Production of a Novel Protopanaxatriol-Type Ginsenoside by Yeast Cell Factories
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. The Engineered Yeast Construction
2.3. Fermentation and Product Extraction of the Engineered Yeasts
2.4. Product Analysis by HPLC, HPLC-ESI-MS and NMR
3. Results
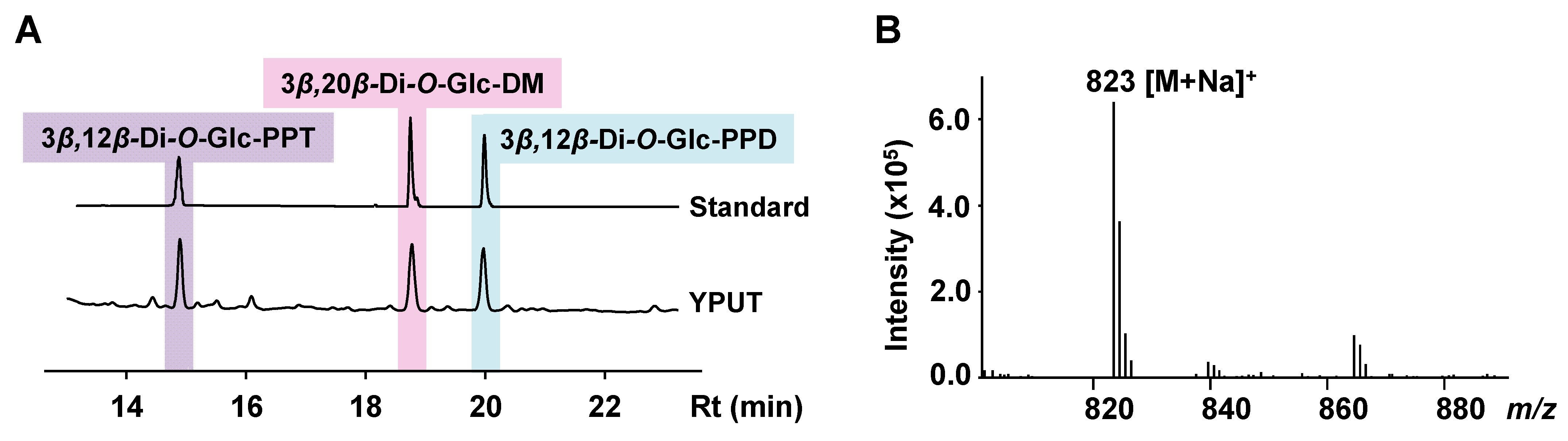
3.1. Constructing 3β,12β-Di-O-Glc-PPT-Producing Yeast
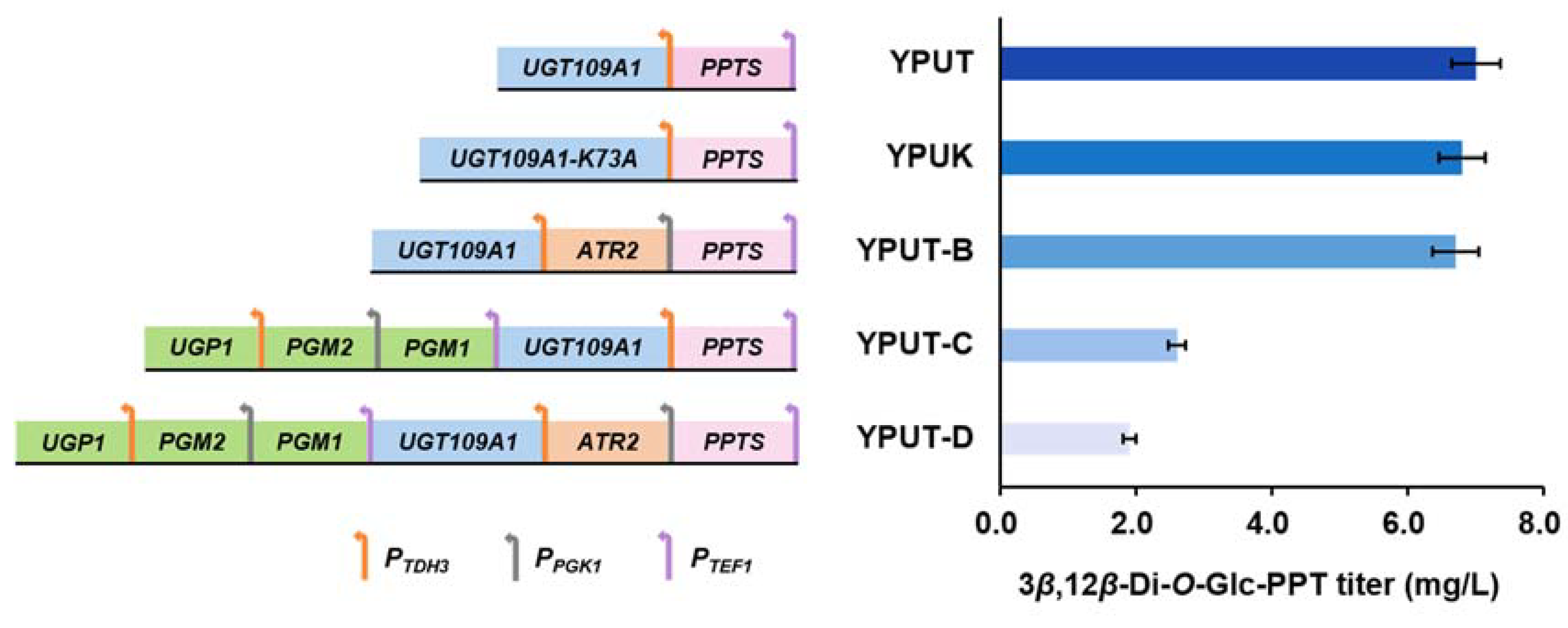
3.2. Improving the Catalytic Efficiency of UGT109A1 to Promote the Glycosylation of PPT
3.3. Improving Precursor Supply for the Production of 3β,12β-Di-O-Glc-PPT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar]
- Mohanan, P.; Yang, T.J.; Song, Y.H. Genes and regulatory mechanisms for ginsenoside biosynthesis. J. Plant Biol. 2023, 66, 87–97. [Google Scholar]
- Ali, M.Y.; Jannat, S.; Rahman, M.M. Ginsenoside derivatives inhibit advanced glycation end-product formation and glucose–fructose mediated protein glycation in vitro via a specific structure–activity relationship. Bioorg. Chem. 2021, 111, 104844. [Google Scholar]
- Ota, T.; Maeda, M.; Odashima, S. Mechanism of action of ginsenoside Rh2: Uptake and metabolism of ginsenoside Rh2 by cultured B16 melanoma cells. J. Pharm. Sci. 1991, 80, 1141–1146. [Google Scholar]
- Chen, Y.; Xu, Y.; Zhu, Y.; Li, X. Anti-cancer effects of ginsenoside compound K on pediatric acute myeloid leukemia cells. Cancer Cell Int. 2013, 13, 24. [Google Scholar]
- Kim, A.D.; Kang, K.A.; Kim, H.S.; Kim, D.H.; Choi, Y.H.; Lee, S.J.; Kim, H.S.; Hyun, J.W. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013, 4, e750. [Google Scholar]
- Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang, Y.; Zhang, H. Anticancer effects of ginsenoside Rg3. Int. J. Mol. Med. 2017, 39, 507–518. [Google Scholar]
- Yang, X.D.; Yang, Y.Y.; Ouyang, D.S.; Yang, G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia. 2015, 100, 208–220. [Google Scholar]
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black ginseng and its saponins: Preparation, phytochemistry and pharmacological effects. Molecules. 2019, 24, 1856. [Google Scholar]
- Liu, J.; Wang, Y.; Yu, Z.; Lv, G.; Huang, X.; Lin, H.; Ma, C.; Lin, Z.; Qu, P. Functional mechanism of ginsenoside compound K on tumor growth and metastasis. Integr. Cancer Ther. 2022, 21, 1–13. [Google Scholar]
- Liu, T.; Zhu, L.; Wang, L. A narrative review of the pharmacology of ginsenoside compound K. Ann. Transl. Med. 2022, 10, 234. [Google Scholar]
- Ge, G.; Yan, Y.; Cai, H. Ginsenoside Rh2 inhibited proliferation by inducing ROS mediated ER stress dependent apoptosis in lung cancer cells. Biol. Pharm. Bull. 2017, 40, 2117–2124. [Google Scholar]
- Li, Q.; Li, B.; Dong, C.; Wang, Y.; Li, Q. 20(S)-Ginsenoside Rh2 suppresses proliferation and migration of hepatocellular carcinoma cells by targeting EZH2 to regulate CDKN2A-2B gene cluster transcription. Eur. J. Pharmacol. 2017, 815, 173–180. [Google Scholar]
- Mook-Jung, I.; Hong, H.-S.; Boo, J.H.; Lee, K.H.; Yun, S.H.; Cheong, M.Y.; Joo, I.; Huh, K.; Jung, M.W. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J. Neurosci. Res. 2001, 63, 509–515. [Google Scholar]
- Wang, X.Y.; Zhang, J.T. NO mediates ginsenoside Rg1-induced long-term potentiation in anesthetized rats. Acta Pharmacol. Sin. 2001, 22, 1099–1102. [Google Scholar]
- Wang, Y.Z.; Chen, J.; Chu, S.F.; Wang, Y.S.; Wang, X.Y.; Chen, N.H.; Zhang, J.T. Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1’s metabolites ginsenoside Rh1 and protopanaxatriol. J. Pharmacol. Sci. 2009, 109, 504–510. [Google Scholar]
- Shang, D.; Li, Z.; Tan, X.; Liu, H.; Tu, Z. Inhibitory effects and molecular mechanisms of ginsenoside Rg1 on the senescence of hematopoietic stem cells. Fundam. Clin. Pharmacol. 2023, 1–9. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Wu, A.; Cao, Y.; Dai, X.; Liang, Y.; Li, X. Ginsenosides in central nervous system diseases: Pharmacological actions, mechanisms, and therapeutics. Phytother. Res. 2022, 36, 1523–1544. [Google Scholar]
- Sala, F.; Mulet, J.; Choi, S.; Jung, S.-Y.; Nah, S.-Y.; Rhim, H.; Valor, L.M.; Criado, M.; Sala, S. Effects of ginsenoside Rg2 on human neuronal nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2002, 301, 1052–1059. [Google Scholar]
- Wang, L.; Yuan, D.; Zhang, D.; Zhang, W.; Liu, C.; Cheng, H.; Song, Y.; Tan, Q. Ginsenoside Re promotes nerve regeneration by facilitating the proliferation, differentiation and migration of schwann cells via the ERK- and JNK-dependent pathway in rat model of sciatic nerve crush injury. Cell. Mol. Neurobiol. 2015, 35, 827–840. [Google Scholar]
- Chen, L.M.; Zhou, X.M.; Cao, Y.L.; Hu, W.X. Neuroprotection of ginsenoside Re in cerebral ischemia-reperfusion injury in rats. J. Asian Nat. Prod. Res. 2008, 10, 439–445. [Google Scholar]
- Tu, T.-H.T.; Sharma, N.; Shin, E.-J.; Tran, H.-Q.; Lee, Y.J.; Jeong, J.H.; Jeong, J.H.; Nah, S.Y.; Tran, H.-Y.P.; Byun, J.K.; et al. Ginsenoside Re protects trimethyltin-induced neurotoxicity via activation of IL-6-mediated phosphoinositol 3-kinase/Akt signaling in mice. Neurochem. Res. 2017, 42, 3125–3139. [Google Scholar]
- Wang, D.D.; Jin, Y.; Wang, C.; Kim, Y.J.; Perez, Z.E.J.; Baek, N.I.; Mathiyalagan, R.; Markus, J.; Yang, D.C. Rare ginsenoside Ia synthesized from F1 by cloning and overexpression of the UDP-glycosyltransferase gene from Bacillus subtilis: Synthesis, characterization, and in vitro melanogenesis inhibition activity in BL6B16 cells. J. Ginseng Res. 2018, 42, 42–49. [Google Scholar]
- Liang, H.; Hu, Z.; Zhang, T.; Gong, T.; Chen, J.; Zhu, P.; Li, Y.; Yang, J. Production of a bioactive unnatural ginsenoside by metabolically engineered yeasts based on a new UDP-glycosyltransferase from Bacillus subtilis. Metab. Eng. 2017, 44, 60–69. [Google Scholar]
- Jiang, F.; Zhou, C.; Li, Y.; Deng, H.; Gong, T.; Chen, J.; Chen, T.; Yang, J.; Zhu, P. Metabolic engineering of yeasts for green and sustainable production of bioactive ginsenosides F2 and 3β,20S-Di-O-Glc-DM. Acta Pharm. Sin. B. 2022, 12, 3167–3176. [Google Scholar]
- Hu, Z.-F.; Gu, A.-D.; Liang, L.; Li, Y.; Gong, T.; Chen, J.-J.; Chen, T.-J.; Yang, J.-L.; Zhu, P. Construction and optimization of microbial cell factories for sustainable production of bioactive dammarenediol-II glucosides. Green Chem. 2019, 21, 3286–3299. [Google Scholar]
- Jung, S.C.; Kim, W.; Park, S.C.; Jeong, J.; Park, M.K.; Lim, S.; Lee, Y.; Im, W.T.; Lee, J.H.; Choi, G.; et al. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014, 55, 2177–2188. [Google Scholar]
- Wang, P.; Wei, W.; Ye, W.; Li, X.; Zhao, W.; Yang, C.; Li, C.; Yan, X.; Zhou, Z. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency. Cell Discov. 2019, 5, 5. [Google Scholar]
- Wang, P.; Wang, J.; Zhao, G.; Yan, X.; Zhou, Z. Systematic optimization of the yeast cell factory for sustainable and high efficiency production of bioactive ginsenoside compound K. Synth. Syst. Biotechnol. 2021, 6, 69–76. [Google Scholar]
- Dai, Z.; Wang, B.; Liu, Y.; Shi, M.; Wang, D.; Zhang, X.; Liu, T.; Huang, L.; Zhang, X. Producing aglycons of ginsenosides in bakers’ yeast. Sci. Rep. 2014, 4, 3698. [Google Scholar]
- Li, X.; Wang, Y.; Fan, Z.; Wang, Y.; Wang, P.; Yan, X.; Zhou, Z. High-level sustainable production of the characteristic protopanaxatriol-type saponins from Panax species in engineered Saccharomyces cerevisiae. Metab. Eng. 2021, 66, 87–97. [Google Scholar]
- Zhou, C.; Chen, T.; Gu, A.; Hu, Z.; Li, Y.; Gong, T.; Chen, J.; Yang, J.; Zhu, P. Combining protein and metabolic engineering to achieve green biosynthesis of 12β-O-Glc-PPD in Saccharomyces cerevisiae. Green Chem. 2023, 25, 1356–1367. [Google Scholar]
- Zhao, Y.; Fan, J.; Wang, C.; Feng, X.; Li, C. Enhancing oleanolic acid production in engineered Saccharomyces cerevisiae. Bioresour. Technol. 2018, 257, 339–343. [Google Scholar]
- Wang, J.H.; Wang, D.; Li, W.X.; Huang, Y.; Dai, Z.B.; Zhang, X.L. Optimization of UDP-glucose supply module and production of ginsenoside F1 in Saccharomyces cerevisiae. Zhongguo Zhong Yao Za Zhi 2019, 44, 4596–4604. [Google Scholar]
- Wang, D.; Wang, J.; Shi, Y.; Li, R.; Fan, F.; Huang, Y.; Li, W.; Chen, N.; Huang, L.; Dai, Z.; et al. Elucidation of the complete biosynthetic pathway of the main triterpene glycosylation products of Panax notoginseng using a synthetic biology platform. Metab. Eng. 2020, 61, 131–140. [Google Scholar]
- Zhou, L.; Li, Z.K.; Li, C.Y.; Liang, Y.Q.; Yang, F. Anticancer properties and pharmaceutical applications of ginsenoside compound K: A review. Chem. Bio. Drug Des. 2021, 99, 286–300. [Google Scholar]
- Matsuda, H.; Samukawa, K.; Kubo, M. Anti-inflammatory activity of ginsenoside Ro1. Planta Med. 1990, 56, 19–23. [Google Scholar]
- Xie, J.T.; Mehendale, S.R.; Li, X.; Quigg, R.; Wang, X.; Wang, C.Z.; Wu, J.A.; Aung, H.H.; Rue, P.A.; Bell, G.I.; et al. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 319–325. [Google Scholar]
- Sun, Y.; Liu, Y.; Chen, K. Roles and mechanisms of ginsenoside in cardiovascular diseases: Progress and perspectives. Sci. China Life Sci. 2016, 59, 292–298. [Google Scholar]
- Park, E.K.; Choo, M.K.; Kim, E.J.; Han, M.J.; Kim, D.H. Antiallergic activity of ginsenoside Rh2. Biol. Pharm. Bull. 2003, 26, 1581–1584. [Google Scholar]
- Liu, T.; Zuo, L.; Guo, D.; Chai, X.; Xu, J.; Cui, Z.; Wang, Z.; Hou, C. Ginsenoside Rg3 regulates DNA damage in non-small cell lung cancer cells by activating VRK1/P53BP1 pathway. Biomed. Pharmacother. 2019, 120, 109483. [Google Scholar]
- Chen, C.; Lv, Q.; Li, Y.; Jin, Y.H. The anti-tumor effect and underlying apoptotic mechanism of ginsenoside Rk1 and Rg5 in human liver cancer cells. Molecules. 2021, 26, 3926. [Google Scholar]
- Nakhjavani, M.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Yool, A.J.; Pei, J.V.; Townsend, A.R.; Hardingham, J.E. Stereoselective anti-cancer activities of ginsenoside Rg3 on triple negative breast cancer cell models. Pharmaceutics. 2019, 12, 117. [Google Scholar]
- Huang, Q.; Zhang, H.; Bai, L.P.; Law, B.Y.K.; Xiong, H.; Zhou, X.; Xiao, R.; Qu, Y.Q.; Mok, S.W.F.; Liu, L.; et al. Novel ginsenoside derivative 20 (S)-Rh2E2 suppresses tumor growth and metastasis in vivo and in vitro via intervention of cancer cell energy metabolism. Cell Death Dis. 2020, 11, 621. [Google Scholar]
- Guo, J.; Zhou, Y.J.; Hillwig, M.L.; Shen, Y.; Yang, L.; Wang, Y.; Zhang, X.; Liu, W.; Peters, R.J.; Chen, X.; et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc. Natl. Acad. Sci. USA. 2013, 110, 12108–12113. [Google Scholar]
- Girvan, H.M.; Munro, A.W. Applications of microbial cytochrome P450 enzymes in biotechnology and synthetic biology. Curr. Opin. Chem. Biol. 2016, 31, 136–145. [Google Scholar]
- Durairaj, P.; Li, S. Functional expression and regulation of eukaryotic cytochrome P450 enzymes in surrogate microbial cell factories. Eng. Microbiol. 2022, 2, 100011. [Google Scholar]
- Jin, Z.; Cong, Y.; Zhu, S.; Xing, R.; Zhang, D.; Yao, X.; Wan, R.; Wang, Y.; Yu, F. Two classes of cytochrome P450 reductase genes and their divergent functions in Camptotheca acuminata Decne. Int. J. Biol. Macromol. 2019, 138, 1098–1108. [Google Scholar]
- Huang, J.; Zha, W.; An, T.; Dong, H.; Huang, Y.; Wang, D.; Yu, R.; Duan, L.; Zhang, X.; Peters, R.J.; et al. Identification of RoCYP01 (CYP716A155) enables construction of engineered yeast for high-yield production of betulinic acid. Appl. Microbiol. Biotechnol. 2019, 103, 7029–7039. [Google Scholar]
- Zhu, M.; Wang, C.; Sun, W.; Zhou, A.; Wang, Y.; Zhang, G.; Zhou, X.; Huo, Y.; Li, C. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants. Metab. Eng. 2018, 45, 43–50. [Google Scholar]

| Strain | Genotype or Characteristic | Target Product |
|---|---|---|
| YPU | PTEF1-synDS-GFP-TCYC1, PTDH3-tPPDS-L18I-TADH2, PTEF2-ATR2-TTPI1, PPGK1-tHMG1-TADH1, PTEF1-HAC1-TCYC1 and TRP1 marker gene integrated into the rDNA site, and PTDH3-IDI1-TTPI1, PPGK1-ERG20-TADH1, PTEF1-ERG9-TCYC1, PPGK1-ERG1-TADH1, PTEF1-ERG7−-TCYC1 and LEU2 marker gene integrated into the δ4 site of Y-ΔHXK2 | PPD |
| YPUT | PTEF1-PPTS-TCYC1, PTDH3-UGT109A1-TADH2 and HIS3 marker gene integrated into the δ1 site of YPU | 3β,12β-Di-O-Glc-PPT |
| YPUK | PTEF1-PPTS-TCYC1, PTDH3-UGT109A1-K73A-TADH2 and HIS3 marker gene integrated into the δ1 site of YPU | 3β,12β-Di-O-Glc-PPT |
| YPUT-B | PTEF1-PPTS-TCYC1, PPGK1-ATR2-TTPI1, PTDH3-UGT109A1-TADH2 and HIS3 marker gene integrated into the δ1 site of YPU | 3β,12β-Di-O-Glc-PPT |
| YPUT-C | PTEF1-PGM1-TCYC1, PPGK1-PGM2-TADH1, PTDH3-UGP1-TADH2, PTEF1-PPTS-TCYC1, PTDH3-UGT109A1-TADH2 and HIS3 marker gene integrated into the δ1 site of YPU | 3β,12β-Di-O-Glc-PPT |
| YPUT-D | PTEF1-PGM1-TCYC1, PPGK1-PGM2-TADH1, PTDH3-UGP1-TADH2, PTEF1-PPTS-TCYC1, PPGK1-ATR2-TTPI1, PTDH3-UGT109A1-TADH2 and HIS3 marker gene integrated into the δ1 site of YPU | 3β,12β-Di-O-Glc-PPT |
| Yield a (mg/L) | YPUT | YPUT-B | YPUT-C | YPUT-D |
|---|---|---|---|---|
| 3β,12β-Di-O-Glc-PPT | 7.0 ± 0.3 | 6.7 ± 0.4 | 2.6 ± 0.1 | 1.9 ± 0.4 |
| 3β,12β-Di-O-Glc-PPD | 4.8 ± 0.2 | 5.2 ± 0.5 | 6.3 ± 0.5 | 7.2 ± 0.4 |
| 3β,20S-Di-O-Glc-DM | 3.8 ± 0.2 | 3.6 ± 0.2 | 3.7 ± 0.2 | 3.1 ± 0.3 |
| PPT | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Gong, T.; Chen, J.; Chen, T.; Yang, J.; Zhu, P. Production of a Novel Protopanaxatriol-Type Ginsenoside by Yeast Cell Factories. Bioengineering 2023, 10, 463. https://doi.org/10.3390/bioengineering10040463
Zhou C, Gong T, Chen J, Chen T, Yang J, Zhu P. Production of a Novel Protopanaxatriol-Type Ginsenoside by Yeast Cell Factories. Bioengineering. 2023; 10(4):463. https://doi.org/10.3390/bioengineering10040463
Chicago/Turabian StyleZhou, Chen, Ting Gong, Jingjing Chen, Tianjiao Chen, Jinling Yang, and Ping Zhu. 2023. "Production of a Novel Protopanaxatriol-Type Ginsenoside by Yeast Cell Factories" Bioengineering 10, no. 4: 463. https://doi.org/10.3390/bioengineering10040463
APA StyleZhou, C., Gong, T., Chen, J., Chen, T., Yang, J., & Zhu, P. (2023). Production of a Novel Protopanaxatriol-Type Ginsenoside by Yeast Cell Factories. Bioengineering, 10(4), 463. https://doi.org/10.3390/bioengineering10040463






