Therapeutic Effects of Robotic-Exoskeleton-Assisted Gait Rehabilitation and Predictive Factors of Significant Improvements in Stroke Patients: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Trial Design
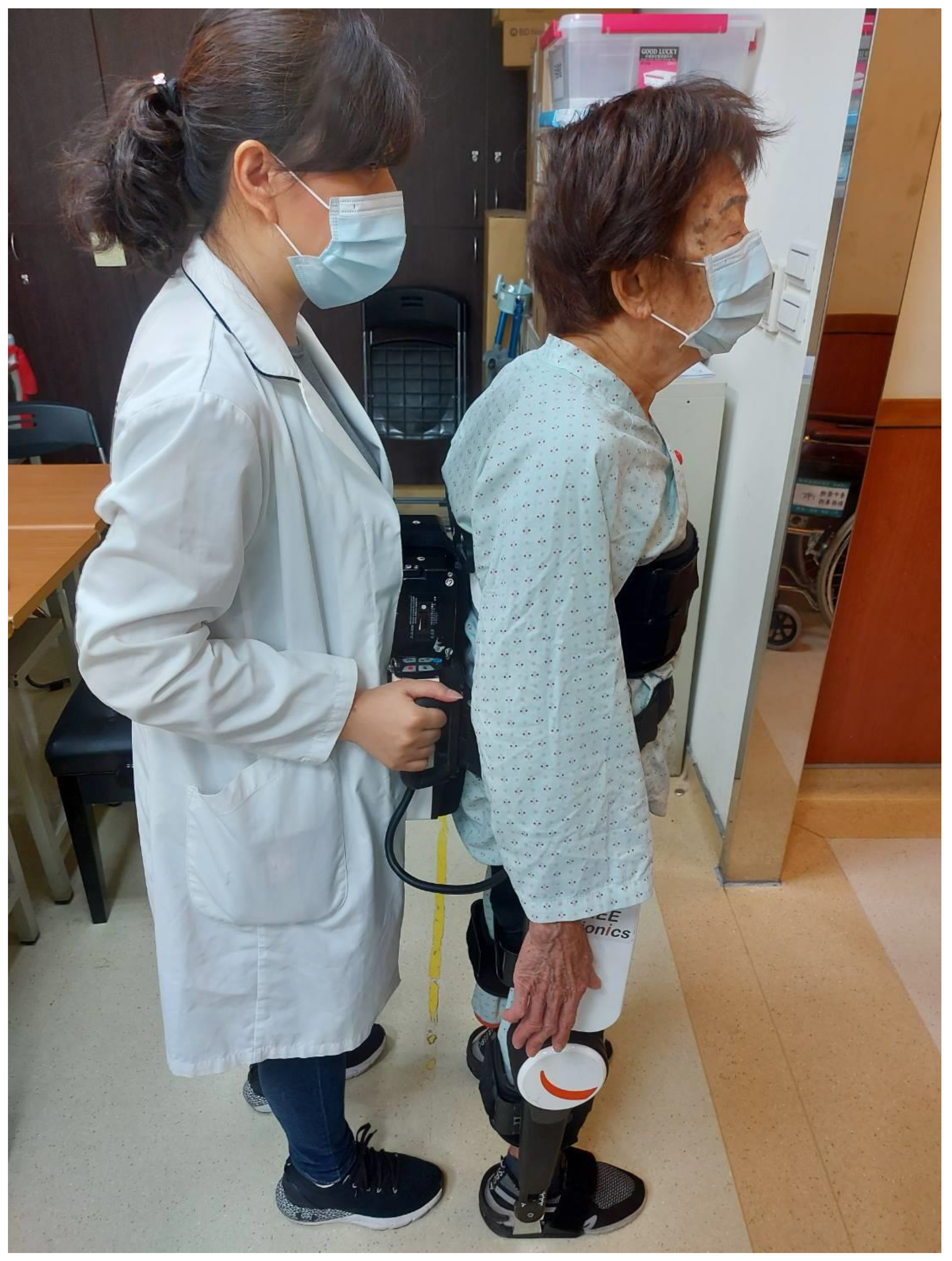
2.2. Interventions
2.3. Assessments
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics of Participants
3.2. Clinical Outcomes
3.2.1. Within-Group Post-Rehabilitation Changes
3.2.2. Between-Group Comparison of Post-Rehabilitation Improvement
3.2.3. Predictors of Improvement Revealed by Univariate and Multivariate Logistic Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 December 2020).
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults—United States, 2005. MMWR Morb. Mortal Wkly. Rep. 2009, 58, 421–426. [Google Scholar]
- Soundy, A.; Liles, C.; Stubbs, B.; Roskell, C. Identifying a Framework for Hope in Order to Establish the Importance of Generalised Hopes for Individuals Who Have Suffered a Stroke. Adv. Med. 2014, 2014, 471874. [Google Scholar] [CrossRef] [PubMed]
- Boudarham, J.; Zory, R.; Genet, F.; Vigné, G.; Bensmail, D.; Roche, N.; Pradon, D. Effects of a knee–ankle–foot orthosis on gait biomechanical characteristics of paretic and non-paretic limbs in hemiplegic patients with genu recurvatum. Clin. Biomech. 2013, 28, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Baumann, M.; Lurbe, K.; Leandro, M.E.; Chau, N. Life satisfaction of two-year post-stroke survivors: Effects of socio-economic factors, motor impairment, Newcastle stroke-specific quality of life measure and World Health Organization quality of life: Bref of informal caregivers in Luxembourg and a rural area in Portugal. Cerebrovasc. Dis. 2012, 33, 219–230. [Google Scholar]
- Bobath, B. Adult Hemiplegia: Evaluation and Treatment, 3rd ed.; Heinemann: Oxford, UK, 1990. [Google Scholar]
- Sawner, K.; LaVigne, J. Brunnstrom’s Movement Therapy in Hemiplegia: A Neurophysiological Approach, 2nd ed.; JB Lippincott: Philadelphia, PA, USA, 1992. [Google Scholar]
- Voss, D.E.; Ionta, M.K.; Myers, B.J. Proprioceptive Neuromuscular Facilitation: Patterns and Techniques, 3rd ed.; Harper & Rows: Philadelphia; PA, USA, 1985. [Google Scholar]
- Carr, J.H.; Shepherd, R.B. Neurological Rehabilitation—Optimizing Motor Performance; Butterworth Heinemann: Oxford, UK, 1998. [Google Scholar]
- Stockmeyer, S.A. An interpretation of the approach of Rood to the treatment of neuromuscular dysfunction. Am. J. Phys. Med. 1967, 46, 900–961. [Google Scholar]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Z.; Wang, J. Robotics in Lower-Limb Rehabilitation after Stroke. Behav. Neurol. 2017, 2017, 3731802. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, 006185. [Google Scholar]
- Zhang, J.F.; Dong, Y.M.; Yang, C.J.; Geng, Y.; Chen, Y.; Yang, Y. 5-Link model based gait trajectory adaption control strategies of the gait rehabilitation exoskeleton for post-stroke patients. Mechatronics 2010, 20, 368–376. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Sorrentino, G.; Cassio, A.; Mazzoli, D.; Andrenelli, E.; Bizzarini, E. Robotic-assisted gait rehabilitation following stroke: A systematic review of current guidelines and practical clinical recommendations. Eur. J. Phys. Rehabil. Med. 2021, 57, 460–471. [Google Scholar] [CrossRef]
- Molteni, F.; Gasperini, G.; Cannaviello, G.; Guanziroli, E. Exoskeleton and End-Effector Robots for Upper and Lower Limbs Rehabilitation: Narrative Review. PM&R 2018, 10 (Suppl. 2), S174–S188. [Google Scholar]
- Macchiavelli, A.; Giffone, A.; Ferrarello, F.; Paci, M. Reliability of the six-minute walk test in individuals with stroke: Systematic review and meta-analysis. Neurol. Sci. 2021, 42, 81–87. [Google Scholar] [CrossRef]
- Chan, P.P.; Si Tou, J.I.; Tse, M.M.; Ng, S.S. Reliability and Validity of the Timed Up and Go Test with a Motor Task in People with Chronic Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 2213–2220. [Google Scholar] [CrossRef]
- Kojima, G.; Masud, T.; Kendrick, D.; Morris, R.; Gawler, S.; Treml, J.; Iliffe, S. Does the timed up and go test predict future falls among british community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015, 3, 38. [Google Scholar] [CrossRef]
- Chen, C.L.; Chang, K.J.; Wu, P.Y.; Chi, C.H.; Chang, S.T.; Cheng, Y.Y. Comparison of the Effects between Isokinetic and Isotonic Strength Training in Subacute Stroke Patients. J. Stroke Cerebrovasc. Dis. 2015, 24, 1317–1323. [Google Scholar] [CrossRef]
- Pickard, A.S.; Johnson, J.A.; Penn, A.; Lau, F.; Noseworthy, T. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke 1999, 30, 1213–1217. [Google Scholar] [CrossRef]
- Hussain, F.; Goecke, R.; Mohammadian, M. Exoskeleton robots for lower limb assistance: A review of materials, actuation, and manufacturing methods. Proc. Inst. Mech. Eng. H 2021, 235, 1375–1385. [Google Scholar] [CrossRef]
- Rosenfeld, J.V.; Wong, Y.T. Neurobionics and the brain-computer interface: Current applications and future horizons. Med. J. Aust. 2017, 206, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Gil-Castillo, J.; Barria, P.; Aguilar Cárdenas, R.; Baleta Abarza, K.; Andrade Gallardo, A.; Biskupovic Mancilla, A. A Robot-Assisted Therapy to Increase Muscle Strength in Hemiplegic Gait Rehabilitation. Front. Neurorobot. 2022, 16, 837494. [Google Scholar] [CrossRef] [PubMed]
- Aprile, I.; Conte, C.; Cruciani, A.; Pecchioli, C.; Castelli, L.; Insalaco, S.; Germanotta, M.; Iacovelli, C. Efficacy of Robot-Assisted Gait Training Combined with Robotic Balance Training in Subacute Stroke Patients: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 5162. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, K.; Dalgas, U.; Overgaard, K.; Pedersen, A.R.; Ørtenblad, N.; Lund, C. Skeletal muscle fiber characteristics and oxidative capacity in hemiparetic stroke survivors. Muscle Nerve 2016, 53, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Choi, W. Effects of Robot-Assisted Gait Training with Body Weight Support on Gait and Balance in Stroke Patients. Int. J. Environ. Res. Public Health 2022, 19, 5814. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, D.Y.; Chun, M.H.; Kim, S.W.; Jeon, H.R.; Hwang, C.H. Effects of robot-(Morning Walk(®)) assisted gait training for patients after stroke: A randomized controlled trial. Clin. Rehabil. 2019, 33, 516–523. [Google Scholar] [CrossRef]
- Yang, X.; Sun, W.; Hou, D.; Wang, T.; Li, C.; Luo, Y.; Zhang, S.; Shen, L.; Liu, W.; Wu, D. The Degree of Plasma Oxidized Low-Density Lipoprotein Level Decrease Is Related to Clinical Outcomes for Patients with Acute Ischemic Stroke. Dis. Markers 2021, 2021, 4998823. [Google Scholar] [CrossRef]
- Nerea, H.; Jean-Luc, B. Low-density lipoprotein-cholesterol-induced endothelial dysfunction and oxidative stress: The role of statins. Antioxid. Redox Signal. 2014, 20, 1216–1237. [Google Scholar]
- Dundar, U.; Toktas, H.; Solak, O.; Ulasli, A.M.; Eroglu, S. A comparative study of conventional physiotherapy versus robotic training combined with physiotherapy in patients with stroke. Top. Stroke Rehabil. 2014, 21, 453–461. [Google Scholar] [CrossRef]
- Bessler, J.; Prange-Lasonder, G.B.; Schaake, L.; Saenz, J.F.; Bidard, C.; Fassi, I.; Valori, M.; Lassen, A.B.; Buurke, J.H. Safety Assessment of Rehabilitation Robots: A Review Identifying Safety Skills and Current Knowledge Gaps. Front. Robot. AI 2021, 8, 602878. [Google Scholar] [CrossRef] [PubMed]

| Experimental Group (n = 17) | Control Group (n = 21) | p-Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age (yr) | 68.0 | (56.5–74.5) | 70.0 | (63.5–74.5) | 0.527 |
| Sex (n,%) | 0.444 | ||||
| Male | 11 | (64.71%) | 11 | (52.38%) | |
| Female | 6 | (35.29%) | 10 | (47.62%) | |
| Height (cm) | 158.00 | (155.50–168.25) | 160.00 | (158.10–165.50) | 0.499 |
| Weight (kg) | 69.40 | (57.65–70.95) | 72.00 | (60.60–77.30) | 0.122 |
| BMI | 24.60 | (21.90–27.35) | 26.70 | (23.56–29.48) | 0.082 |
| NIHSS score | 6 | (3–10) | 7 | (4.5–11.5) | 0.594 |
| HbA1C (%) | 6.30 | (5.60–7.15) | 6.60 | (6.20–7.55) | 0.181 |
| LDL (mg/dL) | 105.00 | (78.00–135.00) | 121.00 | (100.50–179.00) | 0.072 |
| Homocysteine (μmol/L) | 11.05 | (7.99–12.52) | 11.00 | (9.50–16.00) | 0.310 |
| Stroke type (n,%) | 1.000 | ||||
| Hemorrhagic | 5 | (29.41%) | 6 | (28.57%) | |
| Ischemic | 12 | (70.59%) | 15 | (71.43%) | |
| Stroke site (n,%) | 0.407 | ||||
| Left | 6 | (35.29%) | 11 | (52.38%) | |
| Right | 10 | (58.82%) | 10 | (47.62%) | |
| Bilateral | 1 | (5.88%) | 0 | (0%) | |
| Pre-rehabilitation data | |||||
| Ext. 60 (N⋅m) | 10.00 | (4.35–20.20) | 10.00 | (8.00–13.50) | 0.755 |
| Flex. 60 (N⋅m) | 9.10 | (6.50–10.90) | 11.00 | (8.50–14.50) | 0.095 |
| Ext. 120 (N⋅m) | 9.90 | (3.95–14.25) | 12.00 | (9.00–14.50) | 0.176 |
| Flex. 120 (N⋅m) | 11.60 | (8.00–15.00) | 15.00 | (10.50–17.50) | 0.066 |
| 6MWT (m) | 10.00 | (6.00–37.50) | 10.00 | (8.50–12.00) | 0.867 |
| TUG (s) | 61.33 | (50.00–115.86) | 57.07 | (41.16–70.29) | 0.107 |
| SF-12 | 108.50 | (71.63–114.78) | 106.90 | (100.61–112.24) | 0.685 |
| Pre-Rehabilitation | Post-Rehabilitation | p-Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Experimental group | |||||
| Ext. 60 (N⋅m) | 10.00 | (4.35–20.20) | 19.00 | (13.20–37.60) | 0.001 ** |
| Flex. 60 (N⋅m) | 9.10 | (6.50–10.90) | 12.00 | (9.25–16.50) | 0.001 ** |
| Ext. 120 (N⋅m) | 9.90 | (3.95–14.25) | 13.70 | (8.00–23.85) | 0.010 * |
| Flex. 120 (N⋅m) | 11.60 | (8.00–15.00) | 13.80 | (12.10–20.25) | 0.001 ** |
| 6 MWT (m) | 10.00 | (6.00–37.50) | 33.00 | (28.50–60.00) | 0.007 ** |
| TUG (s) | 61.33 | (50.00–115.86) | 44.18 | (33.65–83.72) | 0.006 ** |
| SF-12 physical domain | 52.49 | (47.52–54.98) | 54.59 | (50.34–60.45) | 0.266 |
| SF-12 mental domain | 65.09 | (59.13–112.92) | 58.09 | (51.86–70.02) | 0.136 |
| SF-12 total score | 108.50 | (71.63–114.78) | 117.71 | (111.88–121.14) | 0.005 ** |
| Control group | |||||
| Ext. 60 (N⋅m) | 10.00 | (8.00–13.50) | 14.00 | (9.00–22.50) | 0.001 ** |
| Flex. 60 (N⋅m) | 11.00 | (8.50–14.50) | 14.00 | (10.00–16.00) | 0.012 * |
| Ext. 120 (N⋅m) | 12.00 | (9.00–14.50) | 14.00 | (9.00–16.00) | 0.140 |
| Flex. 120 (N⋅m) | 15.00 | (10.50–17.50) | 16.00 | (9.50–23.50) | 0.120 |
| 6 MWT (m) | 10.00 | (8.50–12.00) | 9.00 | (8.00–12.00) | 0.392 |
| TUG (s) | 57.07 | (41.16–70.29) | 55.89 | (35.76–61.32) | 0.038 * |
| SF-12 physical domain | 53.28 | (47.22–55.26) | 50.34 | (42.58–53.51) | 0.017 * |
| SF-12 mental domain | 54.53 | (51.78–60.06) | 55.34 | (49.69–58.82) | 0.259 |
| SF-12 total score | 106.90 | (100.61–112.24) | 103.30 | (96.85–110.15) | 0.016 * |
| Experimental Group (n = 17) | Control Group (n = 21) | p-Value | Mean Difference | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Effect Size (d) | |||
| Ext. 60 (N⋅m) | 9.48 | 9.18 | 4.38 | 6.89 | 0.068 | 0.62 | 5.1 |
| Flex. 60 (N⋅m) | 4.38 | 3.61 | 2.05 | 3.4 | 0.048 * | 0.67 | 2.33 |
| Ext. 120 (N⋅m) | 4.14 | 6.36 | 1.67 | 4.54 | 0.171 | 0.46 | 2.47 |
| Flex. 120 (N⋅m) | 3.92 | 3.83 | 2.76 | 8.3 | 0.598 | 0.17 | 1.16 |
| 6 MWT (m) | 25.59 | 49.3 | −0.19 | 1.08 | 0.047 * | 0.7 | 25.78 |
| TUG (s) | −22.77 | 38.07 | −3.4 | 5.75 | 0.054 | 0.68 | −19.37 |
| SF-12 physical domain | 4.22 | 13.56 | −2.54 | 5.08 | 0.066 | 0.63 | 6.75 |
| SF-12 mental domain | −20.98 | 33.12 | −0.95 | 4.63 | 0.025 * | 0.8 | −20.03 |
| SF-12 total score | 19.46 | 25.97 | −3.48 | 6.44 | 0.002 ** | 1.16 | 22.93 |
| 6 Min Walk Test | Timed Up-and-Go Test | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Training type | ||||||
| Experimental | 31.87 | (5.09–199.48) | <0.001 ** | 0.34 | (0.09–1.27) | 0.107 |
| Control | 1.00 | 1.00 | ||||
| Age (yr) | 0.99 | (0.93–1.05) | 0.678 | 0.99 | (0.93–1.04) | 0.615 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 0.65 | (0.18–2.37) | 0.512 | 0.42 | (0.11–1.56) | 0.193 |
| BMI | 0.85 | (0.70–1.03) | 0.092 | 1.09 | (0.90–1.30) | 0.380 |
| NIHSS score | 1.03 | (0.91–1.18) | 0.614 | 1.02 | (0.89–1.16) | 0.813 |
| HbA1C (%) | 0.77 | (0.42–1.44) | 0.417 | 1.11 | (0.61–2.02) | 0.738 |
| LDL (mg/dL) | 0.99 | (0.97–1.10) | 0.132 | 1.02 | (1.00–1.04) | 0.033 * |
| Homocysteine (μmol/L) | 0.93 | (0.81–1.08) | 0.347 | 1.04 | (0.90–1.20) | 0.583 |
| Stroke type | ||||||
| Hemorrhagic | 1.00 | 1.00 | ||||
| Ischemic | 1.29 | (0.32–5.28) | 0.721 | 2.19 | (0.52–9.27) | 0.288 |
| Stroke site | ||||||
| Left | 1.00 | 1.00 | ||||
| Right | 1.90 | (0.52–6.96) | 0.330 | 0.81 | (0.22–2.91) | 0.744 |
| Pre-rehabilitation data | ||||||
| Ext. 60 (N⋅m) | 1.03 | (0.97–1.10) | 0.305 | 1.02 | (0.96–1.08) | 0.514 |
| Flex. 60 (N⋅m) | 0.97 | (0.86–1.09) | 0.592 | 1.01 | (0.90–1.13) | 0.874 |
| Ext. 120 (N⋅m) | 1.03 | (0.94–1.12) | 0.511 | 1.03 | (0.95–1.13) | 0.471 |
| Flex. 120 (N⋅m) | 1.00 | (0.92–1.09) | 0.989 | 0.99 | (0.91–1.08) | 0.848 |
| 6 MWT (m) | 0.99 | (0.96–1.03) | 0.756 | 1.01 | (0.97–1.05) | 0.620 |
| TUG (s) | 1.02 | (1.00–1.04) | 0.134 | 0.99 | (0.97–1.01) | 0.271 |
| SF-12 physical domain | 1.03 | (0.91–1.17) | 0.603 | 0.96 | (0.85–1.09) | 0.540 |
| SF-12 mental domain | 1.03 | (1.00–1.07) | 0.078 | 1.00 | (0.97–1.02) | 0.760 |
| SF-12 total score | 0.98 | (0.94–1.02) | 0.296 | 0.98 | (0.95–1.02) | 0.430 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Training type | ||||||
| Experimental | 14.93 | (3.01–74.04) | 0.001 ** | 32.09 | (2.82–364.82) | 0.005 ** |
| Control | 1.00 | |||||
| Age (yr) | 1.03 | (0.97–1.09) | 0.378 | |||
| Sex | ||||||
| Male | 1.00 | |||||
| Female | 0.65 | (0.18–2.37) | 0.512 | |||
| BMI | 0.87 | (0.72–1.05) | 0.156 | |||
| NIHSS score | 0.88 | (0.76–1.02) | 0.097 | |||
| HbA1C (%) | 0.74 | (0.76–1.02) | 0.353 | |||
| LDL (mg/dL) | 0.99 | (0.98–1.00) | 0.184 | |||
| Homocysteine (μmol/L) | 1.07 | (0.93–1.24) | 0.341 | |||
| Stroke type | ||||||
| Hemorrhagic | 1.00 | |||||
| Ischemic | 0.77 | (0.19–3.16) | 0.721 | |||
| Stroke site | ||||||
| Left | 1.00 | |||||
| Right | 2.98 | (0.93–1.24) | 0.107 | |||
| Pre-rehabilitation data | ||||||
| Ext. 60 (N⋅m) | 1.06 | (0.99–1.14) | 0.108 | |||
| Flex. 60 (N⋅m) | 1.10 | (0.95–1.27) | 0.198 | |||
| Ext. 120 (N⋅m) | 1.10 | (0.98–1.23) | 0.103 | |||
| Flex. 120 (N⋅m) | 1.00 | (0.92–1.10) | 0.926 | |||
| 6 MWT (m) | 1.03 | (0.98–1.07) | 0.284 | |||
| TUG (s) | 1.01 | (0.99–1.03) | 0.358 | |||
| SF-12 physical domain | 0.88 | (0.77–1.01) | 0.076 | |||
| SF-12 mental domain | 1.08 | (1.00–1.17) | 0.048 * | |||
| SF-12 total score | 0.95 | (0.90–0.997) | 0.039 * | 0.93 | (0.85–1.02) | 0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Ko, L.-W.; Hsu, C.-Y.; Cheng, Y.-Y. Therapeutic Effects of Robotic-Exoskeleton-Assisted Gait Rehabilitation and Predictive Factors of Significant Improvements in Stroke Patients: A Randomized Controlled Trial. Bioengineering 2023, 10, 585. https://doi.org/10.3390/bioengineering10050585
Lee Y-H, Ko L-W, Hsu C-Y, Cheng Y-Y. Therapeutic Effects of Robotic-Exoskeleton-Assisted Gait Rehabilitation and Predictive Factors of Significant Improvements in Stroke Patients: A Randomized Controlled Trial. Bioengineering. 2023; 10(5):585. https://doi.org/10.3390/bioengineering10050585
Chicago/Turabian StyleLee, Yi-Heng, Li-Wei Ko, Chiann-Yi Hsu, and Yuan-Yang Cheng. 2023. "Therapeutic Effects of Robotic-Exoskeleton-Assisted Gait Rehabilitation and Predictive Factors of Significant Improvements in Stroke Patients: A Randomized Controlled Trial" Bioengineering 10, no. 5: 585. https://doi.org/10.3390/bioengineering10050585
APA StyleLee, Y.-H., Ko, L.-W., Hsu, C.-Y., & Cheng, Y.-Y. (2023). Therapeutic Effects of Robotic-Exoskeleton-Assisted Gait Rehabilitation and Predictive Factors of Significant Improvements in Stroke Patients: A Randomized Controlled Trial. Bioengineering, 10(5), 585. https://doi.org/10.3390/bioengineering10050585







