Doxorubicin and Quercetin Double Loading in Modified MCM-41 Lowered Cardiotoxicity in H9c2 Cardioblast Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Carboxylation of MCM-41
2.2.2. Quercetin and Doxorubicin Loading
2.2.3. Encapsulation Efficiency
2.2.4. Particle Size, Polydispersity Index, Zeta Potential
2.2.5. FTIR
2.2.6. TEM
2.2.7. Low-Temperature Nitrogen Adsorption
2.2.8. X-ray Powder Diffraction
2.2.9. In Vitro Drug Release
2.2.10. Release Kinetics
2.2.11. Cell Culturing and In Vitro Cell Viability Studies
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation Efficiency
3.2. Particle Size, Polydispersity Index, Zeta Potential
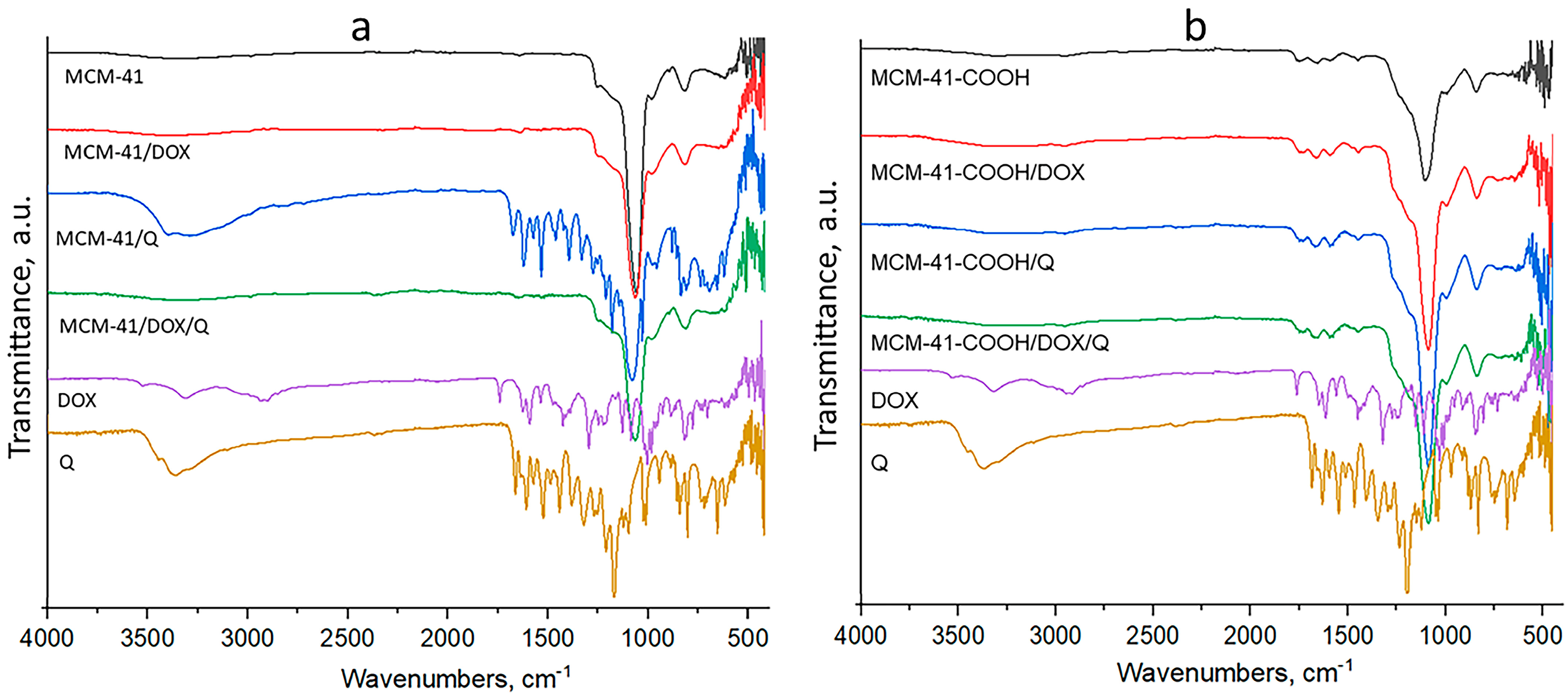
3.3. FTIR
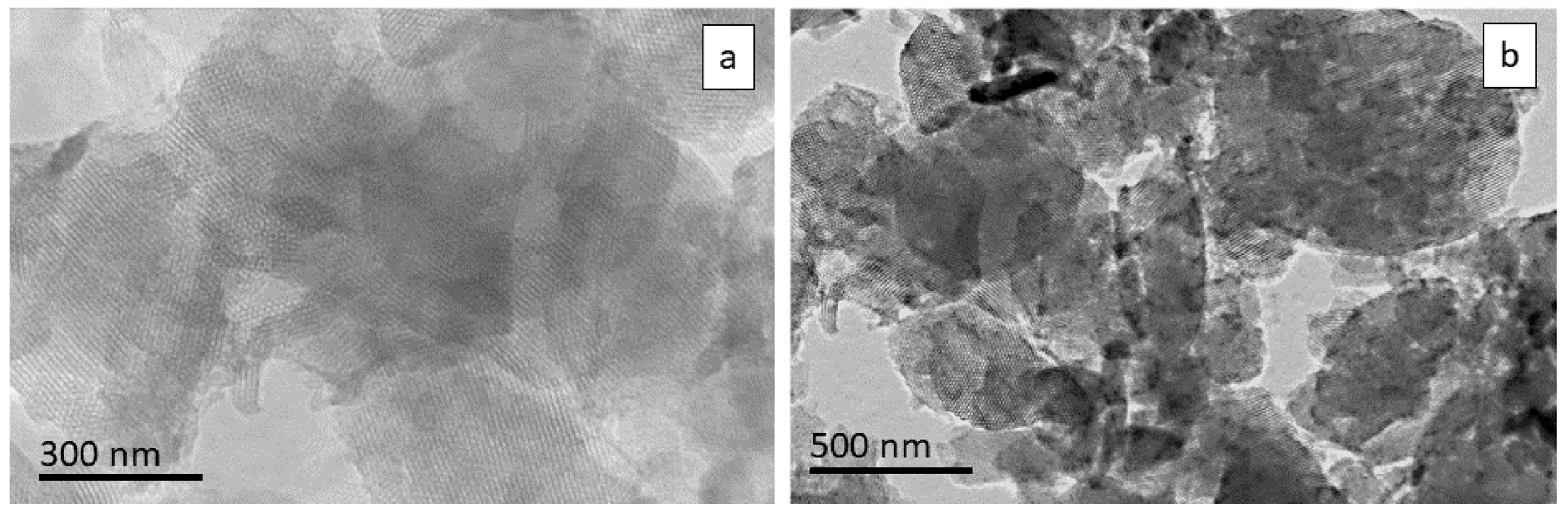
3.4. TEM
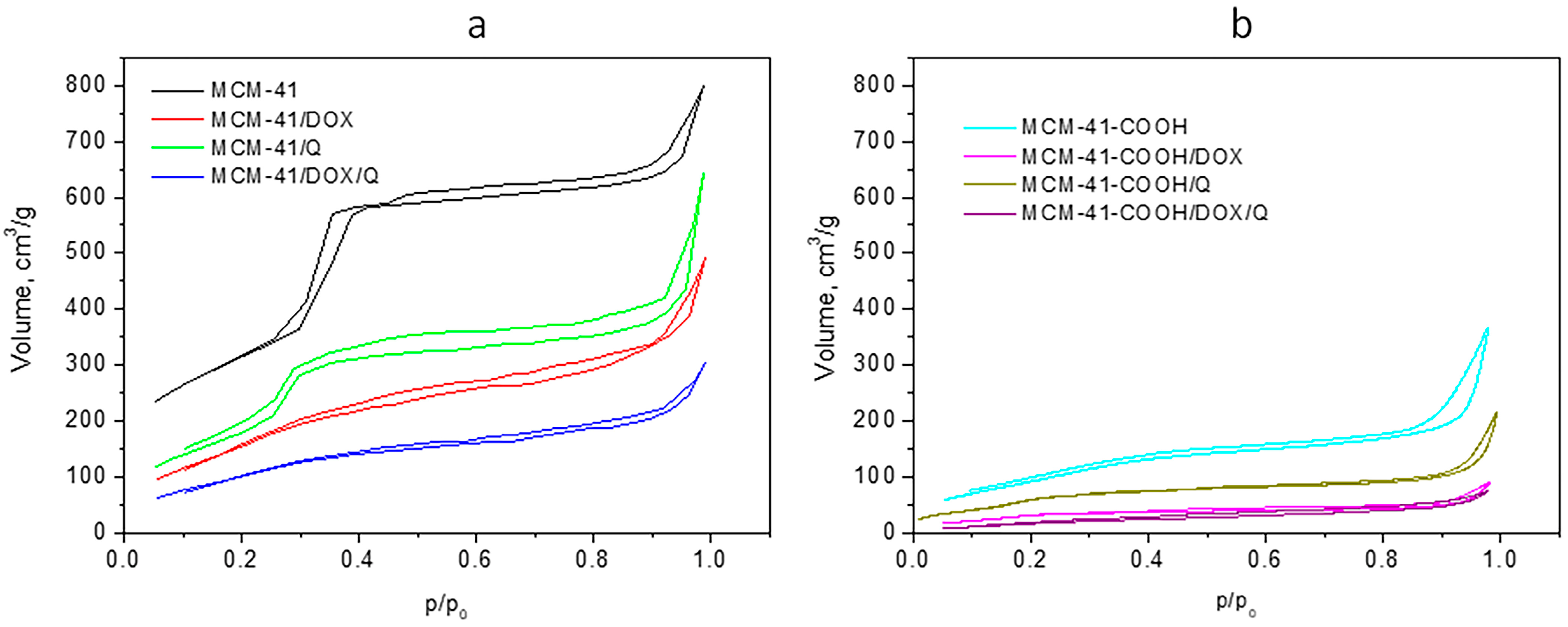
3.5. Low-Temperature Nitrogen Adsorption
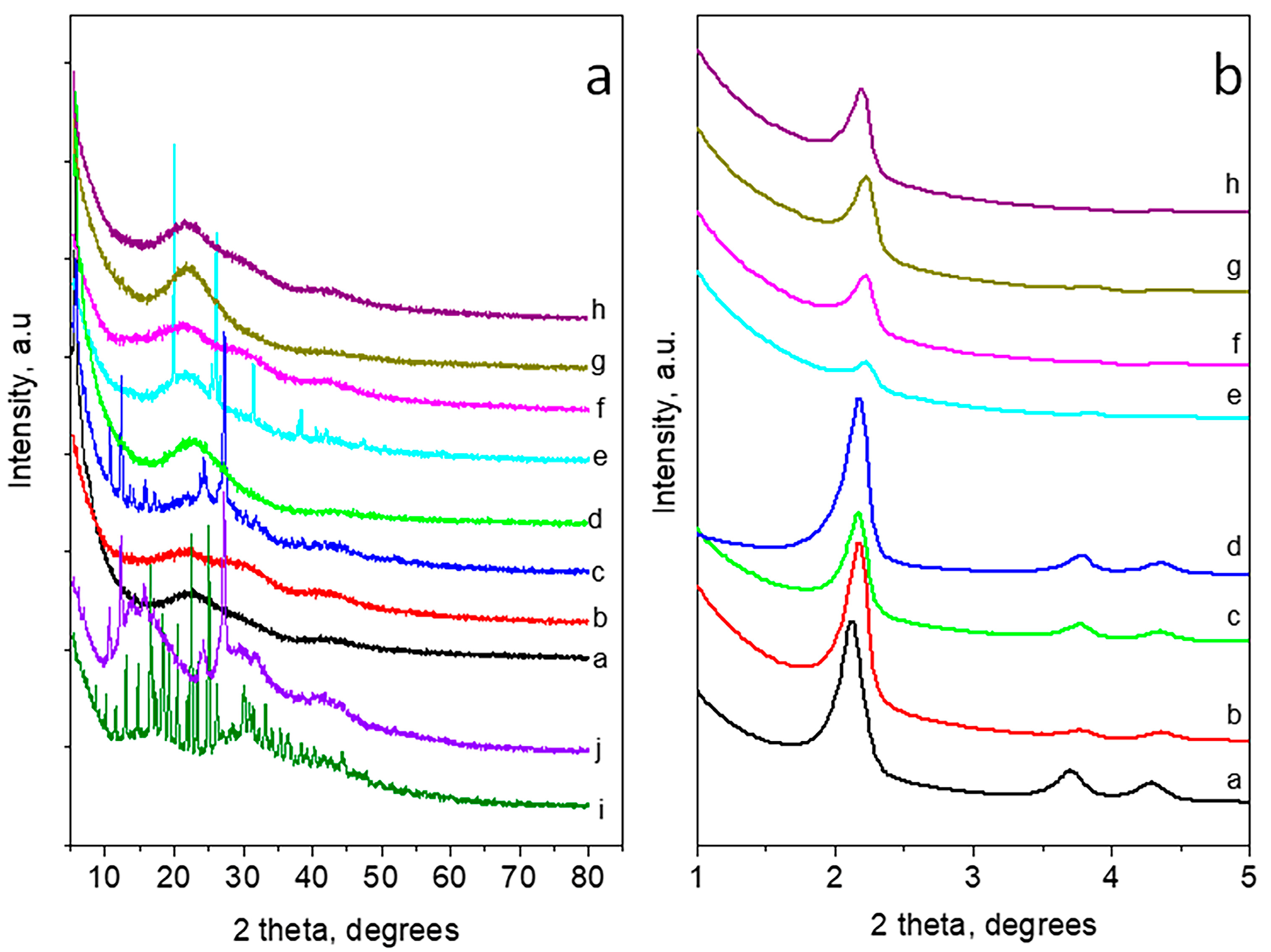
3.6. X-ray Powder Diffraction
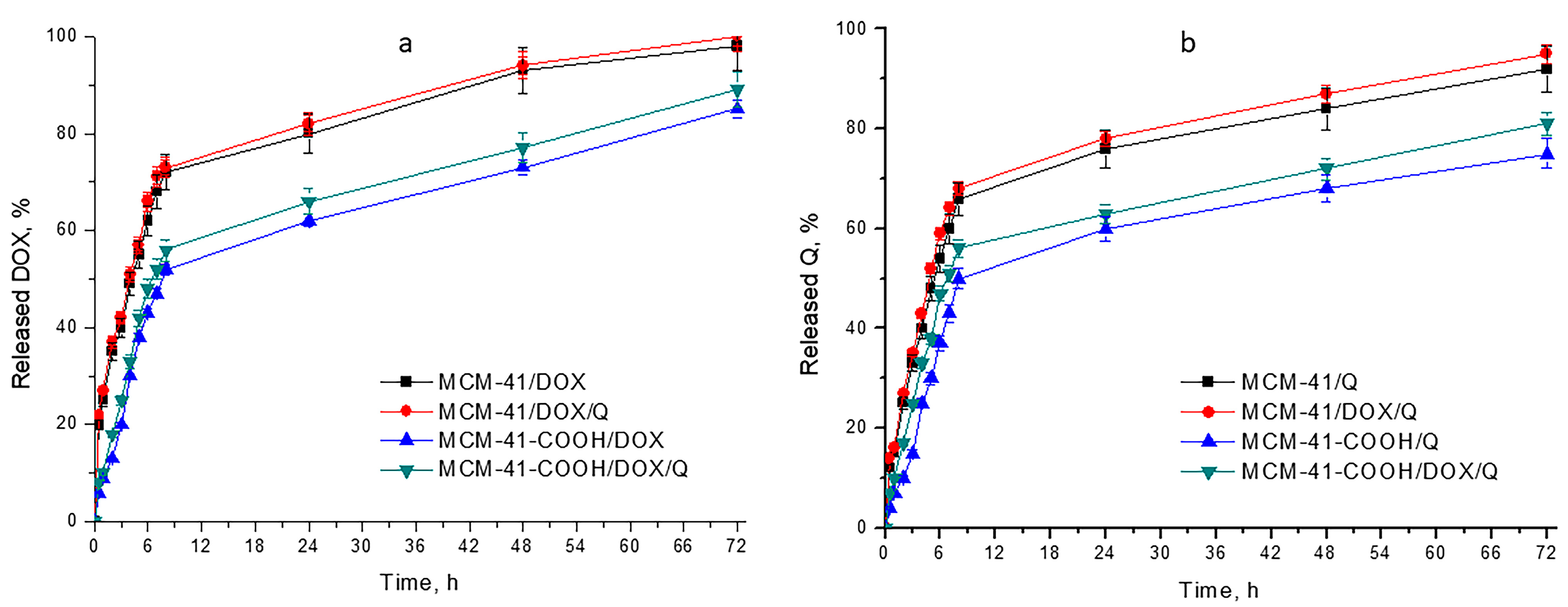
3.7. In Vitro Drug Release
3.8. Kinetic Models
3.9. In Vitro Cytotoxicity Studies on H9c2 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgiadis, N.; Tsarouhas, K.; Rezaee, R.; Nepka, H.; Kass, G.E.N.; Dorne, J.-L.C.M.; Stagos, D.; Toutouzas, K.; Spandidos, D.A.; Kouretas, D.; et al. What Is Considered Cardiotoxicity of Anthracyclines in Animal Studies. Oncol. Rep. 2020, 44, 798–818. [Google Scholar] [CrossRef] [PubMed]
- Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is It like Doxorubicin in Breast Cancer? A Clinical Review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Bober, P.; Alexovič, M.; Tomková, Z.; Kilík, R.; Sabo, J. RHOA and MDia1 Promotes Apoptosis of Breast Cancer Cells via a High Dose of Doxorubicin Treatment. Open Life Sci. 2019, 14, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Denard, B.; Lee, C.; Ye, J. Doxorubicin Blocks Proliferation of Cancer Cells through Proteolytic Activation of CREB3L1. eLife 2012, 1, e00090. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.I.; Ai, D.I. Cardiotoxicity Associated with Targeted Cancer Therapies. Mol. Clin. Oncol. 2016, 4, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Sahebkar, A. Protective Effects of Curcumin against Doxorubicin-Induced Toxicity and Resistance: A Review. Crit. Rev. Oncol. Hematol. 2018, 122, 30–51. [Google Scholar] [CrossRef]
- Ajzashokouhi, A.H.; Bostan, H.B.; Jomezadeh, V.; Hayes, A.W.; Karimi, G. A Review on the Cardioprotective Mechanisms of Metformin against Doxorubicin. Hum. Exp. Toxicol. 2020, 39, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.; Amgalan, D.; Kitsis, R.N. Grounding Cardio-Oncology in Basic and Clinical Science. Circulation 2017, 136, 3–5. [Google Scholar] [CrossRef]
- Wenningmann, N.; Knapp, M.; Ande, A.; Vaidya, T.R.; Ait-Oudhia, S. Insights into Doxorubicin-Induced Cardiotoxicity: Molecular Mechanisms, Preventive Strategies, and Early Monitoring. Mol. Pharmacol. 2019, 96, 219–232. [Google Scholar] [CrossRef]
- Wang, H.Y.J.; Jackson, S.N.; Woods, A.S. Direct MALDI-MS Analysis of Cardiolipin from Rat Organs Sections. J. Am. Soc. Mass Spectrom. 2007, 18, 567–577. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Niu, T.; Wang, H.; Li, B.; Shao, L.; Lai, Y.; Li, H.; Janicki, J.S.; Wang, X.L.; et al. Nrf2 Deficiency Exaggerates Doxorubicin-Induced Cardiotoxicity and Cardiac Dysfunction. Oxid. Med. Cell. Longev. 2014, 2014, 748524. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kumar, J.M.; Kuncha, M.; Borkar, R.M.; Srinivas, R.; Sistla, R. Baicalein Alleviates Doxorubicin-Induced Cardiotoxicity via Suppression of Myocardial Oxidative Stress and Apoptosis in Mice. Life Sci. 2016, 144, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Dua, T.K.; Das, S.; De Feo, V.; Dewanjee, S. Wheat Phenolics Suppress Doxorubicin-Induced Cardiotoxicity via Inhibition of Oxidative Stress, MAP Kinase Activation, NF-ΚB Pathway, PI3K/Akt/MTOR Impairment, and Cardiac Apoptosis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 125, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wang, Z. Genistein Protects against Doxorubicin-Induced Cardiotoxicity through Nrf-2/HO-1 Signaling in Mice Model. Environ. Toxicol. 2019, 34, 645–651. [Google Scholar] [CrossRef]
- Bharathi Priya, L.; Baskaran, R.; Huang, C.-Y.; Vijaya Padma, V. Neferine Modulates IGF-1R/Nrf2 Signaling in Doxorubicin Treated H9c2 Cardiomyoblasts. J. Cell. Biochem. 2018, 119, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and Solution Thermodynamic Properties of Quercetin and Quercetin Dihydrate in Subcritical Water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-Derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for Their Antioxidant Activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Russo, G.L.; Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Iannitti, R.; Palumbo, R. Quercetin: A Pleiotropic Kinase Inhibitor against Cancer. Cancer Treat. Res. 2014, 159, 185–205. [Google Scholar] [CrossRef]
- A Review of Quercetin: Antioxidant and Anticancer Properties—WJPPS! Available online: https://www.yumpu.com/en/document/view/16462888/a-review-of-quercetin-antioxidant-and-anticancer-properties-wjpps (accessed on 22 April 2023).
- Molina, M.F.; Sanchez-Reus, I.; Iglesias, I.; Benedi, J. Quercetin, a Flavonoid Antioxidant, Prevents and Protects against Ethanol-Induced Oxidative Stress in Mouse Liver. Biol. Pharm. Bull. 2003, 26, 1398–1402. [Google Scholar] [CrossRef]
- Gu, Y.; Li, J.; Li, Y.; Song, L.; Li, D.; Peng, L.; Wan, Y.; Hua, S. Nanomicelles Loaded with Doxorubicin and Curcumin for Alleviating Multidrug Resistance in Lung Cancer. Int. J. Nanomed. 2016, 11, 5757–5770. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, M.; Yang, T.; Ding, X.; Bao, X.; Ding, Y.; Xiong, H.; Wu, Y.; Wang, W.; Zhou, J. A Self-Assembled System for Tumor-Targeted Co-Delivery of Drug and Gene. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, M.; Yu, L.; Zhao, Y.; He, N.; Yang, X. Antitumor Activities of Quercetin and Quercetin-5′,8-Disulfonate in Human Colon and Breast Cancer Cell Lines. Food Chem. Toxicol. 2012, 50, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Fatease, A.A.; Shah, V.; Nguyen, D.X.; Cote, B.; LeBlanc, N.; Rao, D.A.; Alani, A.W.G. Chemosensitization and Mitigation of Adriamycin-Induced Cardiotoxicity Using Combinational Polymeric Micelles for Co-Delivery of Quercetin/Resveratrol and Resveratrol/Curcumin in Ovarian Cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 39–48. [Google Scholar] [CrossRef]
- Gadde, S. Multi-Drug Delivery Nanocarriers for Combination Therapy. MedChemComm 2015, 6, 1916–1929. [Google Scholar] [CrossRef]
- Preparation, Characterization of Hydrophilic and Hydrophobic Drug in Combine Loaded Chitosan/Cyclodextrin Nanoparticles and in Vitro Release Study—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0927776510006193 (accessed on 17 May 2023).
- Liu, Q.; Zhang, J.; Sun, W.; Xie, Q.R.; Xia, W.; Gu, H. Delivering Hydrophilic and Hydrophobic Chemotherapeutics Simultaneously by Magnetic Mesoporous Silica Nanoparticles to Inhibit Cancer Cells. Int. J. Nanomed. 2012, 7, 999–1013. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous Silica Nanoparticles for Therapeutic/Diagnostic Applications. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Li, H.-X.; Davis, M.E. Studies on Mesoporous Materials: I. Synthesis and Characterization of MCM-41. Microporous Mater. 1993, 2, 17–26. [Google Scholar] [CrossRef]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The Biocompatibility of Mesoporous Silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Pagano, C.; Latterini, L.; Marmottini, F.; Ricci, M.; Rossi, C. MCM-41 for Furosemide Dissolution Improvement. Microporous Mesoporous Mater. 2012, 147, 343. [Google Scholar] [CrossRef]
- Tzankov, B.; Voycheva, C.; Aluani, D.; Yordanov, Y.; Avramova, K.; Tzankova, V.; Spassova, I.; Kovacheva, D.; Yoncheva, K. Improvement of Dissolution of Poorly Soluble Glimepiride by Loading on Two Types of Mesoporous Silica Carriers. J. Solid State Chem. 2019, 271, 253–259. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Na, J.; Lee, C.-H.; Sun, Z.; Wang, S.-B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.-Z.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, e1907035. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, M.; Sinatra, L. PH-Responsive Porphyrin-Silica Nanoparticles Conjugate via Ionic Self-Assembly. J. Porous Mater. 2021, 28, 183–189. [Google Scholar] [CrossRef]
- Zaharudin, N.S.; Mohamed Isa, E.D.; Ahmad, H.; Abdul Rahman, M.B.; Jumbri, K. Functionalized Mesoporous Silica Nanoparticles Templated by Pyridinium Ionic Liquid for Hydrophilic and Hydrophobic Drug Release Application. J. Saudi Chem. Soc. 2020, 24, 289–302. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Stoica Guzun, A.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef]
- Exploiting the Zwitterionic Properties of Lomefloxacin to Tailor Its Delivery from Functionalized MCM-41 Silica|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Exploiting-the-zwitterionic-properties-of-to-tailor-Deaconu-Brezoiu/8564fa7b0d4f25e35afd790533e239a1507a7828 (accessed on 22 April 2023).
- She, X.; Chen, L.; Li, C.; He, C.; He, L.; Kong, L. Functionalization of Hollow Mesoporous Silica Nanoparticles for Improved 5-FU Loading. J. Nanomater. 2015, 2015, e872035. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Wu, C.-E.; Yang, Y.-C.; Kao, H.-M. PH Responsive Selective Protein Adsorption by Carboxylic Acid Functionalized Large Pore Mesoporous Silica Nanoparticles SBA-1. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 344–356. [Google Scholar] [CrossRef]
- Molecular Simulation Approach to the Rational Design of Self-Assembled Nanoparticles for Enhanced Peroral Delivery of Doxorubicin—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0144861719304953?via%3Dihub (accessed on 17 May 2023).
- Popova, M.D.; Szegedi, Á.; Kolev, I.N.; Mihály, J.; Tzankov, B.S.; Momekov, G.T.; Lambov, N.G.; Yoncheva, K.P. Carboxylic Modified Spherical Mesoporous Silicas as Drug Delivery Carriers. Int. J. Pharm. 2012, 436, 778–785. [Google Scholar] [CrossRef]
- Trzeciak, K.; Chotera-Ouda, A.; Bak-Sypien, I.I.; Potrzebowski, M.J. Mesoporous Silica Particles as Drug Delivery Systems—The State of the Art in Loading Methods and the Recent Progress in Analytical Techniques for Monitoring These Processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ronhovde, C.J.; Baer, J.; Larsen, S.C. Effects of Pore Topology and Iron Oxide Core on Doxorubicin Loading and Release from Mesoporous Silica Nanoparticles. J. Nanoparticle Res. 2017, 19, 215. [Google Scholar] [CrossRef]
- Patel, K.; Sundara Raj, B.; Chen, Y.; Lou, X. Cytotoxicity of Folic Acid Conjugated Hollow Silica Nanoparticles toward Caco2 and 3T3 Cells, with and without Encapsulated DOX. Colloids Surf. B Biointerfaces 2016, 140, 213–222. [Google Scholar] [CrossRef]
- Size-Controlled Functionalized Mesoporous Silica Nanoparticles for Tunable Drug Release and Enhanced Anti-Tumoral Activity | Chemistry of Materials. Available online: https://pubs.acs.org/doi/10.1021/acs.chemmater.6b00877 (accessed on 17 May 2023).
- Song, F.; Li, Y.; Wang, S.; Zhang, L.; Chen, Q. Multifunctional Dual-Mesoporous Silica Nanoparticles Loaded with a Protein and Dual Antitumor Drugs as a Targeted Delivery System. New J. Chem. 2019, 43, 17284–17297. [Google Scholar] [CrossRef]
- Dasgupta, D.; Das, M.; Thakore, S.; Patel, A.; Kumar, S.; Seshadri, S. Development of a Controlled Sustainable Anticancer Drug Delivery Nanosystem Comprising Doxorubicin and Functionalized MCM-48. J. Drug Deliv. Sci. Technol. 2022, 72, 103419. [Google Scholar] [CrossRef]
- Santos, E.C.S.; dos Santos, T.C.; Fernandes, T.S.; Jorge, F.L.; Nascimento, V.; Madriaga, V.G.C.; Cordeiro, P.S.; Checca, N.R.; Costa, N.M.D.; Pinto, L.F.R.; et al. A Reversible, Switchable PH-Driven Quaternary Ammonium Pillar[5]Arene Nanogate for Mesoporous Silica Nanoparticles. J. Mater. Chem. B 2020, 8, 703–714. [Google Scholar] [CrossRef]
- Adsorption of Antitumor Antibiotic Doxorubicin on MCM-41-Type Silica Surface—Nadiia V Roik, Lyudmila A Belyakova, Marina O Dziazko. 2017. Available online: https://journals.sagepub.com/doi/10.1177/0263617416669504 (accessed on 17 May 2023).
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-Bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive Mesoporous Silica Nanoparticles as a Carrier for Skin Delivery of Quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef]
- Ghanimati, M.; Jabbari, M.; Farajtabar, A.; Nabavi-Amri, S.A. Adsorption Kinetics and Isotherms of Bioactive Antioxidant Quercetin onto Amino-Functionalized Silica Nanoparticles in Aqueous Ethanol Solutions. New J. Chem. 2017, 41, 8451–8458. [Google Scholar] [CrossRef]
- Berlier, G.; Gastaldi, L.; Ugazio, E.; Miletto, I.; Iliade, P.; Sapino, S. Stabilization of Quercetin Flavonoid in MCM-41 Mesoporous Silica: Positive Effect of Surface Functionalization. J. Colloid Interface Sci. 2013, 393, 109–118. [Google Scholar] [CrossRef]
- Tzankov, B.; Voycheva, C.; Tosheva, A.; Aluani, D.; Tzankova, V.; Spassova, I.; Kovacheva, D.; Avramova, K.; Tzankova, D.; Yoncheva, K. Novel Olegels for Topical Delivery of Quercetin Based on Mesoporous Silica MCM-41 and HMS Particles. JDDST 2023. submitted. [Google Scholar]
- Hartono, S.B.; Hadisoewignyo, L.; Yang, Y.; Meka, A.K.; Antaresti; Yu, C. Amine Functionalized Cubic Mesoporous Silica Nanoparticles as an Oral Delivery System for Curcumin Bioavailability Enhancement. Nanotechnology 2016, 27, 505605. [Google Scholar] [CrossRef]
- Sábio, R.M.; Meneguin, A.B.; Martins dos Santos, A.; Monteiro, A.S.; Chorilli, M. Exploiting Mesoporous Silica Nanoparticles as Versatile Drug Carriers for Several Routes of Administration. Microporous Mesoporous Mater. 2021, 312, 110774. [Google Scholar] [CrossRef]
- Romero, A.A.; Alba, M.D.; Zhou, W.; Klinowski, J. Synthesis and Characterization of the Mesoporous Silicate Molecular Sieve MCM-48. J. Phys. Chem. B 1997, 101, 5294–5300. [Google Scholar] [CrossRef]
- Yoncheva, K.; Tzankov, B.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Frosini, M.; Valoti, M.; Tzankova, V. Encapsulation of Doxorubicin in Chitosan-Alginate Nanoparticles Improves Its Stability and Cytotoxicity in Resistant Lymphoma L5178 MDR Cells. J. Drug Deliv. Sci. Technol. 2020, 59, 101870. [Google Scholar] [CrossRef]
- Maghsoudi, M.; Abbasian, M.; Farhadi, K.; Mahmoodzadeh, F.; Ghorbani, M.; Hoseinzadeh, M. Mesoporous Si-MCM-41/Polymer as a PH-Responsive Drug Delivery System for Cancer Therapy. ChemistrySelect 2020, 5, 11901–11909. [Google Scholar] [CrossRef]
- Mahmoodzadeh, F.; Hosseinzadeh, M.; Jannat, B.; Ghorbani, M. Fabrication and Characterization of Gold Nanospheres-Cored PH-Sensitive Thiol-Ended Triblock Copolymer: A Smart Drug Delivery System for Cancer Therapy. Polym. Adv. Technol. 2019, 30, 1344–1355. [Google Scholar] [CrossRef]
- Hazra, M.; Dasgupta Mandal, D.; Mandal, T.; Bhuniya, S.; Ghosh, M. Designing Polymeric Microparticulate Drug Delivery System for Hydrophobic Drug Quercetin. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2015, 23, 429–436. [Google Scholar] [CrossRef]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Sousa, E.; Doadrio, J.C.; Doadrio, A.L.; Pariente, J.P.; Martínez, A.; Babonneau, F.; Vallet-Regí, M. Influence of Mesoporous Structure Type on the Controlled Delivery of Drugs: Release of Ibuprofen from MCM-48, SBA-15 and Functionalized SBA-15. J. Sol-Gel Sci. Technol. 2009, 50, 421–429. [Google Scholar] [CrossRef]
- Lim, E.-B.; Vy, T.A.; Lee, S.-W. Comparative Release Kinetics of Small Drugs (Ibuprofen and Acetaminophen) from Multifunctional Mesoporous Silica Nanoparticles. J. Mater. Chem. B 2020, 8, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.A.; Lee, S.-W. A Prominent Anchoring Effect on the Kinetic Control of Drug Release from Mesoporous Silica Nanoparticles (MSNs). J. Colloid Interface Sci. 2018, 510, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative Stress Injury in Doxorubicin-Induced Cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef] [PubMed]

| DOX-EE (% ± SD) | Q-EE (% ± SD) | Z-Potential, mV | PDI | Average Size, nm | |
|---|---|---|---|---|---|
| MCM-41 | - | - | −24.0 | 0.36 | 415 ± 18 |
| MCM-41/DOX | 41 ± 4.9% | - | −22.7 | 0.45 | 480 ± 14 |
| MCM-41/Q | - | 38 ± 3.9% | −21.9 | 0.50 | 486 ± 6 |
| MCM-41/DOX/Q | 43± 4.1% | 37 ± 4.5% | −22.1 | 0.56 | 490 ± 12 |
| MCM-41-COOH | - | - | −29.1 | 0.31 | 580 ± 16 |
| MCM-41-COOH/DOX | 48 ± 3.7% | - | −26.6 | 0.35 | 603 ± 24 |
| MCM-41-COOH/Q | - | 36 ± 4.4% | −24.9 | 0.36 | 606 ± 15 |
| MCM-41-COOH/DOX/Q | 49 ± 4.3% | 36 ± 4.0% | −25.1 | 0.42 | 616 ± 7 |
| Sample | SBET m2/g | Vt cm3/g | Dav nm |
|---|---|---|---|
| MCM-41 | 1139 | 1.24 | 4.0 |
| MCM-41/DOX | 658 | 0.76 | 4.6 |
| MCM-41/Q | 860 | 0.88 | 4.0 |
| MCM-41/DOX/Q | 420 | 0.47 | 4.5 |
| MCM-41-COOH | 380 | 0.61 | 6.4 |
| MCM-41-COOH/DOX | 222 | 0.17 | 3.1 |
| MCM-41-COOH/Q | 267 | 0.34 | 5.1 |
| MCM-41-COOH/DOX/Q | 75 | 0.12 | 6.6 |
| pH 1.2 | pH 5.0 | pH 6.8 | |
|---|---|---|---|
| T50 of DOX, h | |||
| MCM-41/DOX | 3.33 ± 0.17 | 3.92 ± 0.32 | 5.1 ± 0.31 |
| MCM-41/DOX/Q | 3.06 ± 0.25 | 3.6 ± 0.24 | 4.68 ± 0.24 |
| MCM-41-COOH/DOX | 6.33 ± 0.37 | 7.45 ± 0.29 | 9.69 ± 0.16 |
| MCM-41-COOH/DOX/Q | 5.16 ± 0.36 | 6.07 ± 0.16 | 7.89 ± 0.31 |
| T50 of Q, h | |||
| MCM-41/Q | 6.76 ± 0.24 | 5.2 ± 0.33 | 4.41 ± 0.22 |
| MCM-41/DOX/Q | 6.08 ± 0.19 | 4.68 ± 0.21 | 3.98 ± 0.18 |
| MCM-41-COOH/Q | 10.67 ± 0.28 | 8.22 ± 0.09 | 6.98 ± 0.31 |
| MCM-41-COOH/DOX/Q | 9.05 ± 0.43 | 6.97 ± 0.14 | 5.91 ± 0.22 |
| Zero-Order Qt = Q0 − k0t | First-Order lnQt = lnQ0 − k1t | Higuchi Qt = kHt1/2 | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | k | R2 | k | R2 | k | R2 | n | |
| DOX release | ||||||||
| MCM-41/DOX | 0.6538 | 0.0066 | 0.4695 | 0.0003 | 0.8252 | 1.2311 | 0.9587 | 0.6877 |
| MCM-41/DOX/Q | 0.6455 | 0.0065 | 0.4698 | 0.0002 | 0.8173 | 1.2205 | 0.9758 | 0.5376 |
| MCM-41-COOH/DOX | 0.7164 | 0.0069 | 0.4298 | 0.0004 | 0.8676 | 1.2668 | 0.9971 | 0.4284 |
| MCM-41-COOH/DOX/Q | 0.6993 | 0.007 | 0.4264 | 0.0004 | 0.8554 | 1.2829 | 0.9982 | 0.4252 |
| Q release | ||||||||
| MCM-41/Q | 0.6416 | 0.0068 | 0.4204 | 0.0003 | 0.8159 | 1.2712 | 0.9596 | 0.6636 |
| MCM-41/DOX/Q | 0.6272 | 0.0068 | 0.4157 | 0.0003 | 0.8024 | 1.2823 | 0.9798 | 0.5185 |
| MCM-41-COOH/Q | 0.6852 | 0.0064 | 0.4124 | 0.0005 | 0.8470 | 1.1937 | 0.9621 | 0.5160 |
| MCM-41-COOH/DOX/Q | 0.6489 | 0.0062 | 0.3958 | 0.0004 | 0.8168 | 1.1652 | 0.9770 | 0.4737 |
| Treatment | IC50 (μM) | 95% Confidence Interval |
|---|---|---|
| DOX | 0.972 | 0.859 to 1.4851 |
| DOX + Q | 1.33 | 0.923 to 1.986 |
| MCM-41/DOX | 1.636 | 1.122 to 2.386 |
| MCM-41/DOX/Q | 3.028 | 2.236 to 4.102 |
| MCM-41-COOH/DOX | 2.756 | 1.849 to 3.264 |
| MCM-41-COOH/DOX/Q | 5.964 | 4.698 to 6.271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voycheva, C.; Popova, T.; Slavkova, M.; Tzankova, V.; Stefanova, D.; Tzankova, D.; Spassova, I.; Kovacheva, D.; Tzankov, B. Doxorubicin and Quercetin Double Loading in Modified MCM-41 Lowered Cardiotoxicity in H9c2 Cardioblast Cells In Vitro. Bioengineering 2023, 10, 637. https://doi.org/10.3390/bioengineering10060637
Voycheva C, Popova T, Slavkova M, Tzankova V, Stefanova D, Tzankova D, Spassova I, Kovacheva D, Tzankov B. Doxorubicin and Quercetin Double Loading in Modified MCM-41 Lowered Cardiotoxicity in H9c2 Cardioblast Cells In Vitro. Bioengineering. 2023; 10(6):637. https://doi.org/10.3390/bioengineering10060637
Chicago/Turabian StyleVoycheva, Christina, Teodora Popova, Marta Slavkova, Virginia Tzankova, Denitsa Stefanova, Diana Tzankova, Ivanka Spassova, Daniela Kovacheva, and Borislav Tzankov. 2023. "Doxorubicin and Quercetin Double Loading in Modified MCM-41 Lowered Cardiotoxicity in H9c2 Cardioblast Cells In Vitro" Bioengineering 10, no. 6: 637. https://doi.org/10.3390/bioengineering10060637
APA StyleVoycheva, C., Popova, T., Slavkova, M., Tzankova, V., Stefanova, D., Tzankova, D., Spassova, I., Kovacheva, D., & Tzankov, B. (2023). Doxorubicin and Quercetin Double Loading in Modified MCM-41 Lowered Cardiotoxicity in H9c2 Cardioblast Cells In Vitro. Bioengineering, 10(6), 637. https://doi.org/10.3390/bioengineering10060637













