Evaluation of the Effect of Photodynamic Therapy on CAM-Grown Sarcomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Sample Preparation of Tumor Tissue
2.3. Primary Cell Culture Preparation
2.4. Short-Term Cell Cultures
2.5. CAM Model and 5-ALA Treatment
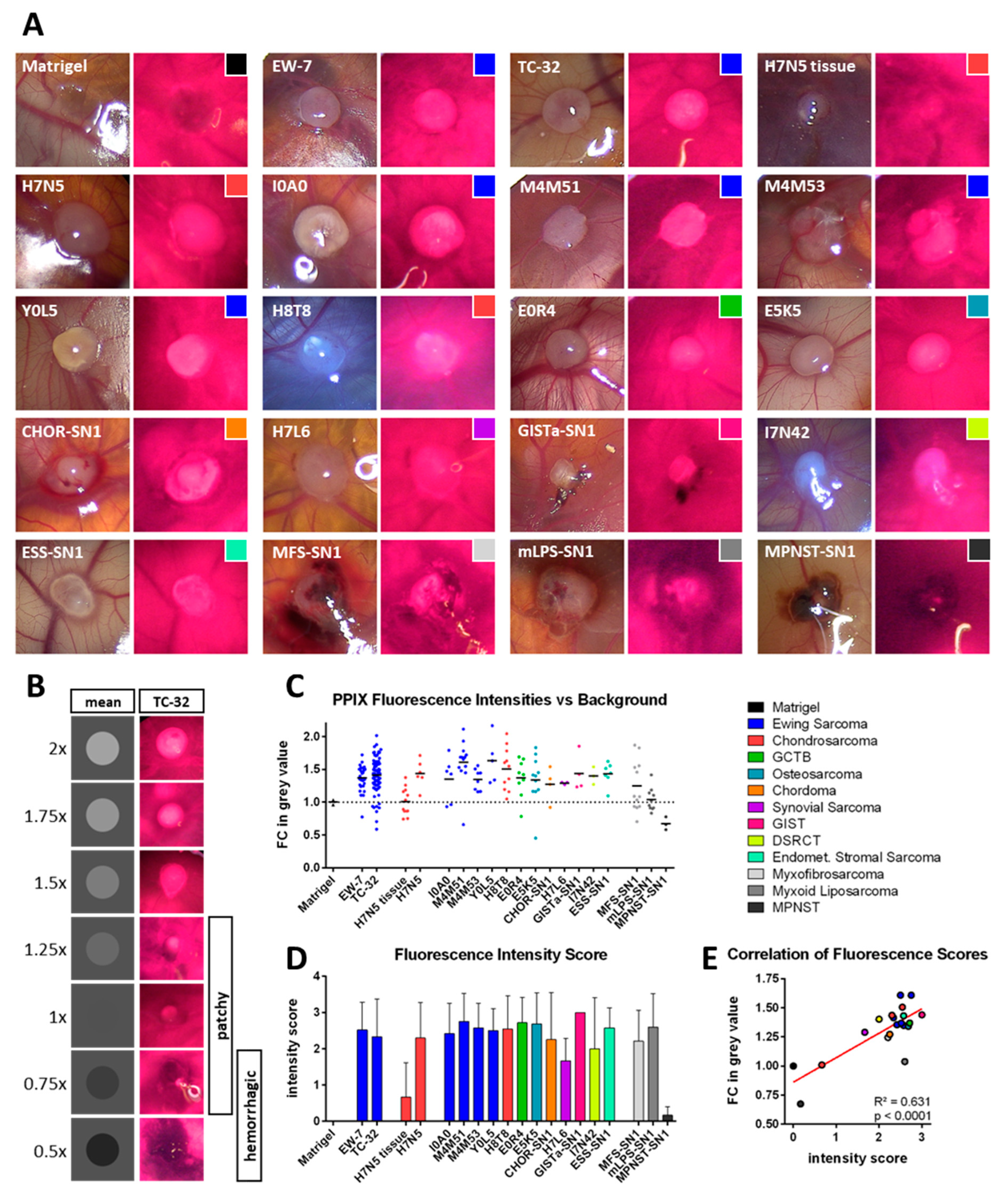
2.6. Photodynamic Diagnosis: PPIX Fluorescence Detection
2.7. Photodynamic Therapy: Red Light Excitation of Tumors
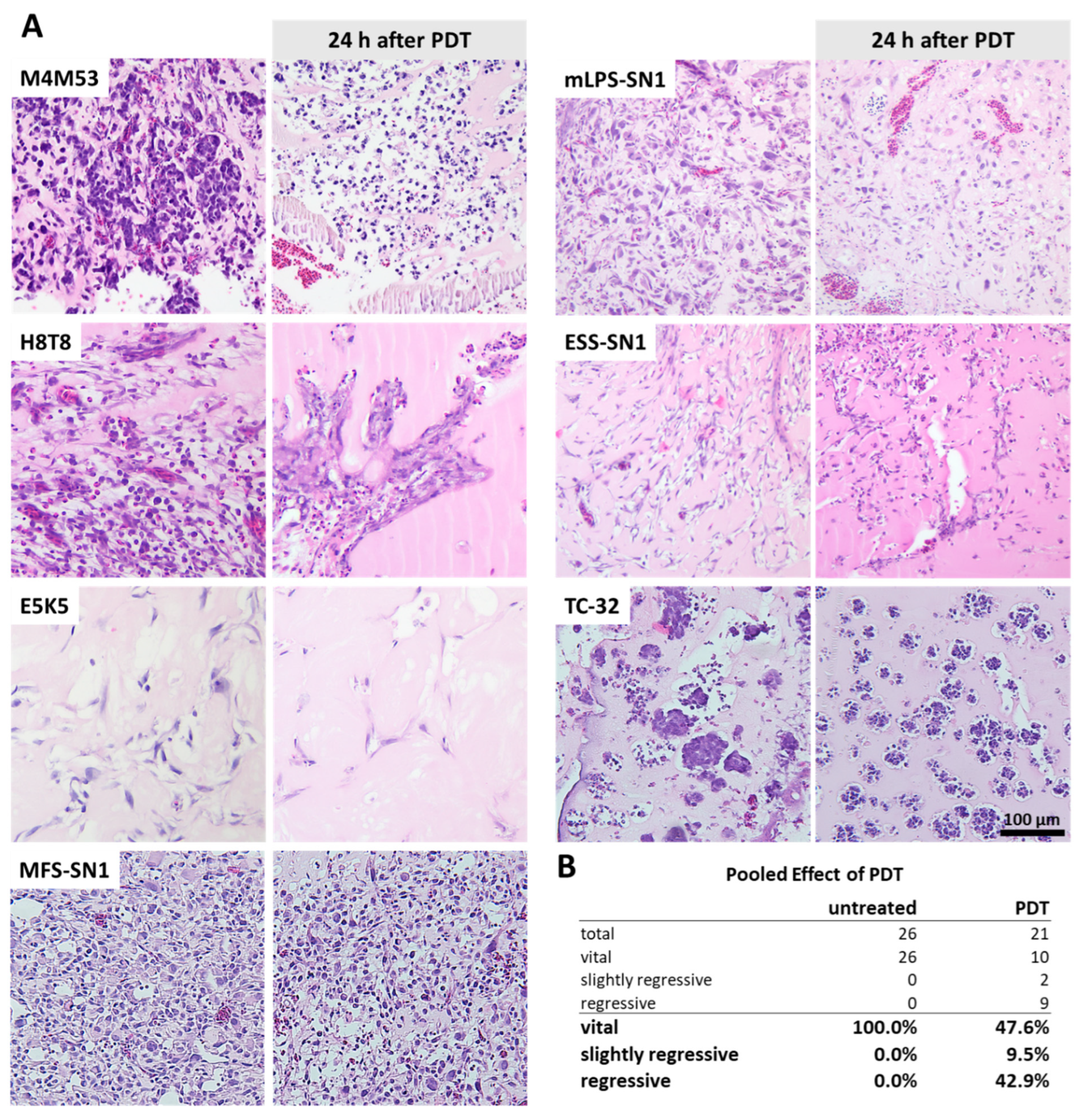
2.8. Histological Evaluation of Tumor Viability and Regression
2.9. Fluorescence Intensity Measurements
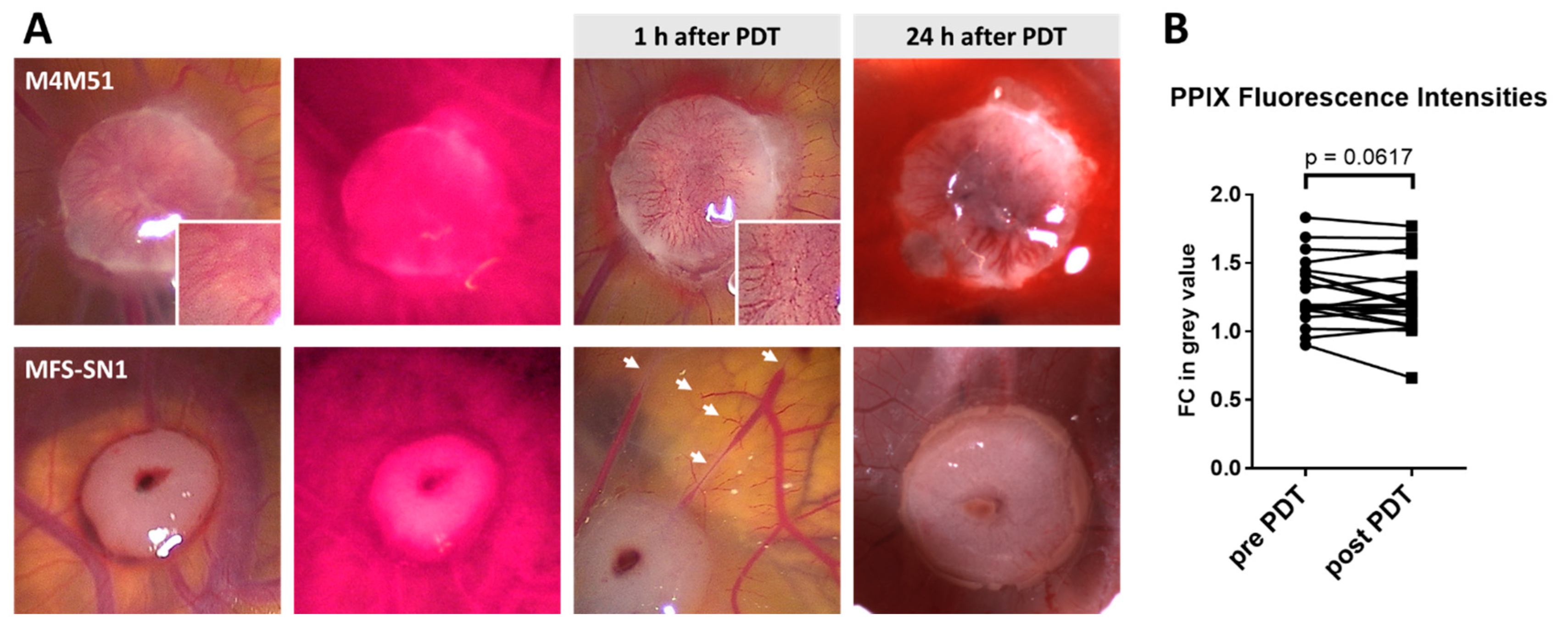
2.10. Analysis of PPIX Fluorescence Intensity Changes after PDT
3. Results
3.1. Chorio-Allantoic Membrane (CAM) Model
3.2. Tumor Models Derived from Patient Material on the CAM
3.3. Photodynamic Diagnostic (PDD)
3.4. Photodynamic Therapy (PDT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pennacchioli, E.; Tosti, G.; Barberis, M.; De Pas, T.M.; Verrecchia, F.; Menicanti, C.; Testori, A.; Mazzarol, G. Sarcoma spreads primarily through the vascular system: Are there biomarkers associated with vascular spread? Clin. Exp. Metastasis 2012, 29, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, S.; Amatruda, J.; Bauer, S.; Collaud, S.; de Álava, E.; DuBois, S.; Hardes, J.; Hartmann, W.; Kovar, H.; Metzler, M.; et al. Ewing sarcoma—Diagnosis, treatment, clinical challenges and future perspectives. J. Clin. Med. 2021, 10, 1685. [Google Scholar] [CrossRef] [PubMed]
- Haglund, K.E.; Raut, C.P.; Nascimento, A.F.; Wang, Q.; George, S.; Baldini, E.H. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int. J. Radiat. Oncol. 2012, 82, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. 2010, 8, 705–718. [Google Scholar]
- Gachiani, J.; Kim, D.; Nelson, A.; Kline, D. Surgical management of malignant peripheral nerve sheath tumors. Neurosurg. Focus 2007, 22, E13. [Google Scholar] [CrossRef]
- Khan, M.; Rankin, K.S.; Todd, R.; Lethbridge, E.; Gerrand, C. Surgical excision and not chemotherapy is the most powerful modality in treating synovial sarcoma: The UK’s North East experience. Arch. Orthop. Trauma Surg. 2018, 139, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; et al. Bone sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef]
- Harati, K.; Goertz, O.; Pieper, A.; Daigeler, A.; Joneidi-Jafari, H.; Niggemann, H.; Stricker, I.; Lehnhardt, M. Soft tissue sarcomas of the extremities: Surgical margins can be close as long as the resected tumor has no ink on it. Oncologist 2017, 22, 1400–1410. [Google Scholar] [CrossRef]
- Van Der Woude, H.-J.; Bloem, J.L.; Hogendoorn, P.C.W. Preoperative evaluation and monitoring chemotherapy in patients with high-grade osteogenic and Ewing’s sarcoma: Review of current imaging modalities. Skelet. Radiol. 1998, 27, 57–71. [Google Scholar] [CrossRef]
- Biermann, J.S.; Chow, W.; Reed, D.; Lucas, D.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Budd, G.T.; Curry, W.T.; et al. NCCN guidelines insights: Bone cancer, version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 155–167. [Google Scholar] [CrossRef]
- Sawamura, C.; Springfield, D.S.; Marcus, K.J.; Perez-Atayde, A.R.; Gebhardt, M.C. Factors predicting local recurrence, metastasis, and survival in pediatric soft tissue sarcoma in extremities. Clin. Orthop. Relat. Res. 2010, 468, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Bosma, S.E.; Rueten-Budde, A.J.; Lancia, C.; Ranft, A.; Dirksen, U.; Krol, A.D.; Gelderblom, H.; Van De Sande, M.A.J.; Dijkstra, P.D.S.; Fiocco, M. Individual risk evaluation for local recurrence and distant metastasis in Ewing sarcoma: A multistate model: A multistate model for Ewing sarcoma. Pediatr. Blood Cancer 2019, 66, e27943. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Hiroshima, Y.; Yano, S.; Zhang, Y.; Matsumoto, Y.; Uehara, F.; Yamamoto, M.; Kimura, H.; Hayashi, K.; Bouvet, M.; et al. Fluorescence-guided surgery improves outcome in an orthotopic osteosarcoma nude-mouse model. J. Orthop. Res. 2014, 32, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Chan, C.D.; Nicoli, F.; Crowley, T.P.; Ghosh, K.M.; Beckingsale, T.; Saleh, D.; Dildey, P.; Gupta, S.; Ragbir, M.; et al. Intraoperative near-infrared fluorescence guided surgery using indocyanine green (ICG) for the resection of sarcomas may reduce the positive margin rate: An extended case series. Cancers 2021, 13, 6284. [Google Scholar] [CrossRef]
- Van Keulen, S.; Hom, M.; White, H.; Rosenthal, E.L.; Baik, F.M. The evolution of fluorescence-guided surgery. Mol. Imaging Biol. 2022, 25, 36–45. [Google Scholar] [CrossRef]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Kaneko, S. Fluorescence-guided resection of malignant glioma with 5-ALA. Int. J. Biomed. Imaging 2016, 2016, 6135293. [Google Scholar] [CrossRef]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M.; 5-ALA Recurrent Glioma Study Group. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase II study. Neurosurgery 2009, 65, 1070–1077. [Google Scholar] [CrossRef]
- Kamp, M.A.; Felsberg, J.; Sadat, H.; Kuzibaev, J.; Steiger, H.-J.; Rapp, M.; Reifenberger, G.; Dibué, M.; Sabel, M. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir. 2015, 157, 207–214. [Google Scholar] [CrossRef]
- Chung, I.W.H.; Eljamel, S. Risk factors for developing oral 5-aminolevulenic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagnosis Photodyn. Ther. 2013, 10, 362–367. [Google Scholar] [CrossRef]
- Peng, Q.; Berg, K.; Moan, J.; Kongshaug, M.; Nesland, J.M. 5-aminolevulinic acid-based photodynamic therapy: Principles and experimental research. Photochem. Photobiol. 1997, 65, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Kenan, S.; Liang, H.; Goodman, H.J.; Jacobs, A.J.; Chan, A.; Grande, D.A.; Levin, A.S. 5-Aminolevulinic acid tumor paint and photodynamic therapy for myxofibrosarcoma: An in vitro study. J. Orthop. Surg. Res. 2020, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Rossi, V.; Baugher, P.J. Aminolevulinic acid-mediated photodynamic therapy causes cell death in MG-63 human osteosarcoma cells. Photomed. Laser Surg. 2016, 34, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Fingar, V.H. Vascular effects of photodynamic therapy. J. Clin. Laser Med. Surg. 1996, 14, 323–328. [Google Scholar] [CrossRef]
- Henderson, B.W.; Fingar, V.H. Oxygen limitation of direct tumor cell kill during photodynamic treatment of a murine tumor model. Photochem. Photobiol. 1989, 49, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Waldow, S.M.; Mang, T.S.; Potter, W.R.; Malone, P.B.; Dougherty, T.J. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res 1985, 45, 572–576. [Google Scholar] [PubMed]
- Peng, Q.; Nesland, J.M. Effects of photodynamic therapy on tumor stroma. Ultrastruct. Pathol. 2004, 28, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane as a model for tumor biology. Exp. Cell Res. 2014, 328, 314–324. [Google Scholar] [CrossRef]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.C.; Harrington, D.A.; Yu, W.; Lewis, M.T.; Sikora, A.G. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and pre-clinical research. Am. J. Cancer Res. 2018, 8, 1642–1660. [Google Scholar]
- Guder, W.K.; Hartmann, W.; Buhles, C.; Burdack, M.; Busch, M.; Dünker, N.; Hardes, J.; Dirksen, U.; Bauer, S.; Streitbürger, A. 5-ALA-mediated fluorescence of musculoskeletal tumors in a chick chorio-allantoic membrane model: Preclinical in vivo qualification analysis as a fluorescence-guided surgery agent in orthopedic oncology. J. Orthop. Surg. Res. 2022, 17, 34. [Google Scholar] [CrossRef]
- Sys, G.M.L.; Lapeire, L.; Stevens, N.; Favoreel, H.; Forsyth, R.; Bracke, M.; De Wever, O. The in ovo CAM-assay as a Xenograft Model for Sarcoma. J. Vis. Exp. 2013, 77, e50522. [Google Scholar] [CrossRef]
- Gomez, P.; Morcuende, J. High-grade sarcomas mimicking traumatic intramuscular hematomas: A report of three cases. Iowa Orthop. J. 2004, 24, 106–110. [Google Scholar] [PubMed]
- Kontogeorgakos, V.A.; Martinez, S.; Dodd, L.; Brigman, B.E. Extremity soft tissue sarcomas presented as hematomas. Arch. Orthop. Trauma Surg. 2009, 130, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. The effect of photodynamic therapy on tumor angiogenesis. Cell Mol. Life Sci. 2009, 66, 2275–2283. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Van Beijnum, J.R.; Van Berkel, M.; Bergh, H.V.D.; Griffioen, A.W. Vascular regrowth following photodynamic therapy in the chicken embryo chorioallantoic membrane. Angiogenesis 2010, 13, 281–292. [Google Scholar] [CrossRef]
- Weiss, A.; Bergh, H.V.D.; Griffioen, A.W.; Nowak-Sliwinska, P. Angiogenesis inhibition for the improvement of photodynamic therapy: The revival of a promising idea. Biochim. Biophys. Acta (BBA) Rev. Cancer 2012, 1826, 53–70. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Kübler, A.C.; Stenzel, W.; Rühling, M.; Meul, B.; Fischer, J.-H. Experimental evaluation of possible side effects of intra-operative photodynamic therapy on rabbit blood vessels and nerves. Lasers Surg. Med. 2003, 33, 247–255. [Google Scholar] [CrossRef]
- Grant, W.E.; Speight, P.M.; MacRobert, A.J.; Hopper, C.; Bown, S.G. Photodynamic therapy of normal rat arteries after photosensitisation using disulphonated aluminium phthalocyanine and 5-aminolaevulinic acid. Br. J. Cancer 1994, 70, 72–78. [Google Scholar] [CrossRef]
- Stolik, S.; Delgado, J.; Pérez, A.; Anasagasti, L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J. Photochem. Photobiol. B 2000, 57, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.K.; Smith, L.F.; Thurgood, R.F.; Kereszti, Z.; Straight, R.C. Intraoperative phototherapy (PDT) and surgical resection in a mouse neuroblastoma model. Lasers Surg. Med. 1990, 10, 275–279. [Google Scholar] [CrossRef] [PubMed]
| ID | Entitity | Age | Sex | Grade | Sample Origin | Pretreatment | Inoculated Eggs | Loss (Egg Death) | Contaminated Eggs | No Visible Tumor | Evaluable Grafts (PDD) | Loss (Death after PDT) | Evaluable Grafts (PDT) | Confirmed Histology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHOR-SN1 | Chordoma | 53 | m | n.a. | resection, Os sacrum; metachronous lymphnodular, osseous and pulmonary metastases | radiotherapy, Imatinib, Sirolimus, Sorafenib, debulking | 4 | 0 | 0 | 0 | 4 | n.d. | n.d. | 3 |
| E0R4 | GCTB | 45 | m | n.a. | curettage, proximal tibia | none | 11 | 0 | 1 | 1 | 9 | n.d. | n.d. | (7) † |
| E5K5 | Osteosarcoma | 20 | m | TNM: cT3 N0 M0 G high-grade | resection of primary site, proximal femur | 2× Doxorubicin/Cisplatin, 3× MTX | 12 | 1 | 0 | 0 | 11 | 2 | 8 ‡ | 10 |
| ESS-SN1 | Endometrial Stromal Sarcoma | 19 | f | TNM: T4 Nx M1 (OTH, PER) Stage: IV (UICC 2016 [8th Edition]) high-grade | palliative resection, middle and lower abdomen | 3× Ifosfamid/Doxorubicin | 10 | 0 | 6 | 0 | 7 | 0 | 7 | 7 |
| GISTa-SN1 | GIST | 67 | m | TNM (initial): pT4 cN0 cM0; current: Tx Nx M1 Stage (initial): IIIb (UICC 2016 [8th Edition]); current: Stage IV | resection of metastasis, right abdominal wall | laparotomy, BLU285 | 5 | 1 | 0 | 0 | 4 | n.d. | n.d. | n.d. |
| H7L6 | Synovialsarcoma | 20 | f | TNM: pT1 L0 V0 Pn0 R0 (UICC 2017 [8th Edition]) Stage: FNCLCC-Grading: 3 + 1 + 0 = 4 (G2) | resection, proximal upper arm | none | 8 | 0 | 5 | 0 | 3 | n.d. | n.d. | n.d. |
| H7N5 | Chondrosarcoma | 53 | f | TNM: pT1 L0 V0 Pn0 R0 (UICC 2017 [8th Edition]) | resection, lateral distal thigh | none | 23 (PDXs) | 5 | 6 | 0 | 12 (PDXs) | n.d. | n.d. | n.d. |
| 13 (CDXs) | 2 | 2 | 3 | 6 (CDXs) | n.d. | n.d. | 7 | |||||||
| H8T8 | Chondrosarcoma | 48 | m | TNM: ypT4 ypNX L0 V0 Pn0 R0 (UICC 2017 [8th Edition]) | resection, dorsal thigh | 3× Doxorubicin/Ifosfamide; radiotherapy | 20 | 4 | 2 | 2 | 11 | 1 | 3 ‡ | 11 |
| I0A0 | Ewing Sarcoma | 20 | f | Stage: IV | metastasectomy of pulmonary metastasis | 6× VIDE, 3× VAI; 4× Temoz./Irinot./Vinc., 1× ICE, 1× VAI; irradiation of the lung; 3× Temoz./Irinot.; 6× Topot./Cycloph. | 13 | 1 | 5 | 1 | 6 | n.d. | n.d. | 5 |
| I7N42 | DSRCT | 21 | f | TNM: Tx N0 M1 (REN, PER) Stage: IV (UICC 2016 [8th Edition]) | resection of pulmonary primarius | 3× VIDE | 4 | 0 | 0 | 1 | 3 | n.d. | n.d. | 1 |
| M4M51 | Ewing Sarcoma | 22 | m | TNM: M0 | ascites fluid, recurrent tumor sites: neuroforamina and Os sacrum | 6× VIDE, 8× VAI | 20 | 3 | 4 | 0 | 13 | n.d. | n.d. | 9 |
| M4M53 | 6× VIDE, 8× VAI; 4× Topotecan/Cyclophosphamide | 8 | 0 | 0 | 0 | 8 | 2 | 4 ‡ | 6 | |||||
| MFS-SN1 | Myxofibrosarcoma | 71 | m | TNM (initial): pT2b N0 M1 (PUL) G3 (MX) TNM (current): pT4, pN0(0/3), L0, V0, Pn0, R1, G high-grade | resection, right adductor approaching femur | resection | 13 | 0 | 1 | 0 | 12 | 2 | 8 ‡ | 10 |
| mLPS-SN1 | Myxoid Liposarcoma | 62 | m | TNM: pT2a/b N0 M1 Stage: IV (UICC 2009 [7th Edition]) | abdominal tumor debulking, Omentum and Colon Transversum | resection, 3× Doxorubicin/Ifosfamide; laparotomy, Ixoten, Trabectidin, Eribulin | 10 | 0 | 0 | 0 | 10 | 2 | 7 ‡ | 9 |
| MPNST-SN1 | MPNST | 66 | f | TNM: rT0 N0 M1 pulm, per Stage: IV (UICC 2016 [8th Edition]) | metastasis, small intestine section | radiotherapy | 6 | 2 | 0 | 1 | 3 | n.d. | n.d. | n.d. |
| Y0L5 | Ewing Sarcoma | 20 | f | TNM: cT2 N0 M0 Stage: IIB (UICC 2016 [8th Edition]) | resection, left femur | 5× VDC, 4× IE; Denosumab | 14 | 2 | 6 | 1 | 5 | n.d. | n.d. | 4 |
| EW-7 | Ewing Sarcoma | 20 * | f * | n.a. | derived from metastatic site: Pleural effusion * | n.a. | 44 | 13 | 3 | 0 | 28 | n.d. | n.d. | 5 |
| TC-32 | Ewing Sarcoma | 17 * | f * | n.a. | derived from sampling site: Bone; left ilium * | n.a. | 99 | 12 | 1 | 4 | 82 | 0 | 10 | 10 |
| PDT Dose [J/cm2] | Viable Eggs [n] | Total [n] | Survivors [%] | Linear Regression Model |
|---|---|---|---|---|
| 0 | 13 | 13 | 100.0 | |
| 10 | 5 | 8 | 62.5 | m = −2.5 ± 0.4 |
| 20 | 3 | 8 | 37.5 | R2 = 0.952 |
| 30 | 2 | 8 | 25.0 | p = 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerkhoff, M.; Grunewald, S.; Schaefer, C.; Zöllner, S.K.; Plaumann, P.; Busch, M.; Dünker, N.; Ketzer, J.; Kersting, J.; Bauer, S.; et al. Evaluation of the Effect of Photodynamic Therapy on CAM-Grown Sarcomas. Bioengineering 2023, 10, 464. https://doi.org/10.3390/bioengineering10040464
Kerkhoff M, Grunewald S, Schaefer C, Zöllner SK, Plaumann P, Busch M, Dünker N, Ketzer J, Kersting J, Bauer S, et al. Evaluation of the Effect of Photodynamic Therapy on CAM-Grown Sarcomas. Bioengineering. 2023; 10(4):464. https://doi.org/10.3390/bioengineering10040464
Chicago/Turabian StyleKerkhoff, Maximilian, Susanne Grunewald, Christiane Schaefer, Stefan K. Zöllner, Pauline Plaumann, Maike Busch, Nicole Dünker, Julia Ketzer, Josephine Kersting, Sebastian Bauer, and et al. 2023. "Evaluation of the Effect of Photodynamic Therapy on CAM-Grown Sarcomas" Bioengineering 10, no. 4: 464. https://doi.org/10.3390/bioengineering10040464
APA StyleKerkhoff, M., Grunewald, S., Schaefer, C., Zöllner, S. K., Plaumann, P., Busch, M., Dünker, N., Ketzer, J., Kersting, J., Bauer, S., Hardes, J., Streitbürger, A., Dirksen, U., Hartmann, W., & Guder, W. K. (2023). Evaluation of the Effect of Photodynamic Therapy on CAM-Grown Sarcomas. Bioengineering, 10(4), 464. https://doi.org/10.3390/bioengineering10040464








