Differences in Water Dynamics between the Hydrated Chitin and Hydrated Chitosan Determined by Quasi-Elastic Neutron Scattering
Abstract
1. Introduction
2. Samples and Experiments
2.1. Chitin Samples
2.2. QENS Measurements
3. Results
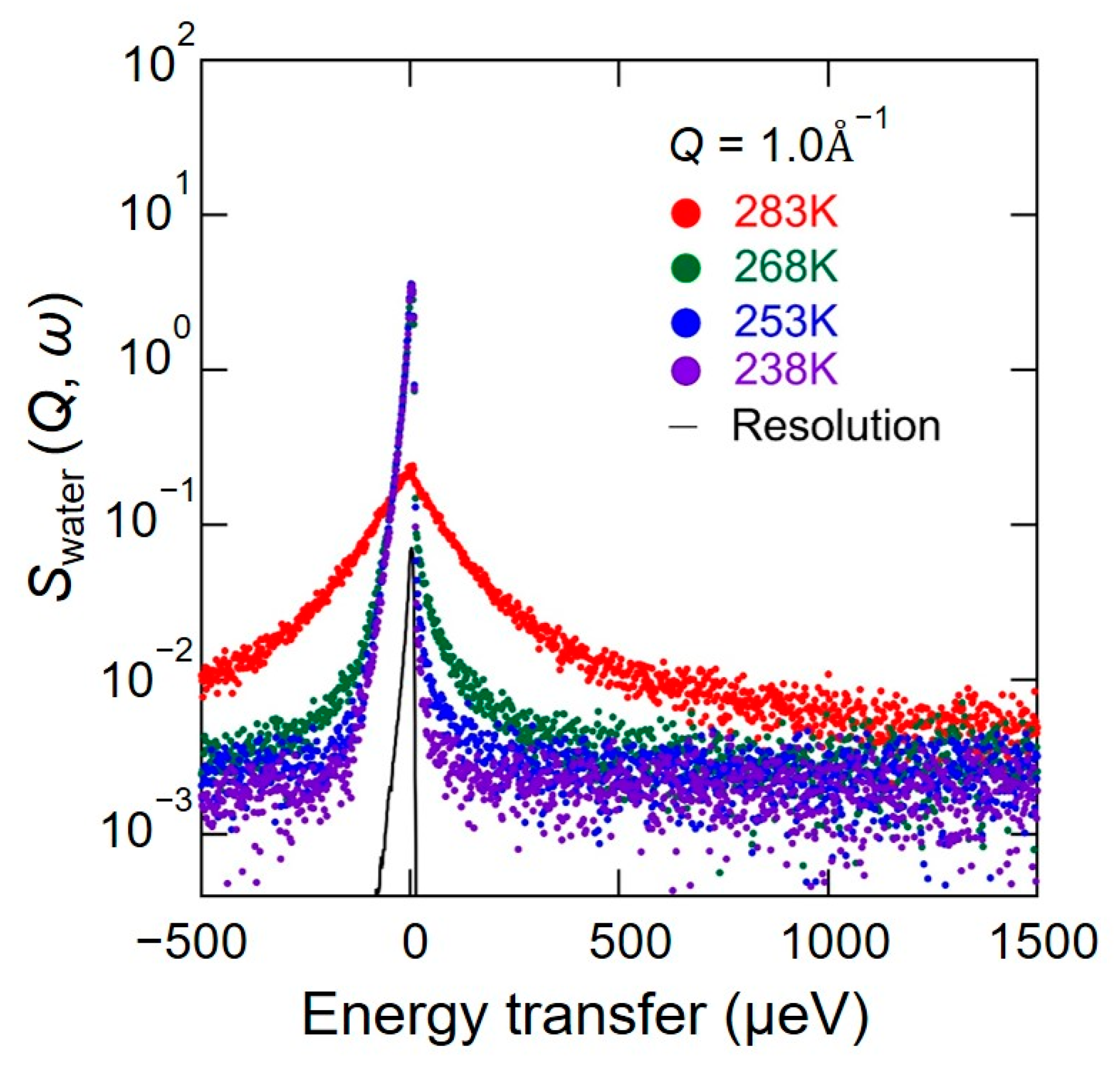
3.1. Quasi-Elastic Neutron Scattering Signals in Chitin; Swater (Q, ω)
3.2. Quasi-Elastic Neutron Scattering Signals in Chitin; Satom (Q, ω)
4. Results of QENS Spectrum Analysis
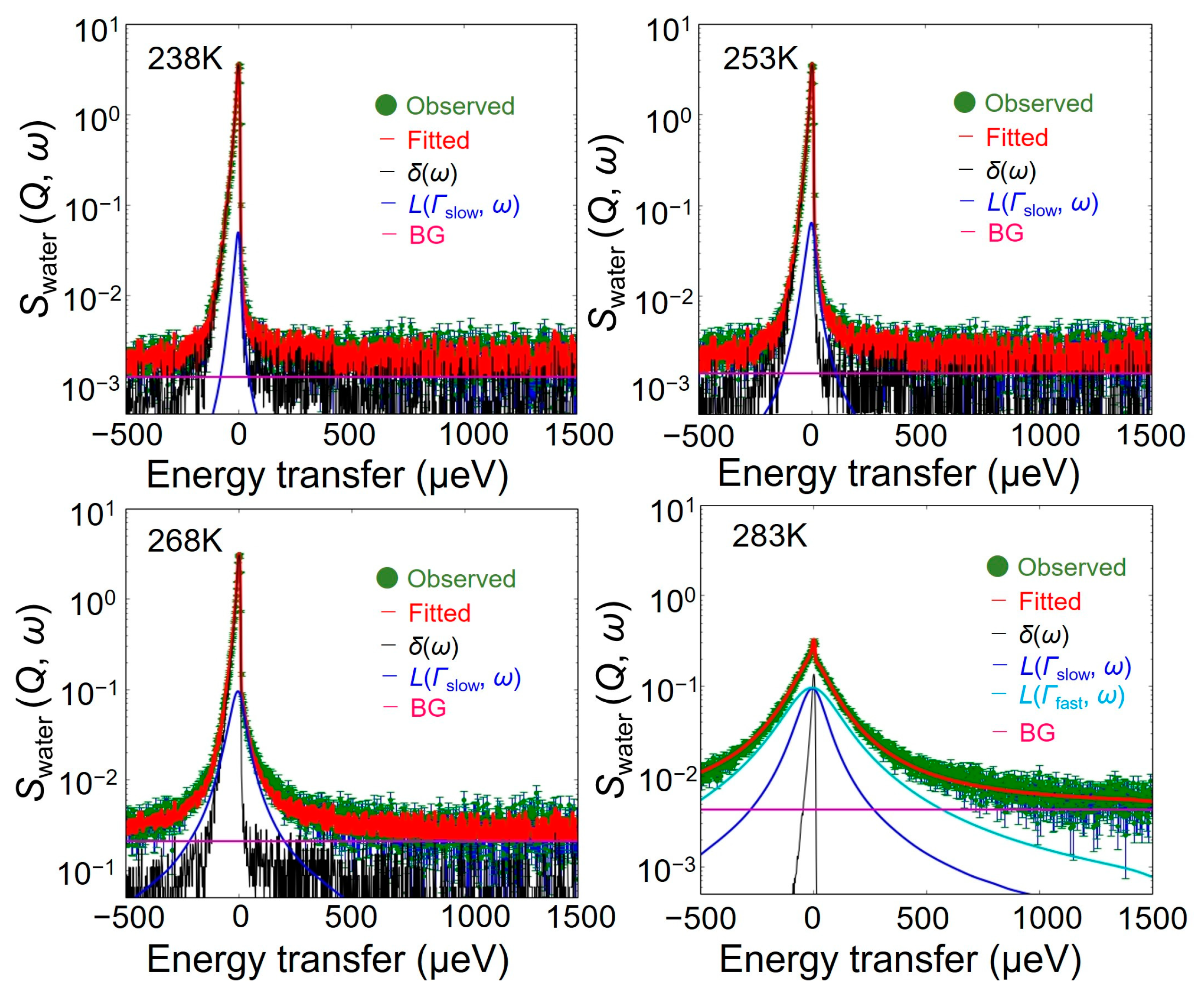
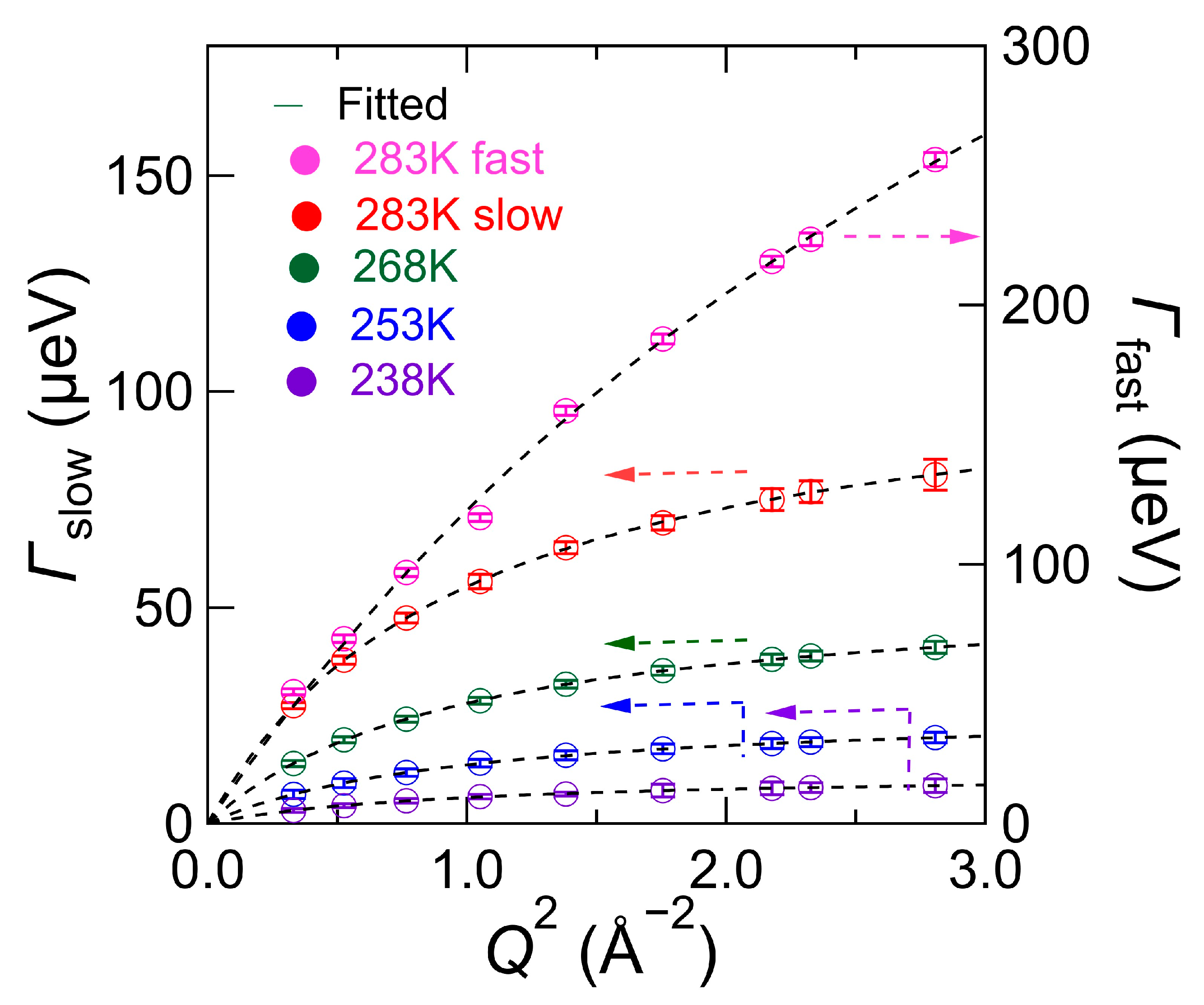
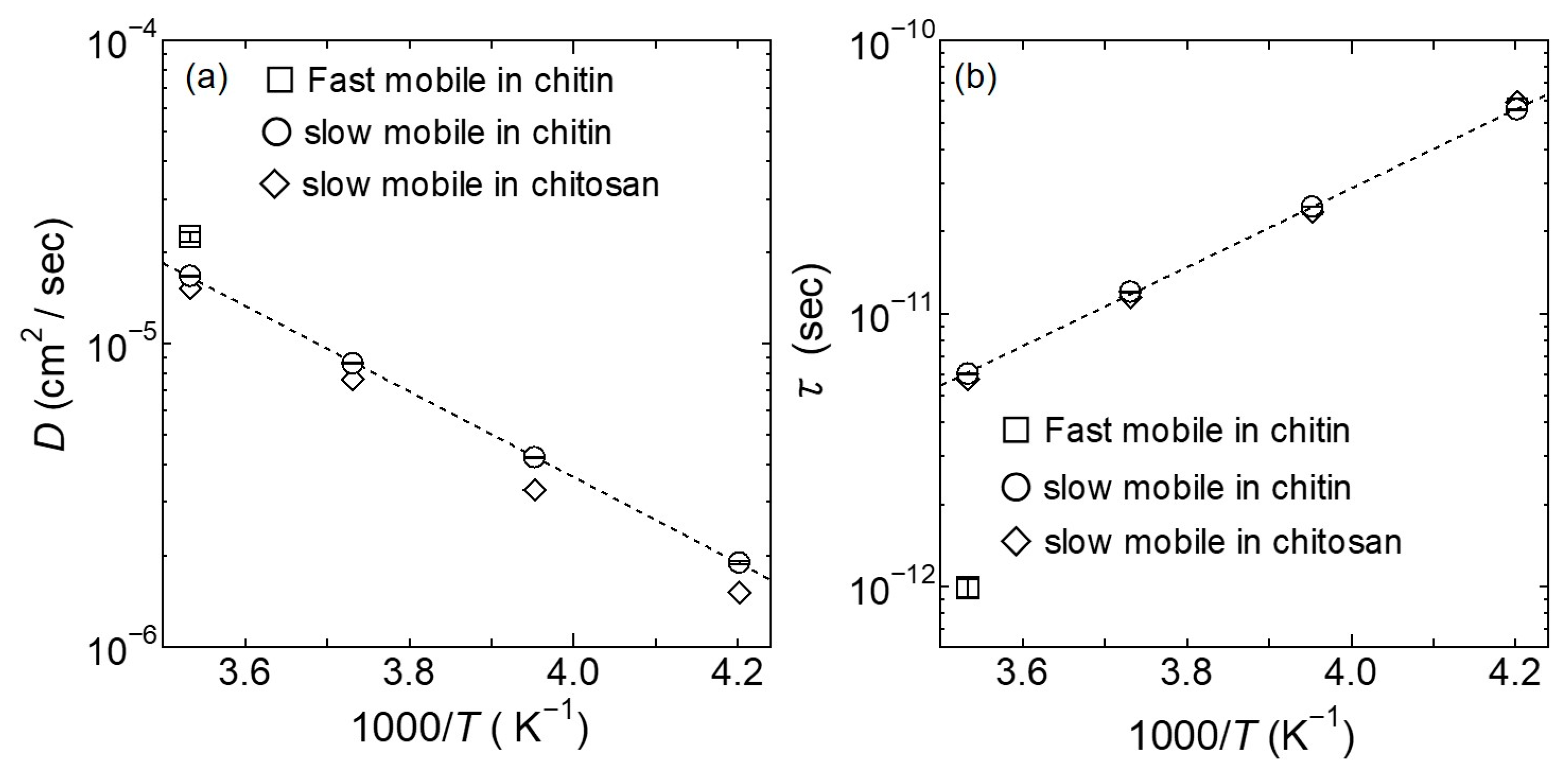
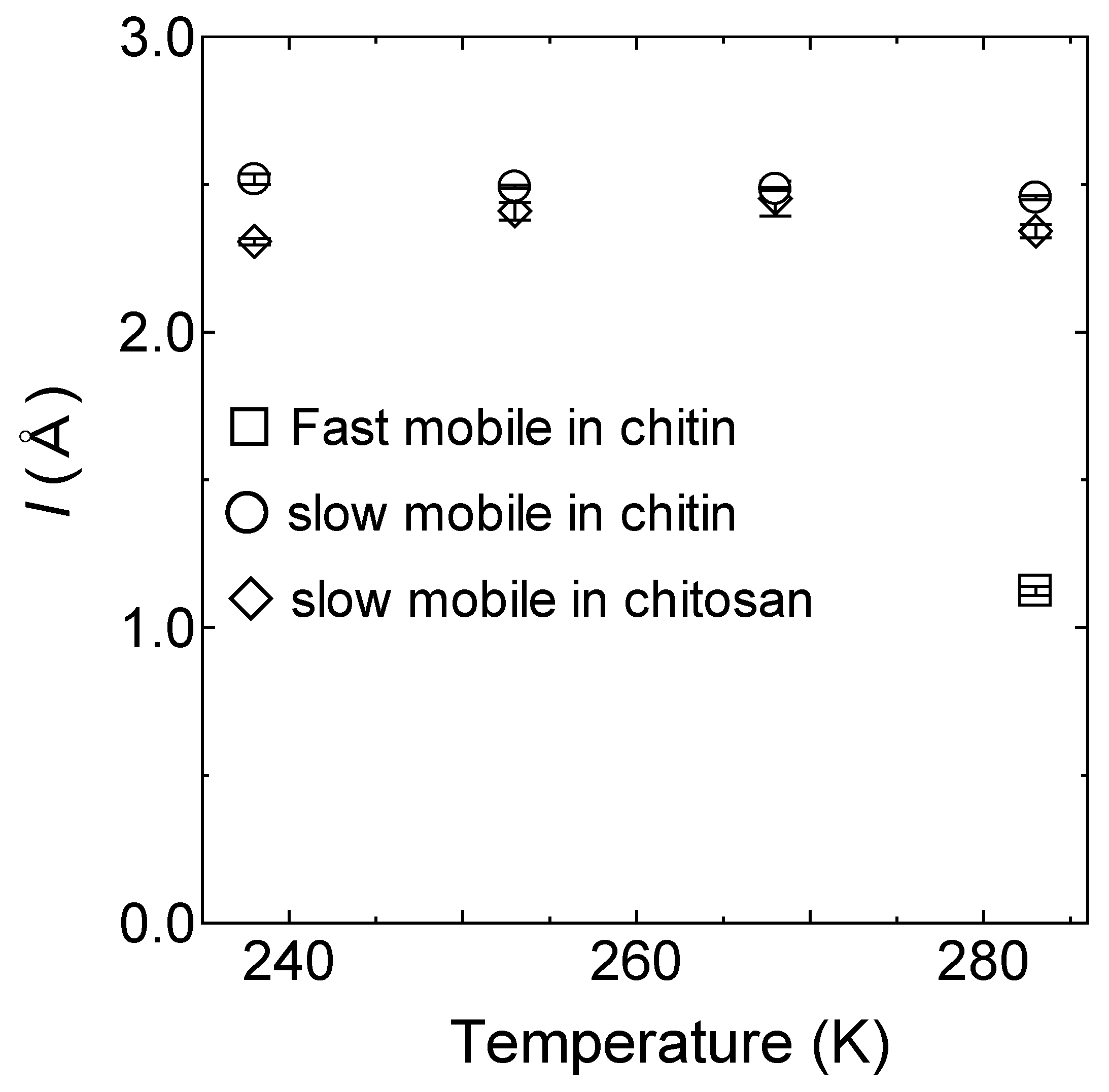
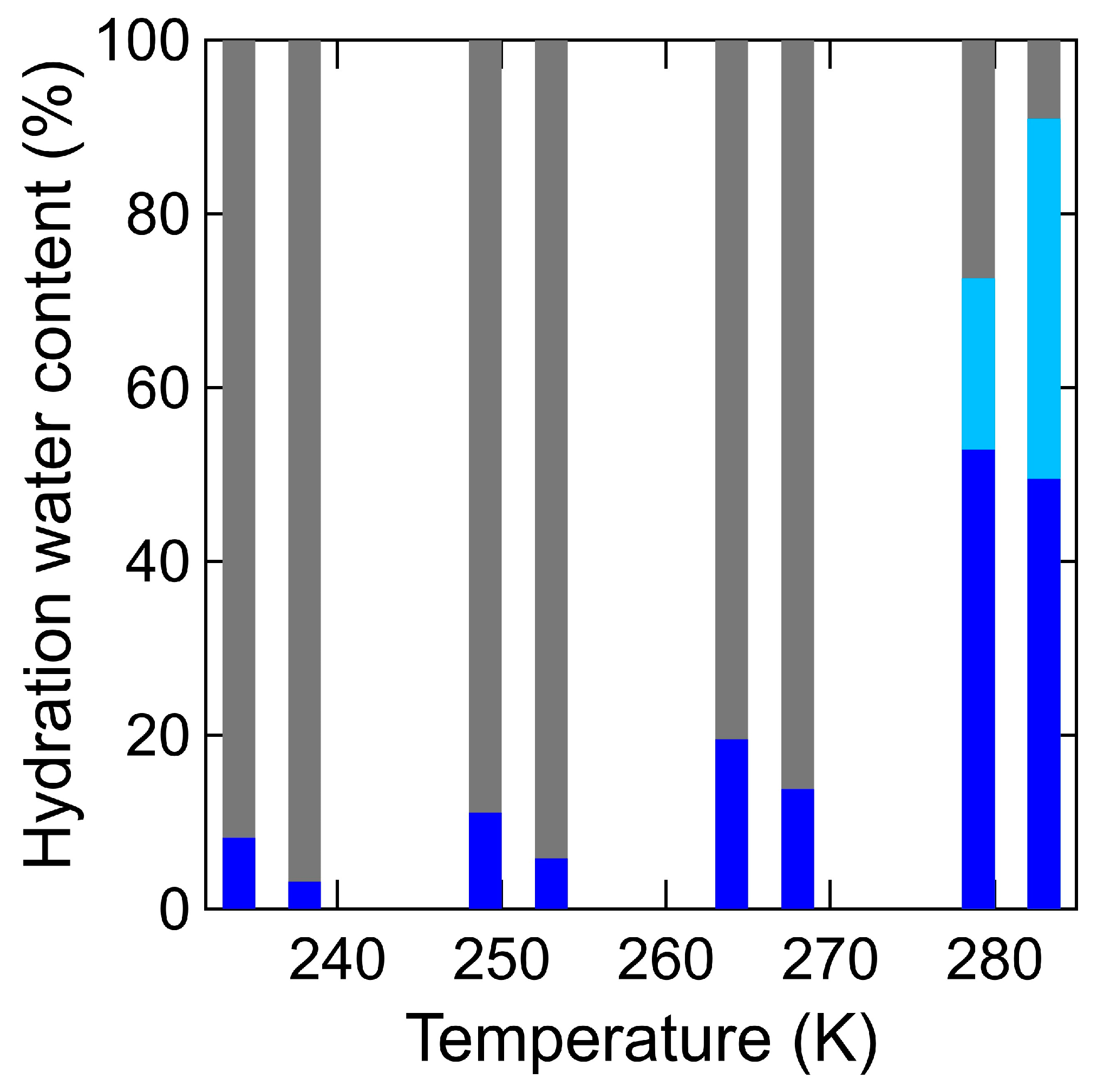
4.1. Swater (Q, ω): Hydration Water Dynamics in the Hydrated Chitin
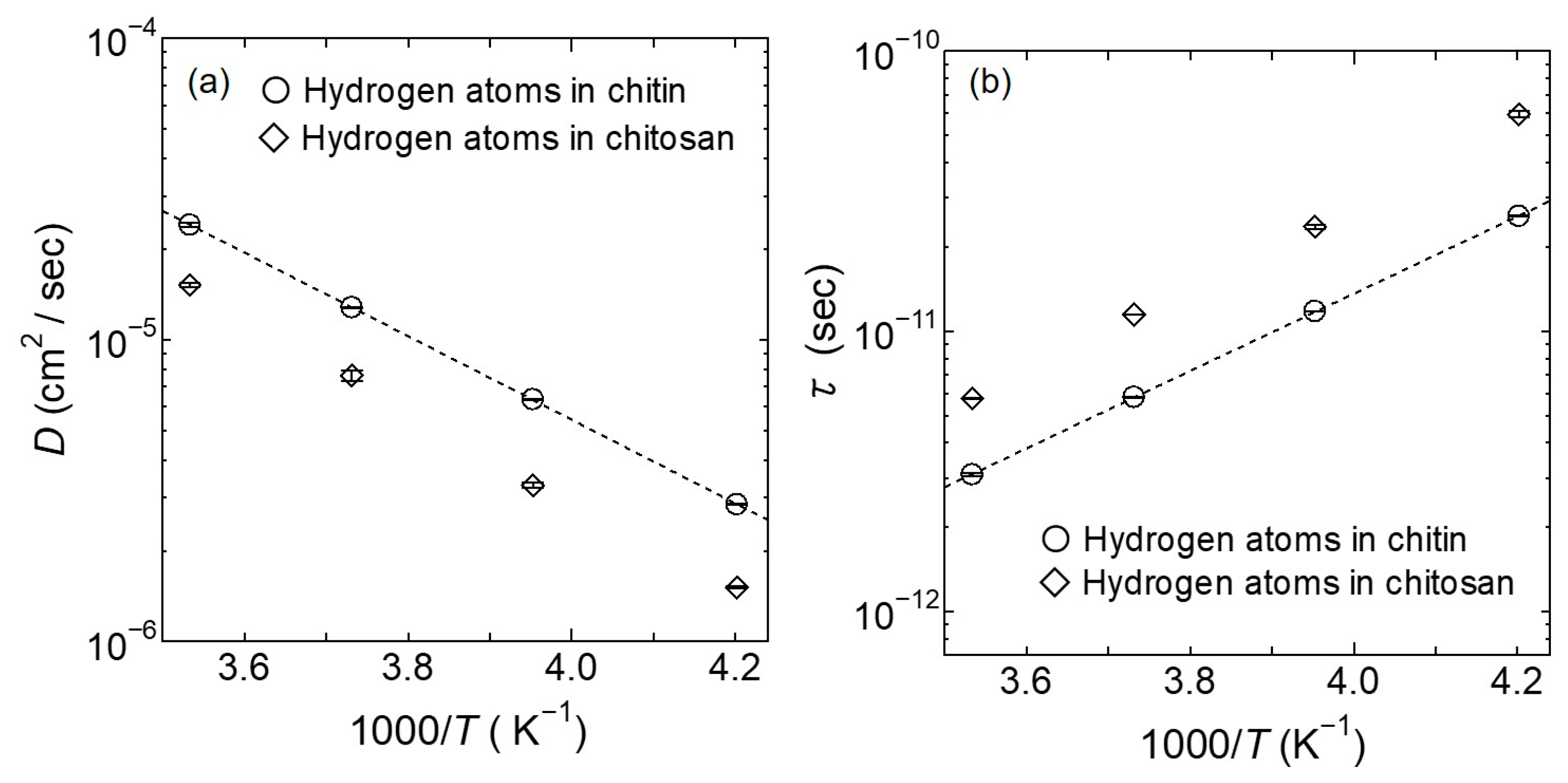
4.2. Satom (Q, ω): Hydrogen Atoms Dynamics in Chitin
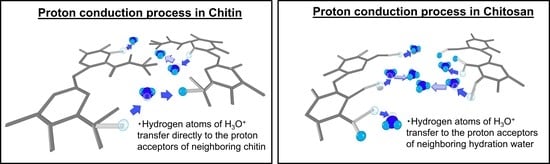
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurita, K. Controlled Functionalization of the Polysaccharide Chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Pokhrel, S.; Yadav, P.N. Functionalization of Chitosan Polymer and Their Applications. J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 450–475. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Matsuo, Y.; Hatori, J. Fuel Cell Based on Natural Sausage Casing. J. Biobased Mater. Bioenergy 2011, 5, 562–564. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ikeda, H.; Kawabata, T.; Hatori, J.; Oyama, H. Collagen-Based Fuel Cell and Its Proton Transfer. Mater. Sci. Appl. 2017, 8, 747–756. [Google Scholar] [CrossRef]
- Matsuo, Y.; Hatori, J.; Oyama, H. Proton Conduction and Impedance Analysis in Submucosa Membrane. In Solid State Ionics; World Scientific: Singapore, 2013; pp. 334–339. [Google Scholar] [CrossRef]
- Matsuo, Y. Bio-fuel cell based on biopolymer electrolyte. J. Fuels Cell Press Technol. 2014, 13, 60–64. [Google Scholar]
- Matsuo, Y.; Ikehata, S. Proton (Chapter. 4) and DNA-Biofuel Cell (Chapter. 14). DNA engineering: Properties and applications. In Conduction; Mizoguchi, K., Sakamoto, H., Eds.; Pan Stanford Publishing: Singapore, 2017; pp. 59–85, 309–314. [Google Scholar]
- Kawabata, T.; Takahashi, Y.; Matsuo, Y. Anomalous Proton Conductivity in Chitin-Chitosan Mixed Compounds. Mater. Sci. Appl. 2019, 11, 1. [Google Scholar] [CrossRef]
- Kawabata, T.; Matsuo, Y. Chitin Based Fuel Cell and Its Proton Conductivity. Mater. Sci. Appl. 2018, 9, 779–789. [Google Scholar] [CrossRef]
- Kawabata, T.; Matsuo, Y. Role of Acetyl Group on Proton Conductivity in Chitin System. J. Mater. 2019, 5, 258–263. [Google Scholar] [CrossRef]
- Kawabata, T. Proton conductivity in chitin system. In Chitin and Chitosan-Physicochemical Properties and Industrial Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Viktorsson, L.; Heinonen, J.T.; Skulason, J.B.; Unnthorsson, R. A Step towards the Hydrogen Economy—A Life Cycle Cost Analysis of a Hydrogen Refueling Station. Energies 2017, 10, 763. [Google Scholar] [CrossRef]
- Ball, M.; Weeda, M. The Hydrogen Economy—Vision or Reality? Int. J. Hydrogen Energy 2015, 40, 7903–7919. [Google Scholar] [CrossRef]
- Hirota, Y.; Tominaga, T.; Kawabata, T.; Kawakita, Y.; Matsuo, Y. Hydrogen Dynamics in Hydrated Chitosan by Quasi-Elastic Neutron Scattering. Bioengineering 2022, 9, 599. [Google Scholar] [CrossRef]
- Bee, M. Quasi-elastic neutron scattering: Principles and applications in solid state chemistry. Biol. Mater. Sci. Adam Hilger Bristol. 1988, 193, 437. [Google Scholar]
- Shibata, K.; Takahashi, N.; Kawakita, Y.; Matsuura, M.; Yamada, T.; Tominaga, T.; Kambara, W.; Kobayashi, M.; Inamura, Y.; Nakatani, T.; et al. The Performance of TOF near Backscattering Spectrometer DNA in MLF, J-PARC. JPS Conf. Proc. 2015, 8, 036022. [Google Scholar] [CrossRef]
- Seto, H.; Itoh, S.; Yokoo, T.; Endo, H.; Nakajima, K.; Shibata, K.; Kajimoto, R.; Ohira-Kawamura, S.; Nakamura, M.; Kawakita, Y.; et al. Inelastic and Quasi-Elastic Neutron Scattering Spectrometers in J-PARC. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 3651–3660. [Google Scholar] [CrossRef]
- Kajimoto, R.; Yokoo, T.; Nakamura, M.; Kawakita, Y.; Matsuura, M.; Endo, H.; Seto, H.; Itoh, S.; Nakajima, K.; Ohira-Kawamura, S. Status of Neutron Spectrometers at J-PARC. Phys. B Condens. Matter 2019, 562, 148–154. [Google Scholar] [CrossRef]
- Kawakita, Y.; Matsuura, M.; Tominaga, T.; Yamada, T.; Tamatsukuri, H.; Nakagawa, H.; Ohuchi, K. Recent Progress on DNA ToF Backscattering Spectrometer in MLF, J-PARC. In EPJ Web of Conferences; EDP Sciences: Les Ulis, France, 2022; p. 02002. [Google Scholar] [CrossRef]
- Tominaga, T.; Kobayashi, M.; Yamada, T.; Matsuura, M.; Kawakita, Y.; Kasai, S. Position-encoded automatic cell elevator for BL02, J-PARC MLF. In Proceedings of the 3rd J-PARC Symposium (J-PARC2019), Tsukuba, Japan, 23–27 September 2021; Volume 33, p. 011095. [Google Scholar]
- Azuah, R.T.; Kneller, L.R.; Qiu, Y.; Tregenna-Piggott, P.L.W.; Brown, C.M.; Copley, J.R.D.; Dimeo, R.M. DAVE: A Comprehensive Software Suite for the Reduction, Visualization, and Analysis of Low Energy Neutron Spectroscopic Data. J. Res. Natl. Inst. Stand. Technol. 2009, 114, 341–358. [Google Scholar] [CrossRef]
- Yamada, T.; Takahashi, N.; Tominaga, T.; Takata, S.I.; Seto, H. Dynamical Behavior of Hydration Water Molecules between Phospholipid Membranes. J. Phys. Chem. B. 2017, 121, 8322–8329. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton Conductivity: Materials and Applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Perrin, J.C.; Lyonnard, S.; Volino, F. Quasielastic Neutron Scattering Study of Water Dynamics in Hydrated Nafion Membranes. J. Phys. Chem. C 2007, 111, 3393–3404. [Google Scholar] [CrossRef]
- Foglia, F.; Lyonnard, S.; Sakai, V.G.; Berrod, Q.; Zanotti, J.M.; Gebel, G.; Clancy, A.J.; McMillan, P.F. Progress in Neutron Techniques: Towards Improved Polymer Electrolyte Membranes for Energy Devices. J. Phys. Condens. Matter 2021, 33, 264005. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.K.; Fraser, A.; Karan, K. Towards the Understanding of Proton Conduction Mechanism in PEMFC Catalyst Layer: Conductivity of Adsorbed Nafion Films. Electrochem. Commun. 2011, 13, 774–777. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K.H. MOFs as Proton Conductors-Challenges and Opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirota, Y.; Tominaga, T.; Kawabata, T.; Kawakita, Y.; Matsuo, Y. Differences in Water Dynamics between the Hydrated Chitin and Hydrated Chitosan Determined by Quasi-Elastic Neutron Scattering. Bioengineering 2023, 10, 622. https://doi.org/10.3390/bioengineering10050622
Hirota Y, Tominaga T, Kawabata T, Kawakita Y, Matsuo Y. Differences in Water Dynamics between the Hydrated Chitin and Hydrated Chitosan Determined by Quasi-Elastic Neutron Scattering. Bioengineering. 2023; 10(5):622. https://doi.org/10.3390/bioengineering10050622
Chicago/Turabian StyleHirota, Yuki, Taiki Tominaga, Takashi Kawabata, Yukinobu Kawakita, and Yasumitsu Matsuo. 2023. "Differences in Water Dynamics between the Hydrated Chitin and Hydrated Chitosan Determined by Quasi-Elastic Neutron Scattering" Bioengineering 10, no. 5: 622. https://doi.org/10.3390/bioengineering10050622
APA StyleHirota, Y., Tominaga, T., Kawabata, T., Kawakita, Y., & Matsuo, Y. (2023). Differences in Water Dynamics between the Hydrated Chitin and Hydrated Chitosan Determined by Quasi-Elastic Neutron Scattering. Bioengineering, 10(5), 622. https://doi.org/10.3390/bioengineering10050622