscope (EDS, S-4700 with a Noran System 7, Hitachi, Tokyo, Japan).
2.5. Surface Roughness
Surface roughness of plays an important role in cell attachment and osteoblasting as ions release in physiological activity [40]. It was reported that good cell attachment was observed at a roughness value of 1–2 µm. Roughness of the HA-ZnO coatings was measured with stylus profilometer (TMR 360, Beijing, China). Samples were cleaned prior to testing. An average of three readings were measured to obtain the actual value.
2.6. Electrochemical Study
In order to assess the corrosion behavior of HA-ZnO coatings in SBF medium, it is necessary to perform electrochemical corrosion testing [41]. Initially, specimens were prepared by masking the desired surface area of the HA-ZnO-coated substrate. The SBF solution was prepared using ISO standards 10993-14 [42]. Electrochemical corrosion testing was carried out on the coated specimens using a potentiostat on Gamry setup (Farmwork 600, Louis Drive Warminster, PA, USA). A three-electrode configuration was utilized for this purpose, which includes the coated specimen as the working electrode, a reference electrode and a counter electrode. Samples were immersed in the SBF solution for a specific period of 24 h, which allowed them to stabilize. Potentiodynamic polarization curves were obtained by applying a potential range that covers the corrosion potential (Ecorr) and measures the corrosion current (Icorr) and corrosion potential (Ecorr) from the curve. Furthermore, the corrosion rate of the coated specimen in SBF solution was measured by applying a small-amplitude sinusoidal potential around the corrosion potential (Ecorr) and recording the corresponding current response. Finally, the results were drawn against the voltage potential 0.2 V and current density 1 × 10−3 A·cm−2. These results were compared with those obtained for uncoated specimens to determine the effectiveness or corrosion rate of the HA-ZnO coating.
2.7. FTIR
Fourier transform infrared (FTIR) spectroscopy (Shimadzu, Kyoto, Japan) setup was used for HA-ZnO powder, utilizing the parameters of transmittance and wave number. The samples were prepared using the standard technique [43], and FTIR measurements were conducted using a single-beam Fourier transform infrared spectrometer. For this purpose, the spectra of FTIR for the HA-ZnO-coated sample was taken in the spectral range from 4000 to 600 cm−1.
2.8. Adhesion Properties
The cross-hatch tape test was used to check the adhesion of the coating using ASTM D3359 and ISO 2409 standards [44]. Two cuts/scratches were made perpendicular to each other with an appropriate cutter, i.e., through the coating into the substrate to draw a lattice pattern. Pressure-sensitive adhesive tape (PSA, self-adhesive) was pasted on it with good contact and pulled back at a 180° angle. Each test was performed three times, and the average value was taken.
2.9. XRD
To investigate the phase composition of HA-ZnO, XRD analysis was carried out using an X-ray powder diffractometer (Malvern PANalytical Aeris, Cambridge, UK) using Cu Kα monochromatic radiation at a scan range of 5° to 90° with a stay time at each step of 4 sec and step size of 0.4. Phase identification was achieved by comparing the diffraction patterns using ICDD (JCPDS) standards.
2.10. In Vitro Study
The bioactivity of the coated samples (size 1 × 1 cm2) in the simulated body fluid were examined by weight loss and pH variation. To examine biocompatibility, the SBF (NaCl 7.996, NaHCO3 0.35, KCl 0.224, K2HPO4·3H2O 0.228, MgCl2·6H2O 0.305, CaCl2 0.278, Na2SO4 0.071 and (CH2OH)3CNH2 6.057) by concentration (g/L) was prepared using kokokubo [45] techniques, as the SBF is a solution utilized for in vitro bio-mineralization studies that imitates the chemical composition of human blood plasma. Its preparation protocol includes making a stock solution of salts, adding them to a volumetric flask, regulating the pH and sterilizing it by filtering [42]. In the current research, the biocompatibility of coated samples were examined for 24 h, 72 h, 120 h and 168 h in SBF solution, and the morphology was checked via SEM. For this purpose, the samples were taken out of the SBF and dried at room temperature to check the weight. In addition, weight gain/loss studies were also considered for HA-ZnO-coated samples in SBF for durations of 24 h, 72 h, 120 h and 168 h.
2.11. Cell Culture Study
The HA-ZnO coating’s in vitro biocompatibility was evaluated using human-derived stem cells. Stem cells are basically the origin of all kinds of cells. Cells were maintained at 37 °C in a humidified environment of 5% CO2 and grown in Dulbecco’s modified eagle’s medium (DMEM; Gibco, Billings, MT, USA) supplemented with 10 vol.% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) and 1 vol.% penicillin–streptomycin (Pen Strep; Sigma-Aldrich). The medium was removed after incubation, and cells were then cleaned with phosphate buffer saline (PBS; Gibco). The cells were separated from the flask wall using trypsin (Sigma-Aldrich, St. Louis, MO, USA) in the following steps. All materials were divided into 1 × 1 cm2 pieces and sterilized for 30 min using ultraviolet light to conduct cell tests. As a control, tissue culture plates (TCP) were utilized. In vitro cellular studies were carried out using an indirect assay (cells were in contact with the extracts of the scaffolds, which were obtained by immersing the coatings in DMEM).
2.12. Antibacterial Study
For the evaluation of antibacterial properties, the agar disc diffusion test was performed against Gram-positive (Staphylococcus aureus; S. aureus) and Gram-negative (Escherichia coli; E. coli) bacterial strains. Petri dishes were filled with 20 mL agar and LB medium inoculated with 20 μL Staphylococcus (S. aureus) and Escherichia coli. E. coli having an optical density of 0.015 (OD600) were spread on agar plates. Coated and uncoated samples were again placed in the agar plate for 24 h and incubated on 37 °C for antibacterial studies. Images of Petri dishes were captured to track the inhibition zone (if it existed). To monitor the inhibition zone in the antibacterial test, sterile filter paper discs were placed onto an agar plate, and an antimicrobial agent was added and incubated. Presence of an inhibition zone was checked and its size determined after incubation [46], and the results were reported.
3. Results and Discussion
3.1. Morphological and Compositional Analysis
Pure HA and ZnO powder was examined using scanning electron microscopy (SEM), as shown in Figure 2. The surface morphology and thickness of the HA-ZnO-coated samples prepared via EPD techniques were examined using SEM. The SEM morphology and EDS of the SS 316L are shown in Figure 3 and Figure 4. The EDS of the SS shows that no impurities existed. The SEM images shows that HA-ZnO coatings were uniform, and no segregation of any particles at any point was detected. The flower-like structure and cluster morphology depicts that the HA and ZnO are properly mixed and are present in the coating, as shown in Figure 5a–c. The images also confirm the proper mixing of HA and ZnO because of the structural distribution among the cluster. Figure 6 represents the color mapping, which leads to the verification of the uniform mixing of the HA powder with the ZnO powder.
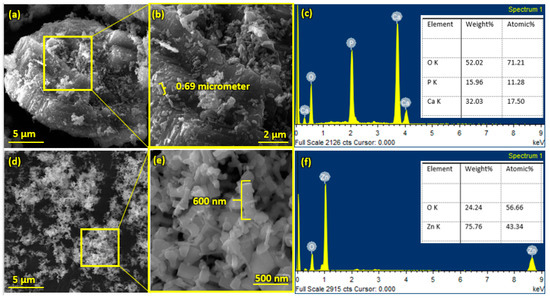
Figure 2.
Scanning electron microscopy and EDS of (a–c) hydroxyapatite (HA) nanopowder and (d–f) zinc oxide (ZnO) nanoparticles.
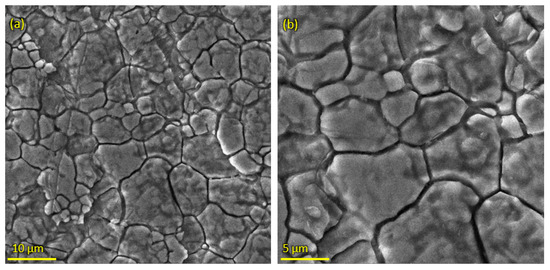
Figure 3.
Scanning electron microscopy of SS 316L substrate (a) at 10 µm and (b) at 5 µm.
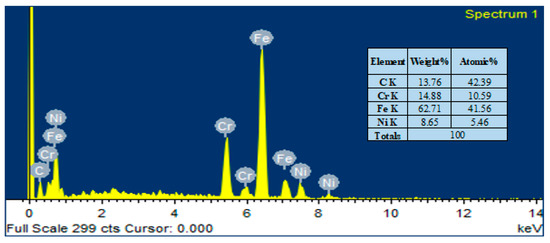
Figure 4.
Elemental compositions (EDS) of SS 316L substrate.
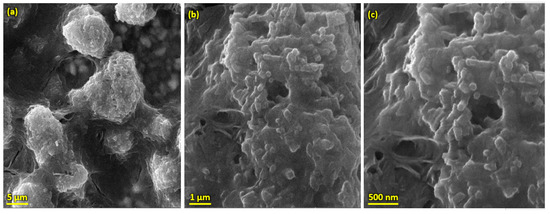
Figure 5.
SEM morphology of HA-ZnO coating on SS 316L substrate (a) at 5 µm, (b) 1 µm and (c) 50 nm.
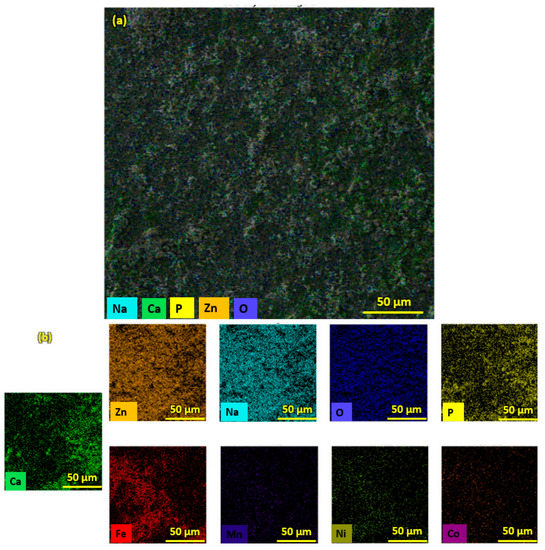
Figure 6.
EDS Color maps of (a) HA-ZnO coating on SS substrate. (b) Elemental compositions of HA-ZnO coating.
The elemental composition analysis of the HA-ZnO coating applied on the SS substrate was carried out using EDX analysis. The EDX analysis shows the presence of Ca, P, O and ZnO elements, as shown in Figure 7. Therefore, the formation of the HA-ZnO coating is confirmed, in addition to some other elements, i.e., Fe and Cr, which showed the composition of the SS.
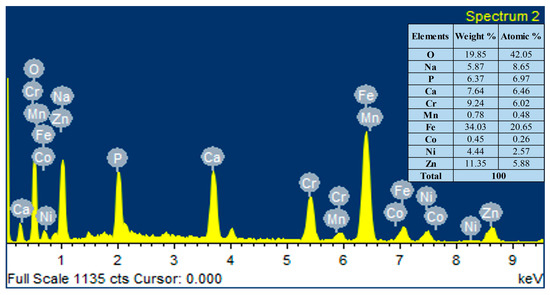
Figure 7.
Elemental compositions (EDS) of HA-ZnO coating on SS 316L substrate.
Figure 8 shows that the coating is uniform, compact and has a thickness of about 30 µm. Similar results were achieved by Krause et al. for a Bioglass® coating on a stainless-steel substrate [47].

Figure 8.
Cross-sectional view and thickness of HA-ZnO coating.
3.2. Surface Roughness Measurement
Surface roughness is an important parameter in biomedical materials for physiological activities. Surface roughness is basically cause by the irregularities present on a material’s surface that cause variations in its features. It is characterized by its roughness average (Ra), which measures the surface’s mean deviation from its nominal height over a specific area. Figure 9 shows that the roughness (Ra) of 316L SS is 0.45 µm, while the Ra of HA-ZnO is 1.21 µm, determined using the average of three sample values. Our values for as-deposited coatings lie in the recommended cell roughness range of 1–2 µm [48], and it was shown that the addition of ZnO enhances the surface roughness to the biomedically recommended range [14].
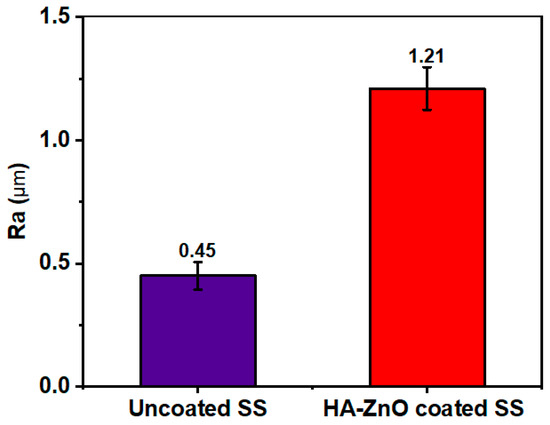
Figure 9.
Surface roughness (Ra) of uncoated SS and HA-ZnO coating.
3.3. Corrosion Study
Electrochemical corrosion testing was conducted for bare and coated SS substrate samples. Figure 10 shows the potentiodynamic polarization curves for the SS substrate and HA-ZnO-coated samples. The data indicate that an increase in the applied potential results in the passivation of the stainless-steel samples, implying that the formation of a stable chromium oxide layer inhibits further corrosion and slows down the corrosion process. On the contrary, while the applied potential is increased, HA-ZnO coatings did not undergo passivation. When examining the cathodic branch of the polarization curve, it was noted that the use of HA-ZnO coatings led to a significant reduction in cathodic current densities. To determine the corrosion current density, a Tafel fit was applied to the polarization curve, as shown in Figure 10. The corrosion rate of SS was 72.27 mpy, while the HA-ZnO coating successfully decreased the corrosion rate to 1.809 mpy, indicating that the bare SS is more vulnerable to corrosion while the HA-ZnO-coated sample is comparatively good against corrosion resistance in simulated body fluid. Due to the high protection it offers against corrosion, this coating may survive until the healing of the injury. As HA-ZnO usually starts interaction with surrounding tissues within 21 days and a very strong interface is found in-between implant and tissues, this bonding makes the implant durable and long-lasting [49].
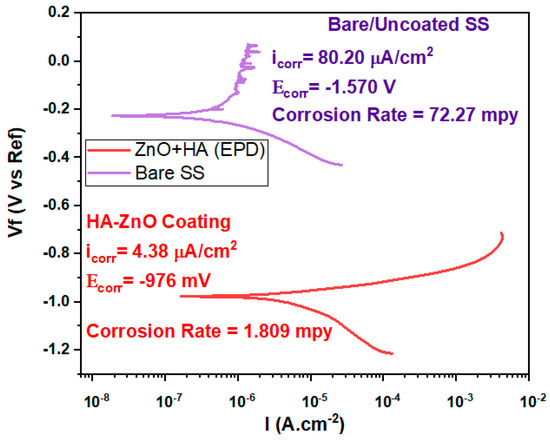
Figure 10.
Tafel plot of bare/uncoated SS and HA-ZnO coating in SBF.
3.4. FTIR Analysis
Figure 11 shows the results of FITR analysis. The peaks confirm the presence of HA and ZnO compositions in the spectra range of 500–600 and at 3500 cm−1. P-O groups are present in the range of 1000–1500 cm-1, while OH is in a hydroxyl group detected from HA, detected at a 3600 cm−1 wave number. Extra peaks were not examined, which is an indication that no other reaction took place. Ziqi Liu et al. also investigated the same peaks on a double-layer Ca-P sandwiched siloxane composite coating on an Mg alloy for bone tissue applications and reported that the corrosion resistance of the coated samples was improved compared to the Mg alloy substrate [50].
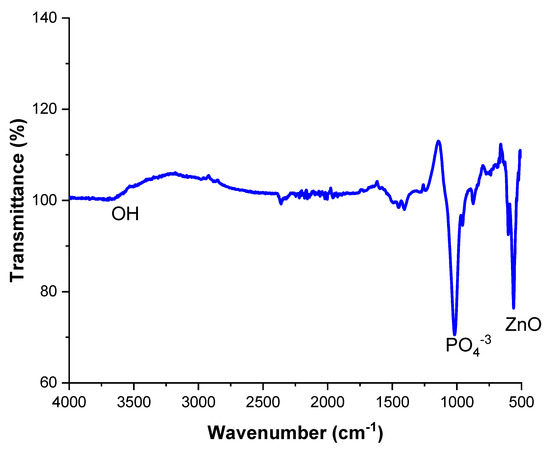
Figure 11.
FTIR Spectra of HA-ZnO EPD coating.
3.5. Adhesion Strength
To examine the adhesive properties of the coating with the substrate, a cross-hatch tape test was conducted. Figure 12 depicts the results of the cross-hatch tape test for the HA-ZnO composite coatings. The adhesion strength of the EPD coatings was rated as “4B”, according to the ASTM D3359 standard, showing that these coated samples are suitable for ortho-biomedical applications. In addition, it assures that the adhesion rate between the substrate and coating is appropriate and can bear the implantation load. The proper encapsulation and cohesion of HA with ZnO was the reason for the good adhesion with the substrate, as investigated by Mokobia et al. [51]. Ahmadi et al. [52] observed that the addition of ZnO has a positive effect on the cohesive properties of the HA-ZnO coating. This is due to ZnO’s ability to facilitate the synthesis of pure HA crystals while also promoting crystal growth and network formation, resulting in increased strength. ZnO nanoparticles are also useful for enhancing loading efficiency since they possess the same HCP crystal structure as HA. However, when too much ZnO is added, irregularly shaped elongated plates may form in the HA-Z8 coating. HA-Z8 results in the incorporation of much more ZnO NPs. It has been reported that as the thickness of the coating increases, the residual stresses also increase, potentially compromising the overall durability of the coating [52].
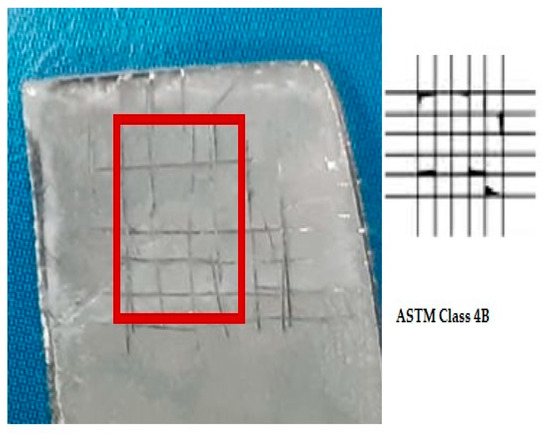
Figure 12.
Cross-hatch tape test result of HA-ZnO coating.
3.6. XRD Analysis
Figure 13 shows the XRD patterns of the HA-ZnO composite sample. The results show the compositions of hydroxyapatite and zinc oxide. The broad diffraction peaks are identical to the standard diffraction patterns of ZnO (PDF#21-1486) and HA (PDF#09-0432). The obtained patterns are in agreement with Kim et al. and Heidari et al. [53,54].
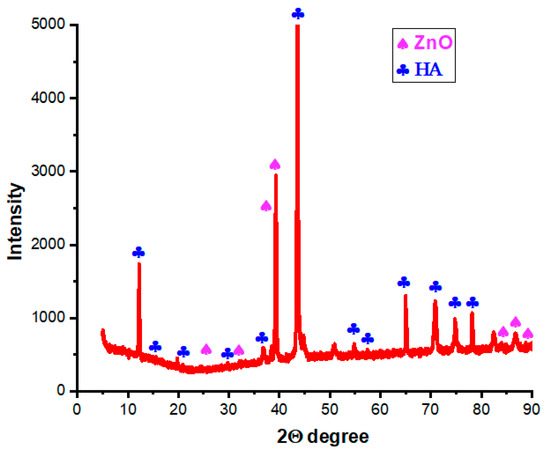
Figure 13.
XRD results of HA-ZnO.
3.7. In Vitro Bioactivity
In order to check and investigate the in vitro bio-activity, coated samples were immersed in SBF solution. The samples were cut into small pieces to be immersed in the SBF solution. pH and weight were taken into account before and after immersion in SBF. Changes in weight were observed after durations of 24 h, 72 h, 120 h and 168 h. The detailed data of the weight and pH are shown in Table 1. It was observed that after 24 h, the sample lost weight due to adjustment or inflammation factors, as stated by Li. et al. [55]. After 168 h, the sample adjusted to the simulated body fluid and gained weight, and its pH also stabilized. These factors lead towards the interaction of cells withing the bodily fluid.

Table 1.
HA-ZnO nanocomposite-coated samples after immersion in SBF.
SEM images of the surface of coated samples were examined during immersion in SBF at different time intervals. Figure 14a–d depict the SEM images after SBF treatment of coated samples for 24 h, 72 h, 120 h and 168 h. Figure 14a indicates that SBF treatment affects the morphology of the coating, as cracks and opening were observed, while Figure 14b–d show that with the passage of time, HA-ZnO shows growth in the form of a cauliflower structure [56], which indicates cell interaction and stability. Figure 15 shows the percentage weight loss of the HA-ZnO-coated samples after immersion in SBF for 24 h, 72 h, 120 h and 168 h.
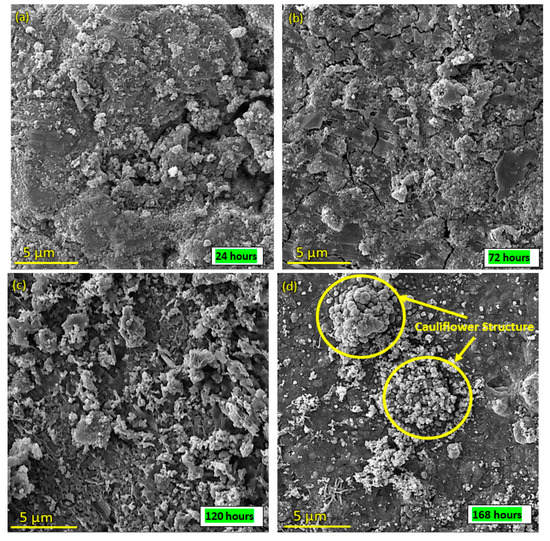
Figure 14.
SEM images of HA-ZnO coating after immersion in SBF for (a) 24 h; (b) 72 h; (c) 120 h; (d) 168 h.
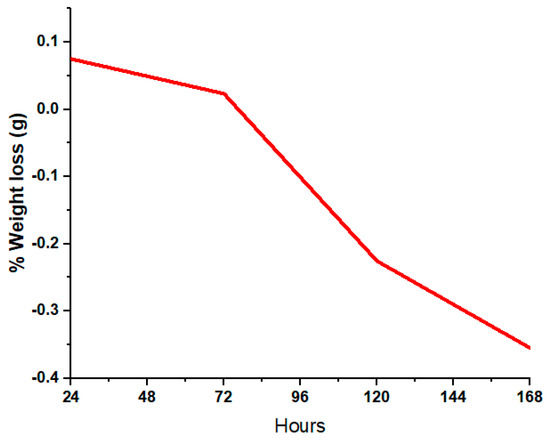
Figure 15.
Percentage weight gain of HA-ZnO-coated samples after immersion in SBF.
3.8. Cell Viability
The viability of the cells on the HA-ZnO coatings compared to the control is depicted in Figure 16. The HA-ZnO layers of the coatings promoted the growth of cells, similar to osteoblasts. The cell density before incubation was 20 × 105. An amount of 50 μL of the cell suspension was added to 50 μL of each sample and incubated for 24 h at 37 °C. After incubation, cells were counted using a hemocytometer, and the obtained cell density was 15.4 × 104, which shows greater than 70% cell viability. Hence, the prepared material is considered to be beneficial for cells. Based on this result, it can be concluded that the cell viability is above 70%, indicating no risk of cytotoxicity. Hence, these coated samples are recommended for use in biomedical implants. Similar results were achieved by T. Alonso et al. [42]. E. Barua et al. also observed the same cell viability in their evaluation of the performance of bone scaffolds made from different hydroxyapatite sources and composed of ZnO-incorporated hydroxyapatite and polymethyl methacrylate tri-components [56]. A. Mehrvarz et al. is in agreement with the cell viability, biocompatibility and antibacterial behavior of electrochemically deposited hydroxyapatite/ZnO porous nanocomposite on a NiTi biomedical alloy [57]. In addition, S. A. Batool is in agreement with the same viability rate for Zn–Mn-doped mesoporous bioactive glass nanoparticle-loaded zein coatings for bioactive and antibacterial orthopedic implants [58].
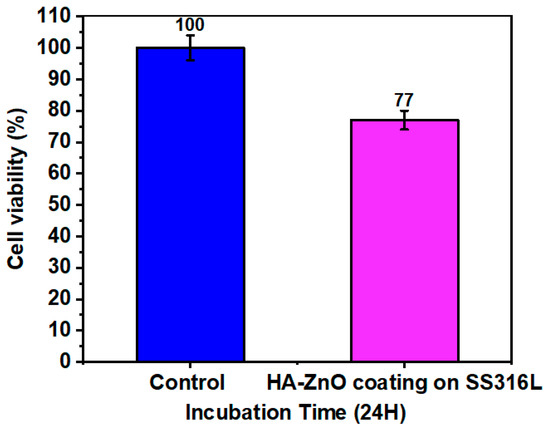
Figure 16.
Cell viability of HA-ZnO-coated sample (value = mean ± SD, N = 3).
3.9. Antibacterial Activity
Figure 17 shows the antibacterial activity of bare/uncoated SS and HA-ZnO-coated samples. The figure shows that the Staphylococcus aureus (Gram-positive) and Escherichia coli (Gram-negative) bacteria attacked the bare sample while the coated sample was protected from bacterial attack, as also mentioned by Venezia at al. for HA-zinc oxide [59]. In the Gram-negative sample, the coated sample was protected and a 26 mm inhibition zone was achieved, while in the Gram-positive bacterial attack, the zone of inhibition was found to be 18 mm. In contrast to this, both bare/uncoated SS samples were observed to be under bacterial attack. The same results were also investigated for ZnO by V. Venezia et al. [59]. Similarly, K. Madhusudana Rao [16] observed that the cell wall of E. coli is generally thin and made up of peptidoglycan, which makes it less susceptible to reactive oxygen species (ROS) generated from ZnO. Reactive oxygen species (ROS) are chemically reactive molecules that contain oxygen, such as superoxide, hydrogen peroxide and hydroxyl radicals [60]. On the other hand, S. aureus has multiple layers of peptidoglycan with numerous pores, making it more vulnerable to ROS and leading to cell disruption [61]. Therefore, ZnO nanoparticles have been found to exhibit greater antibacterial activity against S. aureus than E. coli. HA-ZnO NCH hydrogels displayed more potent antibacterial activity against E. coli than S. aureus [16].
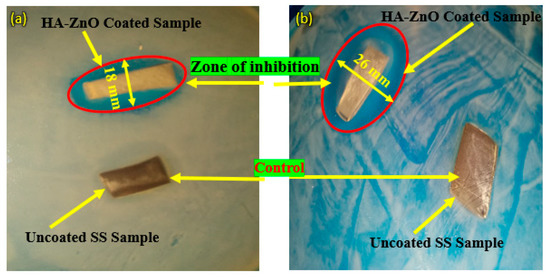
Figure 17.
Antibacterial activity of SS 316L and HA-ZnO-coated samples in (a) Staphylococcus aureus (Gram-positive) medium and (b) Escherichia coli (Gram-negative) medium.
4. Conclusions
In this paper, we have studied the morphology, roughness, corrosion resistance and adhesion properties of HA-ZnO coatings deposited on an SS 316 substrate via EPD, revealing the in vitro and antibacterial performance of such coatings. The resultant coatings appeared to be uniform and evenly distributed, the porous structure of HA being filled with ZnO. The coating’s roughness was improved due to the cohesiveness and surface adoptability of ZnO, making it suitable for use in an SBF environment. The observed improvements in adhesion and inhibition zone collectively reflect the suitability of HA-ZnO coatings prepared via EPD for bio-implant applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering10060693/s1: Figure S1. Experimental set-up for electrophoretic deposition of HA-ZnO on SS 316L; Figure S2. HA-ZnO Coated on SS 316L Substrate.
Author Contributions
Conceptualization, M.Y. and M.A.B.; software, W.A., R.M.U. and R.Z.; validation, M.Y., M.A.B., R.M.U. and W.A.; formal analysis, R.Z.; investigation, R.M.U. and R.Z.; resources, M.A.B., S.A.A. and M.D.; data curation, M.Y., S.A.A. and M.D.; writing—original draft preparation, W.A.; writing—review and editing, M.A.B.; visualization, M.Y.; supervision, M.Y.; project administration, M.Y., S.A.A. and M.D.; funding acquisition, S.A.A. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This Research is funded by the Research Supporting Project Number (RSPD2023R585, King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
All samples were consumed.
References
- Wright, M.; Uddin, A. Organic—inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells 2012, 107, 87–111. [Google Scholar] [CrossRef]
- Lacefield, W. Hydroxyapatite coatings. Ann. N. Y. Acad. Sci. 1988, 523, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Pereira, R.M.; Rodrigues, K.F.; Ribas, R.G.; da Silva, D.M.; Thim, G.P. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceram. Int. 2021, 47, 2999–3012. [Google Scholar] [CrossRef]
- Cheang, P.; Khor, K. Addressing processing problems associated with plasma spraying of hydroxyapatite coatings. Biomaterials 1996, 17, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.R.; Ke, S.; Best, S.; Bonfield, W. Effect of powder characteristics on the sinterability of hydroxyapatite powders. J. Mater. Sci. Mater. Med. 2001, 12, 163–171. [Google Scholar] [CrossRef]
- Wang, Y.; Khor, K.; Cheang, P. Thermal spraying of functionally graded calcium phosphate coatings for biomedical implants. J. Therm. Spray Technol. 1998, 7, 50–57. [Google Scholar] [CrossRef]
- Kweh, S.; Khor, K.; Cheang, P. Plasma-sprayed hydroxyapatite (HA) coatings with flame-spheroidized feedstock: Microstructure and mechanical properties. Biomaterials 2000, 21, 1223–1234. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lee, Y.-T.; Huang, T.-C.; Lin, G.-S.; Chen, Y.-W.; Lee, B.-S.; Tung, K.-L. In vitro bioactivity and antibacterial activity of strontium-, magnesium-, and zinc-multidoped hydroxyapatite porous coatings applied via atmospheric plasma spraying. ACS Appl. Bio Mater. 2021, 4, 2523–2533. [Google Scholar] [CrossRef]
- Goldmann, W.H. Biosensitive and antibacterial coatings on metallic material for medical applications. Cell Biol. Int. 2021, 45, 1624–1632. [Google Scholar] [CrossRef]
- Sabzi, M.; Far, S.M.; Dezfuli, S.M. Characterization of bioactivity behavior and corrosion responses of hydroxyapatite-ZnO nanostructured coating deposited on NiTi shape memory alloy. Ceram. Int. 2018, 44, 21395–21405. [Google Scholar] [CrossRef]
- Kand, A.J.T.; Afaghi, F.; Tabrizi, A.T.; Aghajani, H.; Kivrak, H.D. Electrochemical evaluation of the hydroxyapatite coating synthesized on the AZ91 by electrophoretic deposition route. Synth. Sinter. 2021, 1, 85–91. [Google Scholar] [CrossRef]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surf. B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ranjit, R.; Kumar, N.; Kumar, M.; Giri, B.S. Nanoparticles based nanosensors: Principles and their applications in active packaging for food quality and safety detection. Biochem. Eng. J. 2023, 193, 108861. [Google Scholar] [CrossRef]
- Bansal, P.; Singh, G.; Sidhu, H.S. Investigation of surface properties and corrosion behavior of plasma sprayed HA/ZnO coatings prepared on AZ31 Mg alloy. Surf. Coat. Technol. 2020, 401, 126241. [Google Scholar] [CrossRef]
- Turlybekuly, A.; Pogrebnjak, A.; Sukhodub, L.; Sukhodub, L.B.; Kistaubayeva, A.; Savitskaya, I.; Shokatayeva, D.; Bondar, O.V.; Shaimardanov, Z.K.; Plotnikov, S.V. Synthesis, characterization, in vitro biocompatibility and antibacterial properties study of nanocomposite materials based on hydroxyapatite-biphasic ZnO micro-and nanoparticles embedded in Alginate matrix. Mater. Sci. Eng. C 2019, 104, 109965. [Google Scholar] [CrossRef]
- Rao, K.M.; Suneetha, M.; Zo, S.; Duck, K.H.; Han, S.S. One-pot synthesis of ZnO nanobelt-like structures in hyaluronan hydrogels for wound dressing applications. Carbohydr. Polym. 2019, 223, 115124. [Google Scholar] [CrossRef]
- Maimaiti, B.; Zhang, N.; Yan, L.; Luo, J.; Xie, C.; Wang, Y.; Ma, C.; Ye, T. Stable ZnO-doped hydroxyapatite nanocoating for anti-infection and osteogenic on titanium. Colloids Surf. B Biointerfaces 2020, 186, 110731. [Google Scholar] [CrossRef]
- Bansal, P.; Upadhyay, L. Effect of turning parameters on tool wear, surface roughness and metal removal rate of alumina reinforced aluminum composite. Procedia Technol. 2016, 23, 304–310. [Google Scholar] [CrossRef]
- Zhou, J.; Li, K.; Wang, B.; Ai, F. Nano-hydroxyapatite/ZnO coating prepared on a biodegradable Mg–Zn–Ca bulk metallic glass by one-step hydrothermal method in acid situation. Ceram. Int. 2020, 46, 6958–6964. [Google Scholar] [CrossRef]
- Akram, W.; Farhan Rafique, A.; Maqsood, N.; Khan, A.; Badshah, S.; Khan, R.U. Characterization of PTFE film on 316L stainless steel deposited through spin coating and its anticorrosion performance in multi acidic mediums. Materials 2020, 13, 388. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yasir, M.; Ur Rehman, M.A. Fabrication and characterization of zein/hydroxyapatite composite coatings for biomedical applications. Surfaces 2020, 3, 18. [Google Scholar] [CrossRef]
- Ali, S.; Abdul Rani, A.M.; Mufti, R.A.; Azam, F.I.; Hastuty, S.; Baig, Z.; Hussain, M.; Shehzad, N. The influence of nitrogen absorption on microstructure, properties and cytotoxicity assessment of 316l stainless steel alloy reinforced with boron and niobium. Processes 2019, 7, 506. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloys Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Varshney, S.; Nigam, A.; Singh, A.; Samanta, S.K.; Mishra, N.; Tewari, R. Antibacterial, structural, and mechanical properties of MgO/ZnO nanocomposites and its HA-based bio-ceramics; synthesized via physio-chemical route for biomedical applications. Mater. Technol. 2022, 37, 2503–2516. [Google Scholar] [CrossRef]
- Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol–gel films on titanium alloy fracture plate material. Biomaterials 2007, 28, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Edupuganti, O.P.; Antoci, V., Jr.; King, S.B.; Jose, B.; Adams, C.S.; Parvizi, J.; Shapiro, I.M.; Zeiger, A.R.; Hickok, N.J.; Wickstrom, E. Covalent bonding of vancomycin to Ti6Al4V alloy pins provides long-term inhibition of Staphylococcus aureus colonization. Bioorganic Med. Chem. Lett. 2007, 17, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Teker, D.; Muhaffel, F.; Menekse, M.; Karaguler, N.G.; Baydogan, M.; Cimenoglu, H. Characteristics of multi-layer coating formed on commercially pure titanium for biomedical applications. Mater. Sci. Eng. C 2015, 48, 579–585. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.; Bettenga, M.; Agrawal, C.; Bumgardner, J.; Ong, J. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Yi, S.-C.; Oh, S.-G. Preparation and antibacterial effects of Ag–SiO2 thin films by sol–gel method. Biomaterials 2003, 24, 4921–4928. [Google Scholar] [CrossRef]
- Ewald, A.; Glückermann, S.K.; Thull, R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. Biomed. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef]
- Seuss, S.; Lehmann, M.; Boccaccini, A.R. Alternating current electrophoretic deposition of antibacterial bioactive glass-chitosan composite coatings. Int. J. Mol. Sci. 2014, 15, 12231–12242. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Ali, A.; Ghauri, F.A.; Aslam, A.; Yaqoob, K.; Wasay, A.; Raffi, M. Electrochemical behavior of graphene coatings deposited on copper metal by electrophoretic deposition and chemical vapor deposition. Surf. Coat. Technol. 2017, 332, 112–119. [Google Scholar] [CrossRef]
- Acheson, J.G.; Gallagher, E.; Ward, J.; McKillop, S.; FitzGibbon, B.; Boyd, A.; Meenan, B.; Lemoine, P.; McGarry, J. Shear testing and failure modelling of calcium phosphate coated AZ31 magnesium alloys for orthopaedic applications. Surf. Coat. Technol. 2022, 429, 127944. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-gel derived hydroxyapatite coatings for titanium implants: A review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Pradeep, D.; Venkatesh, C.; Nithin, H. Review on tribological and mechanical behavior in HVOF thermal-sprayed composite coatings. J. Bio-Tribo-Corros. 2022, 8, 30. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Peng, H.-K.; Ren, H.-T.; Lou, C.-W.; Lin, J.-H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Saadati, A.; Khiarak, B.N.; Zahraei, A.A.; Nourbakhsh, A.; Mohammadzadeh, H. Electrochemical characterization of electrophoretically deposited hydroxyapatite/chitosan/graphene oxide composite coating on Mg substrate. Surf. Interfaces 2021, 25, 101290. [Google Scholar] [CrossRef]
- Hanif, M.B.; Gao, J.-T.; Shaheen, K.; Wang, Y.-P.; Yasir, M.; Li, C.-J.; Li, C.-X. Microstructural analysis of highly active cathode material La0. 7Sr0. 3Ti0. 15Fe0. 65Ni0. 2O3-δ (LSTFN) by optimizing different processing parameters. Ceram. Int. 2021, 47, 10893–10904. [Google Scholar] [CrossRef]
- Damiano, M. Surface Profile: Research and Insight. J. Prot. Coat. Linings 2020, 37, 3–4. [Google Scholar]
- Ahmed, U.; Yi, L.; Fei, L.F.; Yasir, M.; Li, C.-J.; Li, C.-X. Enhancement of corrosion resistance and tribological properties of LA43M Mg alloy by cold-sprayed aluminum coatings reinforced with alumina and carbon nanotubes. J. Therm. Spray Technol. 2021, 30, 668–679. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, A.A.; Allam, M.A.; Moharram, M.A. FTIR spectroscopic, thermal and XRD characterization of hydroxyapatite from new natural sources. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 56–60. [Google Scholar] [CrossRef] [PubMed]
- D3359-08; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2003.
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Sayed, M.A.; El-Rahman, T.M.A.; Abdelsalam, H.; Ali, A.M.; Hamdy, M.M.; Badr, Y.A.; El Rahman, N.H.A.; El-Latif, S.M.A.; Mostafa, S.H.; Mohamed, S.S. Attractive study of the antimicrobial, antiviral, and cytotoxic activity of novel synthesized silver chromite nanocomposites. BMC Chem. 2022, 16, 39. [Google Scholar] [CrossRef]
- Krause, D.; Thomas, B.; Leinenbach, C.; Eifler, D.; Minay, E.J.; Boccaccini, A.R. The electrophoretic deposition of Bioglass® particles on stainless steel and Nitinol substrates. Surf. Coat. Technol. 2006, 200, 4835–4845. [Google Scholar] [CrossRef]
- Huang, C.-L.; Huang, K.-T.; Lee, T.-M. The biological responses of osteoblasts on titanium: Effect of oxygen level and surface roughness. J. Formos. Med. Assoc. 2023; in press. [Google Scholar] [CrossRef]
- Zuhdi, S.A. Analysis of Mechanical Properties of Biodegradable Implants Material: Pure Zinc and Stainless Steel 316L for Bone External Fixation; Universitas Andalas: Padang, Indonesia, 2023. [Google Scholar]
- Liu, Z.; Wang, T.; Xu, Y.; Liang, C.; Li, G.; Guo, Y.; Zhang, Z.; Lian, J.; Ren, L. Double-layer calcium phosphate sandwiched siloxane composite coating to enhance corrosion resistance and biocompatibility of magnesium alloys for bone tissue engineering. Prog. Org. Coat. 2023, 177, 107417. [Google Scholar] [CrossRef]
- Mokobia, K.E.; Ifijen, I.H.; Ikhuoria, E.U. ZnO-NPs-coated implants with osteogenic properties for enhanced osseointegration. In TMS 2023 152nd Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2023; pp. 288–300. [Google Scholar]
- Ahmadi, R.; Afshar, A. In vitro study: Bond strength, electrochemical and biocompatibility evaluations of TiO2/Al2O3 reinforced hydroxyapatite sol–gel coatings on 316L SS. Surf. Coat. Technol. 2021, 405, 126594. [Google Scholar] [CrossRef]
- Heidari, F.; Bahrololoom, M.E.; Vashaee, D.; Tayebi, L. In situ preparation of iron oxide nanoparticles in natural hydroxyapatite/chitosan matrix for bone tissue engineering application. Ceram. Int. 2015, 41, 3094–3100. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Bazargan-Lari, R.; Vashaee, D.; Kotturi, H.; Tayebi, L. Mechanical properties of natural chitosan/hydroxyapatite/magnetite nanocomposites for tissue engineering applications. Mater. Sci. Eng. C 2016, 65, 338–344. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Zheng, W.; Yang, J.; Jiang, N. Scaffold-based tissue engineering strategies for soft–hard interface regeneration. Regen. Biomater. 2023, 10, rbac091. [Google Scholar] [CrossRef]
- Barua, E.; Das, A.; Deoghare, A.B.; Pamu, D.; Deb, P.; Lala, S.D.; Chatterjee, S. Performance of ZnO-Incorporated Hydroxyapatite/Polymethyl Methacrylate Tri-Component Composite Bone Scaffolds Fabricated from Varying Sources of Hydroxyapatite. J. Mater. Eng. Perform. 2023, 1–16. [Google Scholar] [CrossRef]
- Mehrvarz, A.; Khalil-Allafi, J.; Khosrowshahi, A.K. Biocompatibility and antibacterial behavior of electrochemically deposited Hydroxyapatite/ZnO porous nanocomposite on NiTi biomedical alloy. Ceram. Int. 2022, 48, 16326–16336. [Google Scholar] [CrossRef]
- Batool, S.A.; Ahmad, K.; Irfan, M.; Ur Rehman, M.A. Zn–Mn-Doped Mesoporous Bioactive Glass Nanoparticle-Loaded Zein Coatings for Bioactive and Antibacterial Orthopedic Implants. J. Funct. Biomater. 2022, 13, 97. [Google Scholar] [CrossRef]
- Venezia, V.; Verrillo, M.; Gallucci, N.; Di Girolamo, R.; Luciani, G.; D’Errico, G.; Paduano, L.; Piccolo, A.; Vitiello, G. Exploiting bioderived humic acids: A molecular combination with ZnO nanoparticles leads to nanostructured hybrid interfaces with enhanced pro-oxidant and antibacterial activity. J. Environ. Chem. Eng. 2023, 11, 108973. [Google Scholar] [CrossRef]
- Biswas, A.; Kar, U.; Jana, N.R. Cytotoxicity of ZnO nanoparticles under dark conditions via oxygen vacancy dependent reactive oxygen species generation. Phys. Chem. Chem. Phys. 2022, 24, 13965–13975. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).