Super-Resolution Ultrasound Localization Microscopy Using High-Frequency Ultrasound to Measure Ocular Perfusion Velocity in the Rat Eye
Abstract
1. Introduction
2. Materials and Methods
2.1. Ultrasound Instrumentation
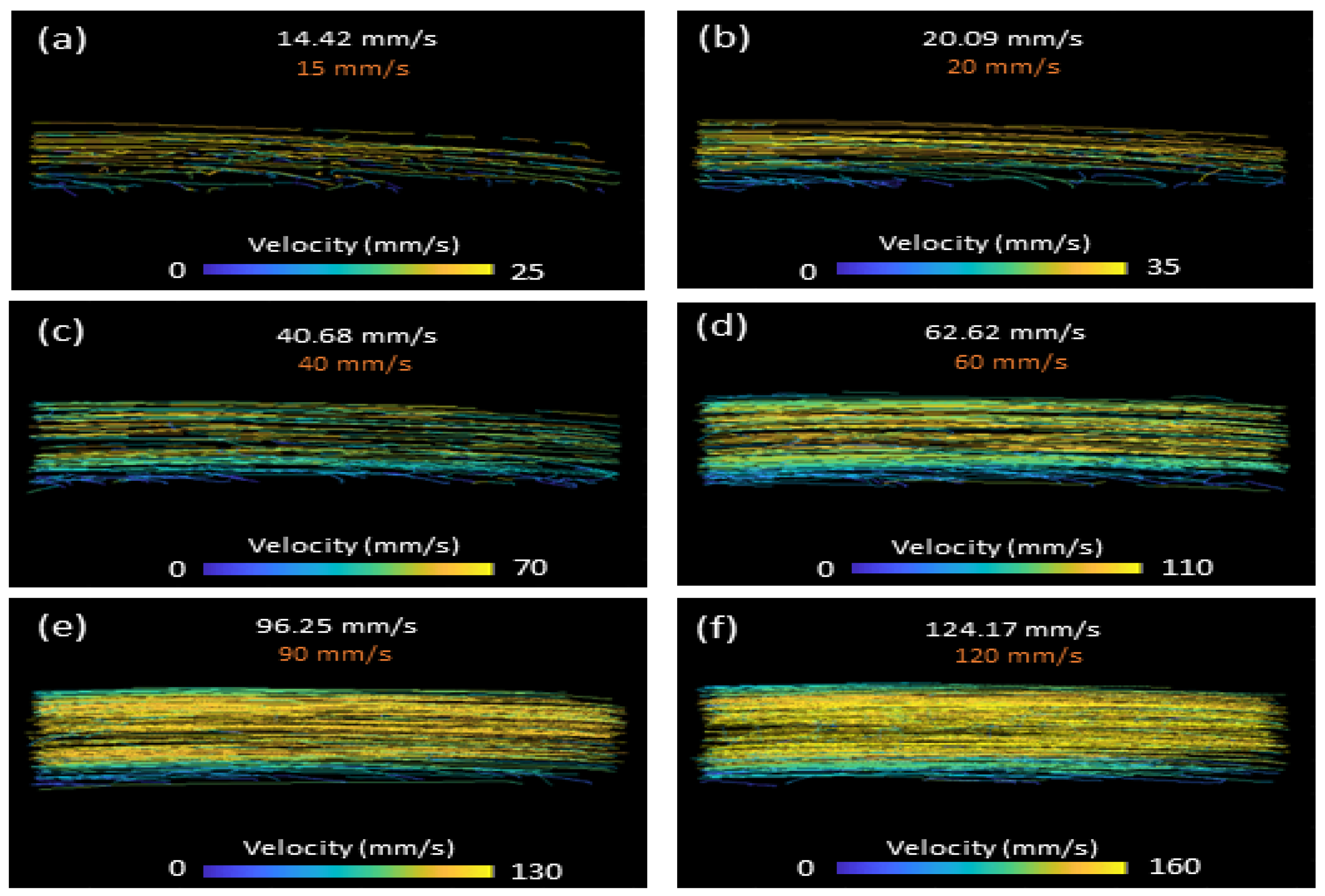
2.2. Controlled Flow Experiments
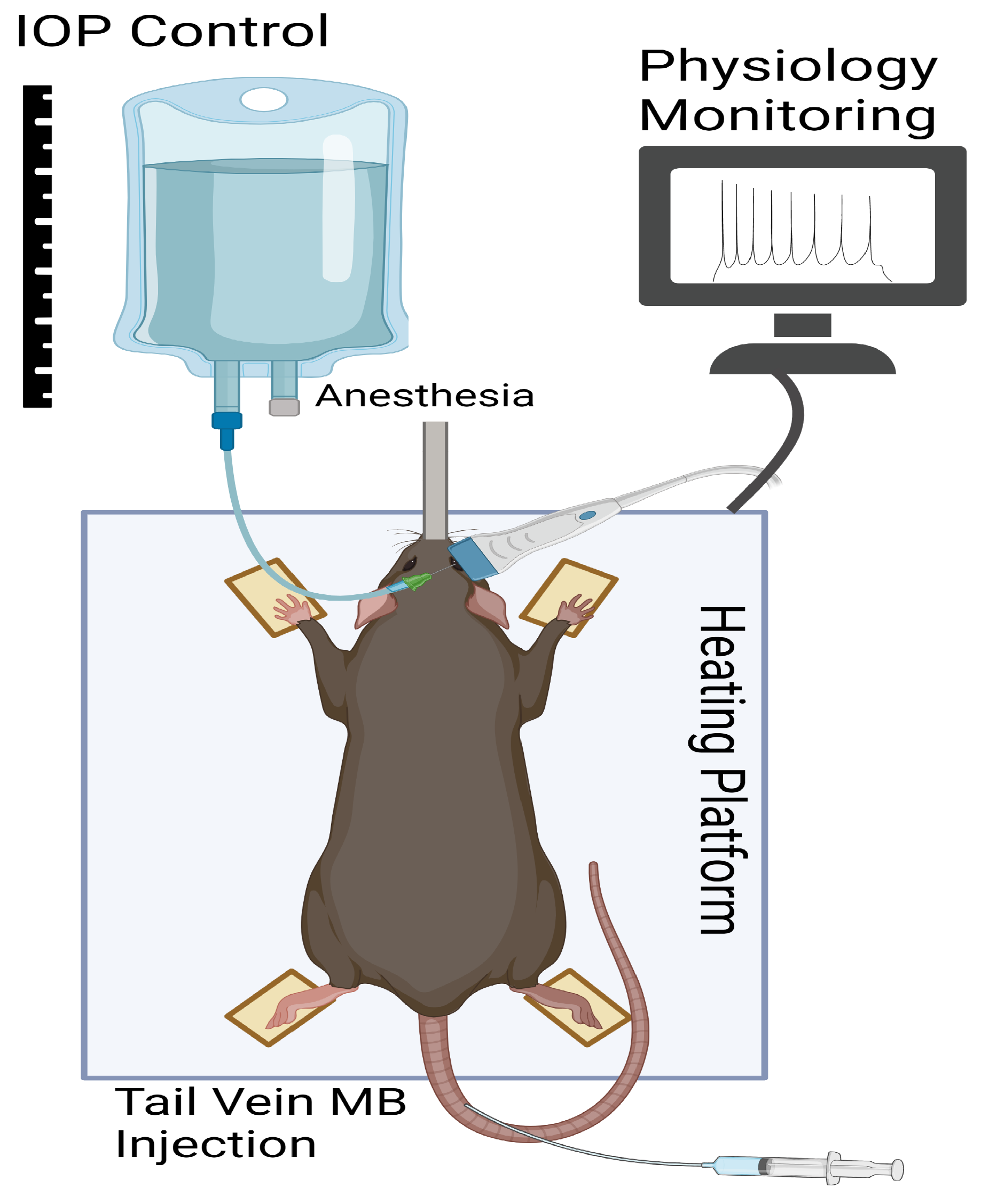
2.3. In Vivo Rat Eye Imaging
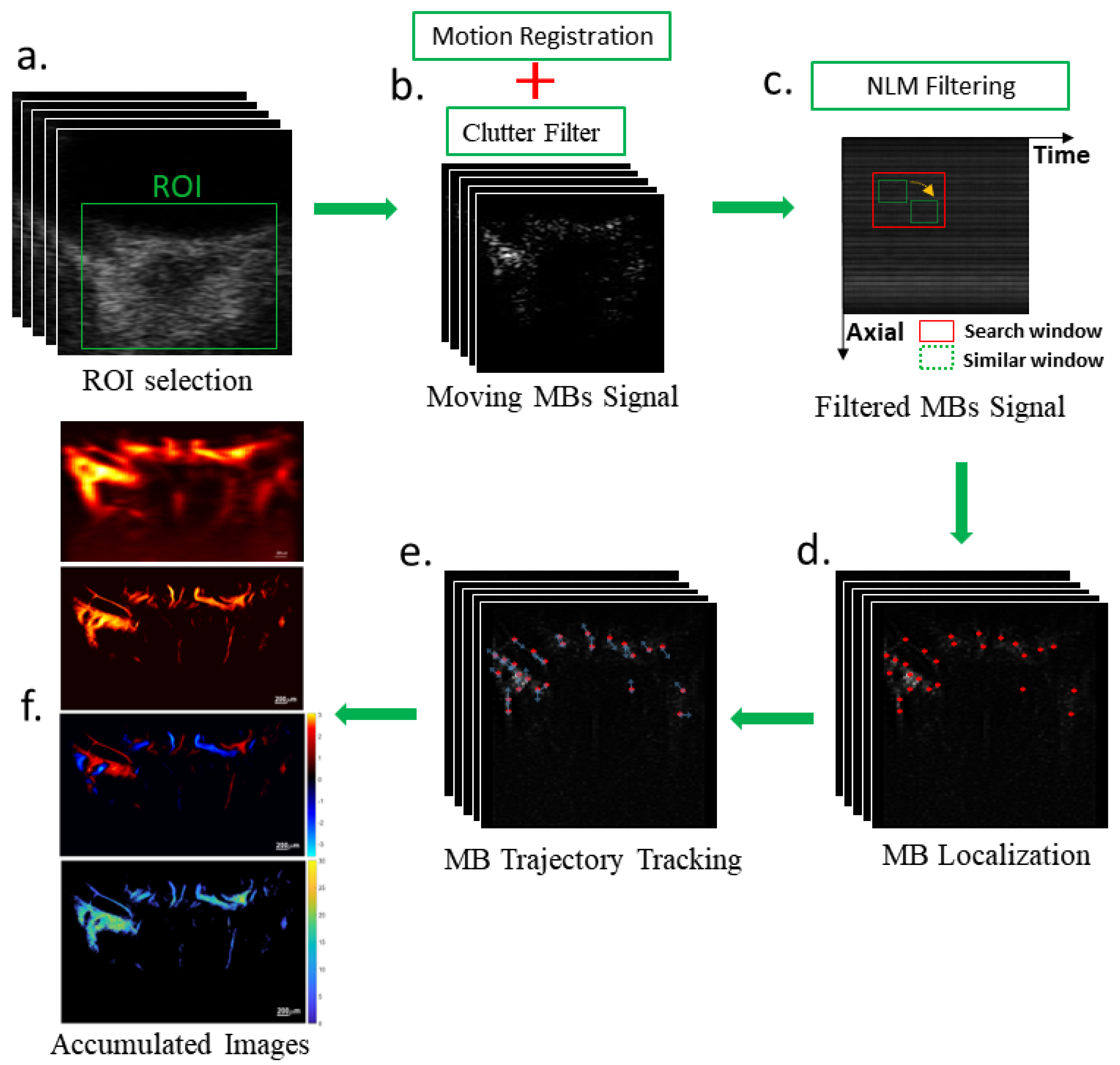
2.4. Microbubble Localization and Tracking Algorithm
2.5. Statistical Analysis
3. Results
3.1. Controlled Flow Results
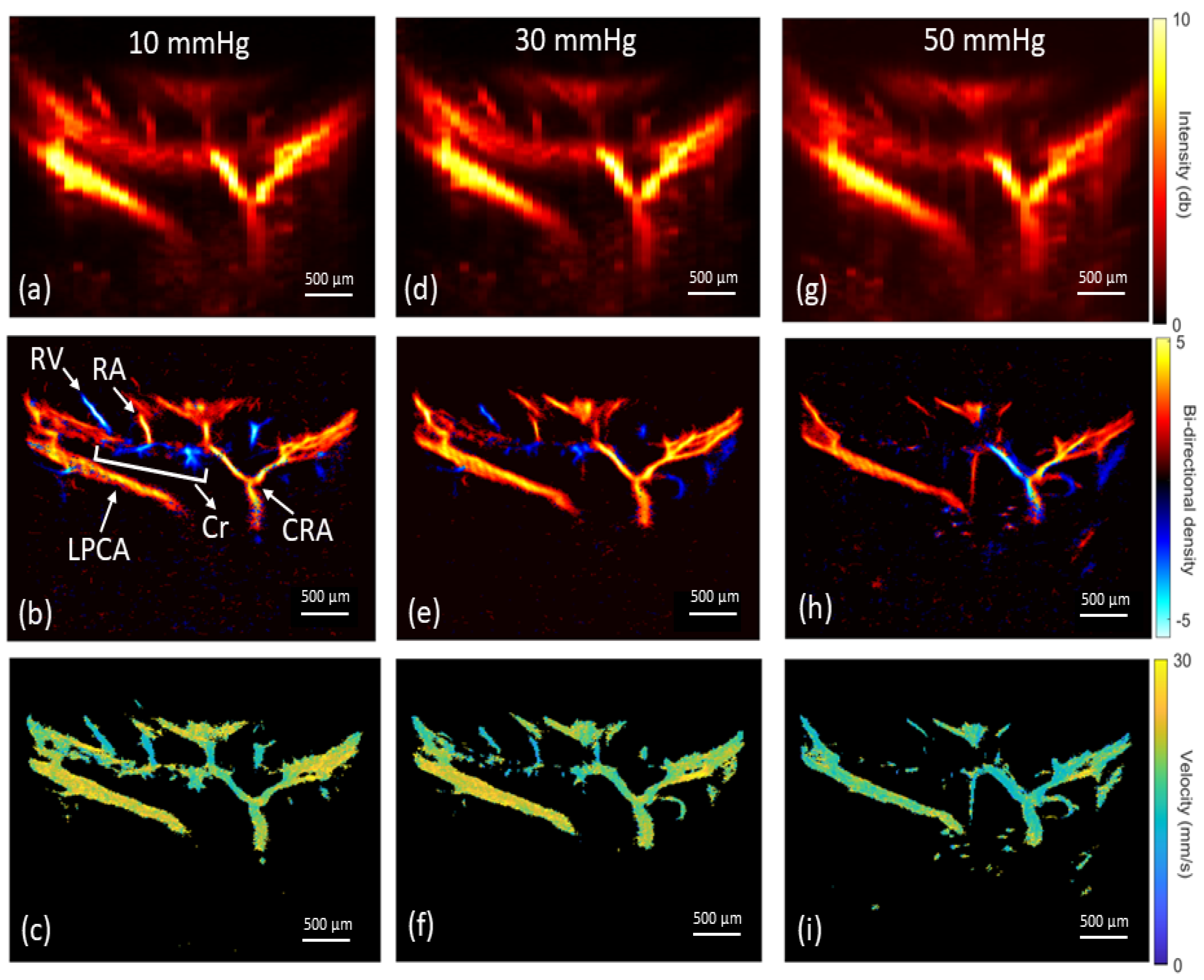
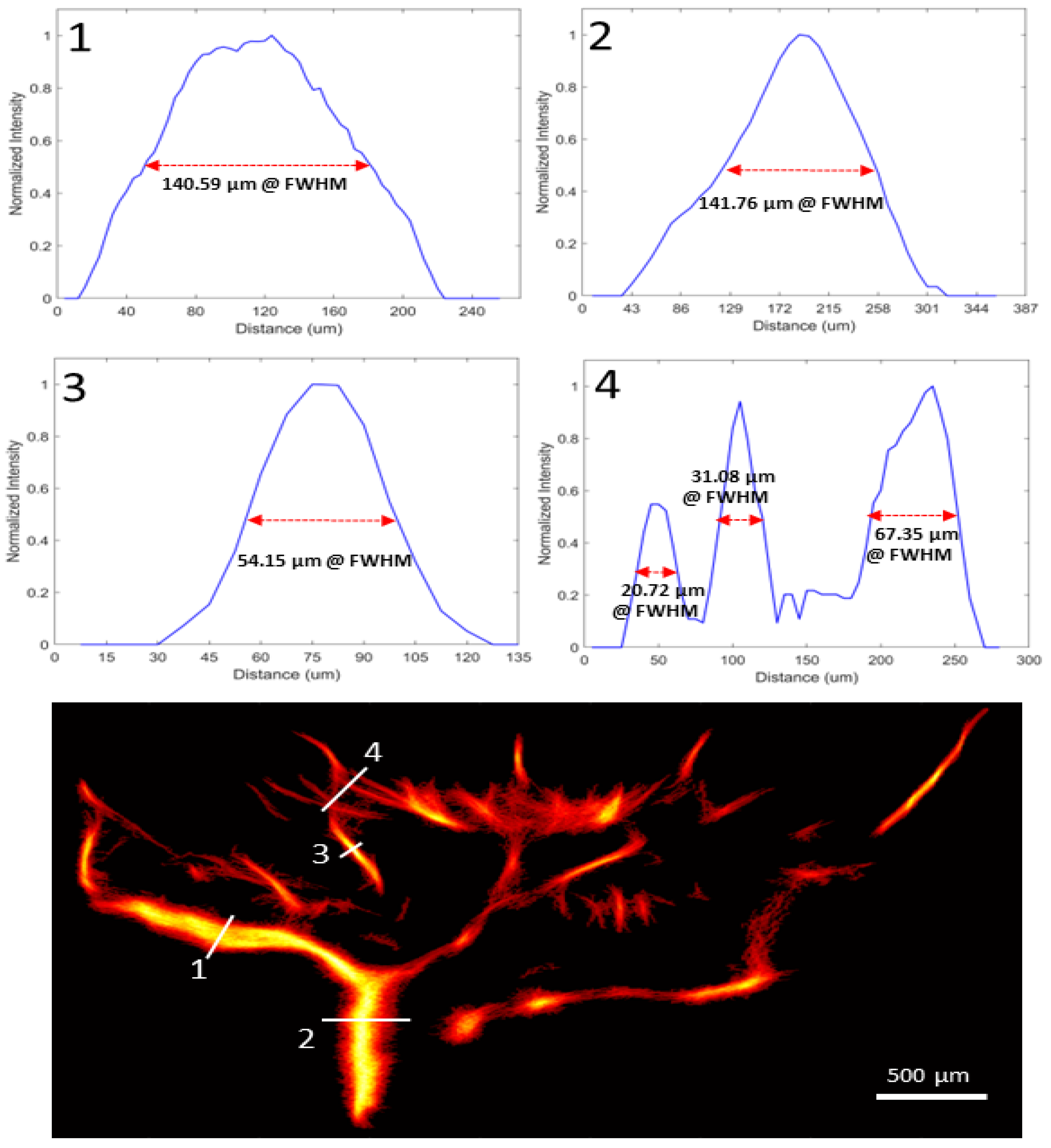
3.2. In Vivo Rat Eye Vasculature Imaging
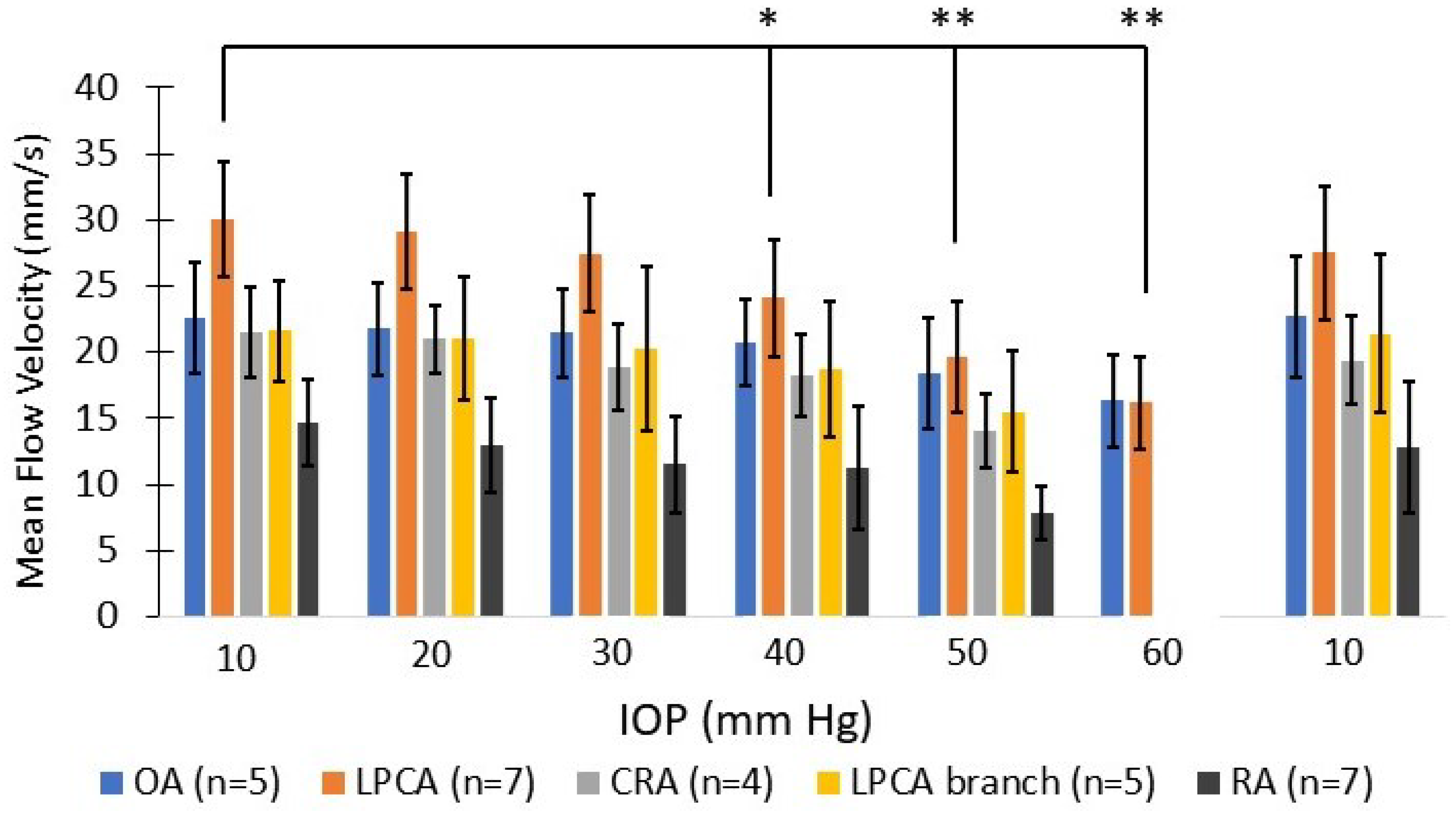
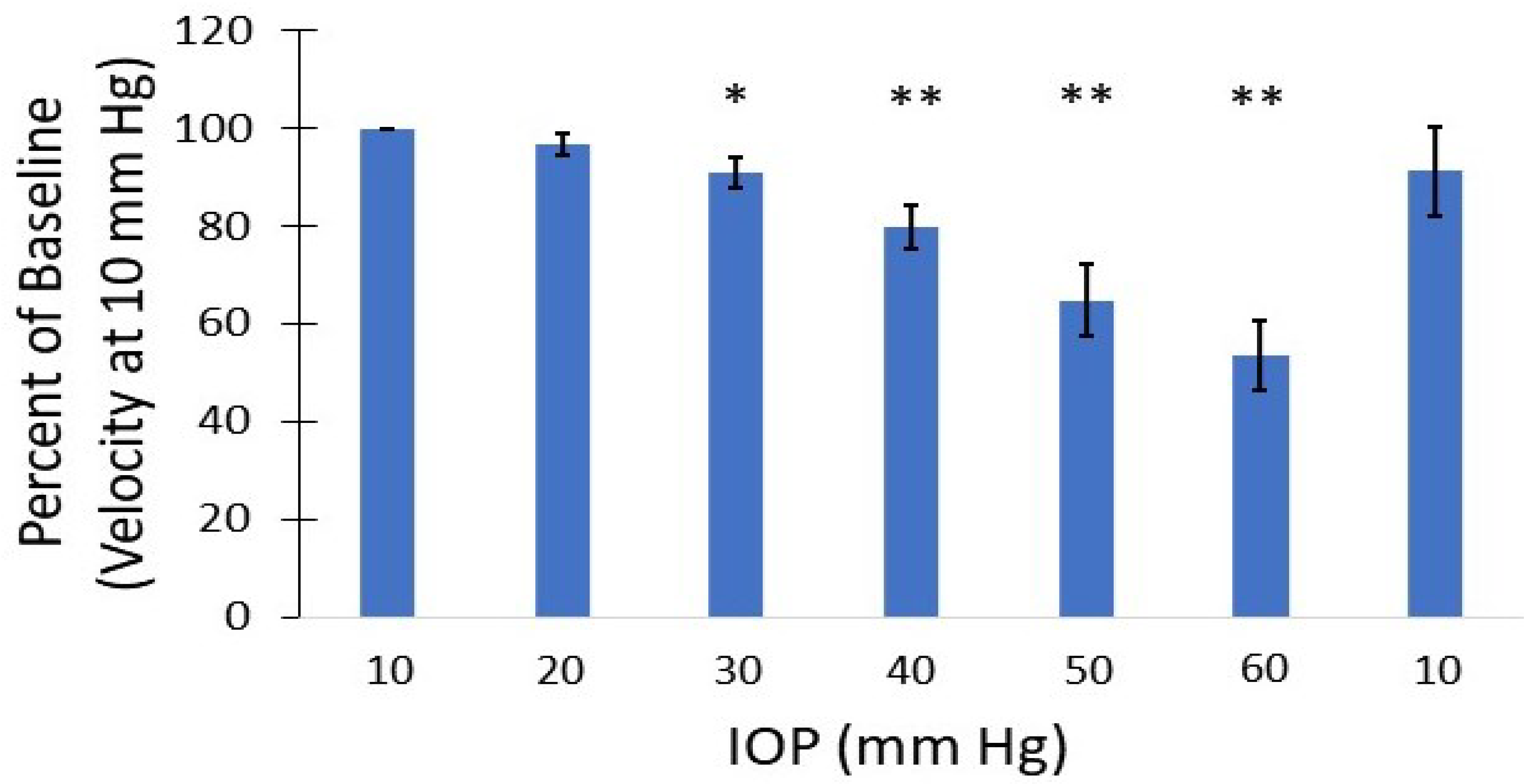
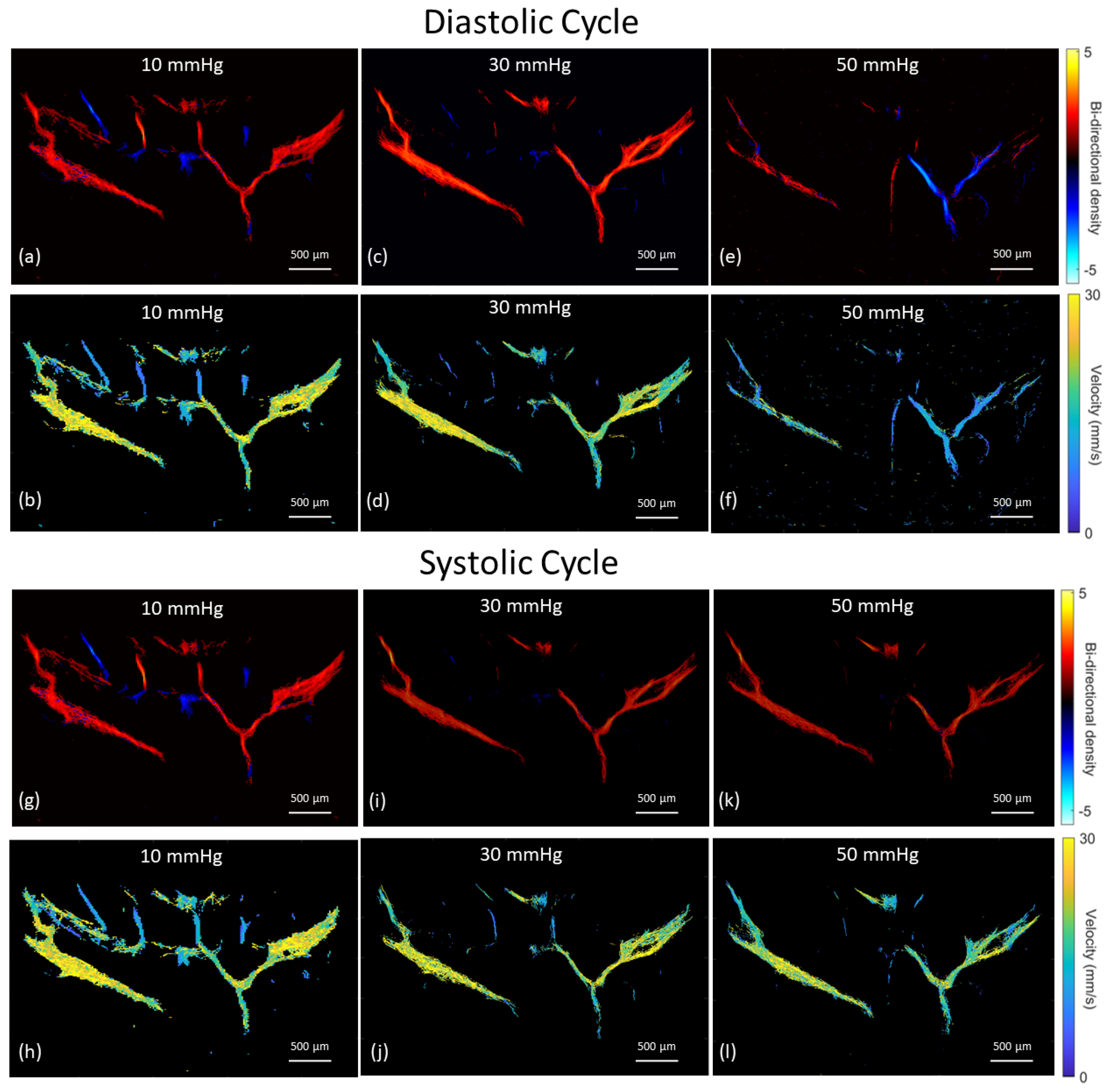
3.3. Vascular Perfusion Changes with Elevated IOP
3.4. 3D Volume Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldberg, I. Relationship between intraocular pressure and preservation of visual field in glaucoma. Surv. Ophthalmol. 2003, 48, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Miglior, S.; Bertuzzi, F. Relationship between intraocular pressure and glaucoma onset and progression. Curr. Opin. Pharmacol. 2013, 13, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, R.D.; Weinreb, R.N. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv. Ophthalmol. 1994, 39, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, C.F.; Downs, J.C.; Bellezza, A.J.; Suh, J.K.F.; Hart, R.T. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retinal Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef]
- Chuangsuwanich, T.; Hung, P.T.; Wang, X.; Liang, L.H.; Schmetterer, L.; Boote, C.; Girard, M.J.A. Morphometric, hemodynamic, and biomechanical factors influencing blood flow and oxygen concentration in the human lamina cribrosa. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3. [Google Scholar] [CrossRef]
- Costa, V.P.; Harris, A.; Anderson, D.; Stodtmeister, R.; Cremasco, F.; Kergoat, H.; Lovasik, J.; Stalmans, I.; Zeitz, O.; Lanzl, I.; et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 2014, 92, 252–266. [Google Scholar] [CrossRef]
- Schmidl, D.; Garhofer, G.; Schmetterer, L. The complex interaction between ocular perfusion pressure and ocular blood flow-relevance for glaucoma. Exp. Eye Res. 2011, 93, 141–155. [Google Scholar] [CrossRef]
- Tielsch, J.M.; Katz, J.; Sommer, A.; Quigley, H.A.; Javitt, J.C. Hypertension, perfusion pressure, and primary open-angle glaucoma: A population-based assessment. Arch. Ophthalmol. 1995, 113, 216–221. [Google Scholar] [CrossRef]
- Bonomi, L.; Marchini, G.; Marraffa, M.; Bernardi, P.; Morbio, R.; Varotto, A. Vascular risk factors for primary open angle glaucoma: The Egna-Neumarkt Study. Ophthalmology 2000, 107, 1287–1293. [Google Scholar] [CrossRef]
- Quigley, H.A.; West, S.K.; Rodriguez, J.; Munoz, B.; Klein, R.; Snyder, R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch. Ophthalmol. 2001, 119, 1819–1826. [Google Scholar] [CrossRef]
- Leske, M.C.; Wu, S.Y.; Hennis, A.; Honkanen, R.; Nemesure, B. Risk factors for incident open-angle glaucoma: The Barbados Eye Studies. Ophthalmology 2008, 115, 85–93. [Google Scholar] [CrossRef]
- Memarzadeh, F.; Ying-Lai, M.; Chung, J.; Azen, S.P.; Varma, R. Blood pressure, perfusion pressure, and open-angle glaucoma: The Los Angeles Latino Eye Study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.A.; Kim, T.W. Influence of choroidal microvasculature dropout on the rate of glaucomatous progression: A prospective study. Ophthalmol. Glauc. 2020, 3, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Sung, K.R.; Uhm, K.B.; Jo, J.; Moon, Y.; Song, M.K.; Song, J.Y. Peripapillary microvascular improvement and lamina cribrosa depth reduction after trabeculectomy in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5993–5999. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, E.J.; Kim, J.A.; Kim, H.; Kim, T.W. Progressive retinal nerve fibre layer thinning and choroidal microvasculature dropout at the location of disc haemorrhage in glaucoma. Br. J. Ophthalmol. 2021, 105, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Kim, T.-W.; Lee, E.J.; Girard, M.J.; Mari, J.M. Microvascular changes in peripapillary and optic nerve head tissues after trabeculectomy in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4614–4621. [Google Scholar] [CrossRef]
- Vinnett, A.; Kandukuri, J.; Le, C.; Cho, K.-A.; Sinha, A.; Asanad, S.; Samuel, A.; Ginger, T.; Victoria, C.; Abhishek, R.; et al. Dynamic alterations in blood flow in glaucoma measured with laser speckle contrast imaging. Ophthalmol. Glauc. 2022, 5, 250–261. [Google Scholar] [CrossRef]
- Quigley, H.A.; Addicks, E.M. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch. Ophthalmol. 1981, 99, 137–143. [Google Scholar] [CrossRef]
- Quigley, H.A. Optic nerve damage in human glaucoma, II: The site of injury and susceptibility of damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Sánchez, M. Predictive value of colour Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta. Ophthalmol. Scand. 2005, 83, 716–722. [Google Scholar]
- Abegão Pinto, L.; Willekens, K.; Van Keer, K.; Shibesh, A.; Molenberghs, G.; Vandewalle, E.; Stalmans, I. Ocular blood flow in glaucoma–the Leuven Eye Study. Acta Ophthalmol. 2016, 94, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Garhöfer, G.; Fuchsjäger-Mayrl, G.; Vass, C.; Pemp, B.; Hommer, A.; Schmetterer, L. Retrobulbar blood flow velocities in open angle glaucoma and their association with mean arterial blood pressure. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6652–6657. [Google Scholar] [CrossRef] [PubMed]
- Plange, N.; Remky, A.; Arend, O. Colour Doppler imaging and fluorescein filling defects of the optic disc in normal tension glaucoma. Br. J. Ophthalmol. 2003, 87, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H.; Urs, R.; Tezel, G.; Yang, X.; Nelson, I.; Ketterling, J.A. Retrobulbar blood flow in rat eyes during acute elevation of intraocular pressure. Exp. Eye Res. 2021, 207, 108606. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Jeffries, K.; Couture, O.; Dayton, P.A.; Eldar, Y.C.; Hynynen, K.; Kiessling, F.; O’Reilly, M.; Pinton, G.F.; Schmitz, G.; Tang, M.X.; et al. Super-resolution ultrasound imaging. Ultrasound Med. Biol. 2020, 46, 865–891. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, W.; Gong, P.; Lok, U.W.; Tang, S.; Yin, T.; Zhang, X.; Zhu, L.; Sang, M.; Song, P.; et al. Super-resolution ultrasound localization microscopy based on a high frame-rate clinical ultrasound scanner: An in-human feasibility study. Phys. Med. Biol. 2021, 66, 8. [Google Scholar] [CrossRef]
- Dencks, S.; Piepenbrock, M.; Opacic, T.; Krauspe, B.; Stickeler, E.; Kiessling, F.; Schmitz, G. Clinical pilot application of super-resolution US imaging in breast cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 517–526. [Google Scholar] [CrossRef]
- Lin, F.; Shelton, S.E.; Espíndola, D.; Rojas, J.D.; Pinton, G.; Dayton, P.A. 3-D ultrasound localization microscopy for identifying microvascular morphology features of tumor angiogenesis at a resolution beyond the diffraction limit of conventional ultrasound. Theranostics 2017, 7, 196–204. [Google Scholar] [CrossRef]
- Ghosh, D.; Xiong, F.; Sirsi, S.R.; Mattrey, R.; Brekken, R.; Kim, J.-W.; Hoyt, K. Monitoring early tumor response to vascular targeted therapy using super-resolution ultrasound imaging. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; pp. 1–4. [Google Scholar]
- Chen, Q.; Yu, J.; Rush, B.M.; Stocker, S.D.; Tan, R.J.; Kim, K. Ultrasound super-resolution imaging provides a noninvasive assessment of renal microvasculature changes during mouse acute kidney injury. Kidney Int. 2020, 98, 355–365. [Google Scholar] [CrossRef]
- Andersen, S.B.; Hoyos, C.A.V.; Taghavi, I.; Gran, F.; Hansen, K.L.; Sørensen, C.M.; Nielsen, M.B. Super-resolution ultrasound imaging of rat kidneys before and after ischemia-reperfusion. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 1169–1172. [Google Scholar]
- Qian, X.; Huang, C.; Li, R.; Song, B.J.; Tchelepi, H.; Shung, K.K.; Shigao, C.; Humayun, M.S.; Zhou, Q. Super-resolution ultrasound localization microscopy for visualization of the ocular blood flow. IEEE Trans. Biomed. Eng. 2022, 69, 1585–1594. [Google Scholar] [CrossRef]
- Morisset, C.; Dizeux, A.; Larrat, B.; Selingue, E.; Boutin, H.; Picaud, S.; Deffieux, T. Retinal functional ultrasound imaging (rfUS) for assessing neurovascular alterations: A pilot study on a rat model of dementia. Sci. Rep. 2022, 12, 19515. [Google Scholar] [CrossRef] [PubMed]
- Arnal, B.; Baranger, J.; Demene, C.; Tanter, M.; Pernot, M. In vivo real-time cavitation imaging in moving organs. Phys. Med. Biol. 2017, 62, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Foroosh, H.; Zerubia, J.B.; Berthod, M. Extension of phase correlation to subpixel registration. IEEE Trans. Image Process 2002, 11, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, J.; Hao, Y.; Jing, L.; He, Q.; Wang, G.; Luo, J. Spatiotemporal nonlocal means based denoising for ultrasound microvascular imaging. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021; pp. 1–4. [Google Scholar]
- Song, P.; Trzasko, J.D.; Manduca, A.; Huang, R.; Kadirvel, R.; Kallmes, D.F.; Chen, S. Improved super-resolution ultrasound microvessel imaging with spatiotemporal nonlocal means filtering and bipartite graph-based microbubble tracking. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 65, 149–167. [Google Scholar] [CrossRef]
- Heiles, B.; Chavignon, A.; Hingot, V.; Lopez, P.; Teston, E.; Couture, O. Performance benchmarking of microbubble-localization algorithms for ultrasound localization microscopy. Nat. Biom. Eng. 2022, 6, 605–616. [Google Scholar] [CrossRef]
- Tang, S.; Song, P.; Trzasko, J.D.; Lowerison, M.; Huang, C.; Gong, P.; Chen, S. Kalman filter-based microbubble tracking for robust super-resolution ultrasound microvessel imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1738–1751. [Google Scholar] [CrossRef]
- Morrison, J.C.; Johnson, E.C.; Cepurna, W.O.; Funk, R.H. Microvasculature of the rat optic nerve head. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1702–1709. [Google Scholar]
- Bhutto, I.A.; Amemiya, T. Microvascular architecture of the rat choroid: Corrosion cast study. Anat. Rec. Off. Publ. Am. Assoc. Anat. 2001, 264, 63–71. [Google Scholar] [CrossRef]
- Pazos, M.; Yang, H.; Gardiner, S.K.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Burgoyne, C.F. Rat optic nerve head anatomy within 3D histomorphometric reconstructions of normal control eyes. Exp. Eye Res. 2015, 139, 1–12. [Google Scholar] [CrossRef]
- Kornfield, T.E.; Newman, E.A. Measurement of retinal blood flow using fluorescently labeled red blood cells. Eneuro 2015, 2, 1–13. [Google Scholar] [CrossRef]
- Wajer, S.D.; Taomoto, M.; McLeod, D.S.; McCally, R.L.; Nishiwaki, H.; Fabry, M.E.; Lutty, G.A. Velocity measurements of normal and sickle red blood cells in the rat retinal and choroidal vasculatures. Microvasc. Res. 2000, 60, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Cepurna, W.; Johnson, E.; Shen, T.; Morrison, J.; Wang, R.K. Volumetric and quantitative imaging of retinal blood flow in rats with optical microangiography. Biomed. Opt. Express 2011, 2, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Hormel, T.T.; Wei, X.; Cepurna, W.; Camino, A.; Guo, Y.; Jia, Y. Monitoring retinal responses to acute intraocular pressure elevation in rats with visible light optical coherence tomography. Neurophotonics 2019, 6, 041104. [Google Scholar] [CrossRef]
- Yang, C.-F.; Chen, M.Y.-C.; Chen, T.I.; Cheng, C.F. Dose-dependent effects of isoflurane on cardiovascular function in rats. Tzu Chi Med. J. 2014, 26, 119–122. [Google Scholar] [CrossRef]
- Puyo, L.; Paques, M.; Atlan, M. Retinal blood flow reversal quantitatively monitored in out-of-plane vessels with laser Doppler holography. Sci. Rep. 2021, 11, 17828. [Google Scholar] [CrossRef] [PubMed]
- Bui, B.V.; He, Z.; Vingrys, A.J.; Nguyen, C.T.; Wong, V.H.; Fortune, B. Using the electroretinogram to understand how intraocular pressure elevation affects the rat retina. J. Ophthalmol. 2013, 2013, 262467. [Google Scholar] [CrossRef] [PubMed]
- Demeulenaere, O.; Bertolo, A.; Pezet, S.; Ialy-Radio, N.; Osmanski, B.; Papadacci, C.; Pernot, M. In vivo whole brain microvascular imaging in mice using transcranial 3D Ultrasound Localization Microscopy. EBioMedicine 2022, 79, 103995. [Google Scholar] [CrossRef]
- Eric, M.; WooJhon, C.; David, A.; Bernhard, B.; Allen, C.; Edward, P.; James, G. Evaluating anesthetic protocols for functional blood flow imaging in the rat eye. J. Biol. Opt. 2013, 22, 016005. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ul Banna, H.; Mitchell, B.; Chen, S.; Palko, J. Super-Resolution Ultrasound Localization Microscopy Using High-Frequency Ultrasound to Measure Ocular Perfusion Velocity in the Rat Eye. Bioengineering 2023, 10, 689. https://doi.org/10.3390/bioengineering10060689
Ul Banna H, Mitchell B, Chen S, Palko J. Super-Resolution Ultrasound Localization Microscopy Using High-Frequency Ultrasound to Measure Ocular Perfusion Velocity in the Rat Eye. Bioengineering. 2023; 10(6):689. https://doi.org/10.3390/bioengineering10060689
Chicago/Turabian StyleUl Banna, Hasan, Benjamin Mitchell, Stephen Chen, and Joel Palko. 2023. "Super-Resolution Ultrasound Localization Microscopy Using High-Frequency Ultrasound to Measure Ocular Perfusion Velocity in the Rat Eye" Bioengineering 10, no. 6: 689. https://doi.org/10.3390/bioengineering10060689
APA StyleUl Banna, H., Mitchell, B., Chen, S., & Palko, J. (2023). Super-Resolution Ultrasound Localization Microscopy Using High-Frequency Ultrasound to Measure Ocular Perfusion Velocity in the Rat Eye. Bioengineering, 10(6), 689. https://doi.org/10.3390/bioengineering10060689





