5-Aminolevulinic Acid as a Theranostic Agent for Tumor Fluorescence Imaging and Photodynamic Therapy
Abstract
1. Introduction
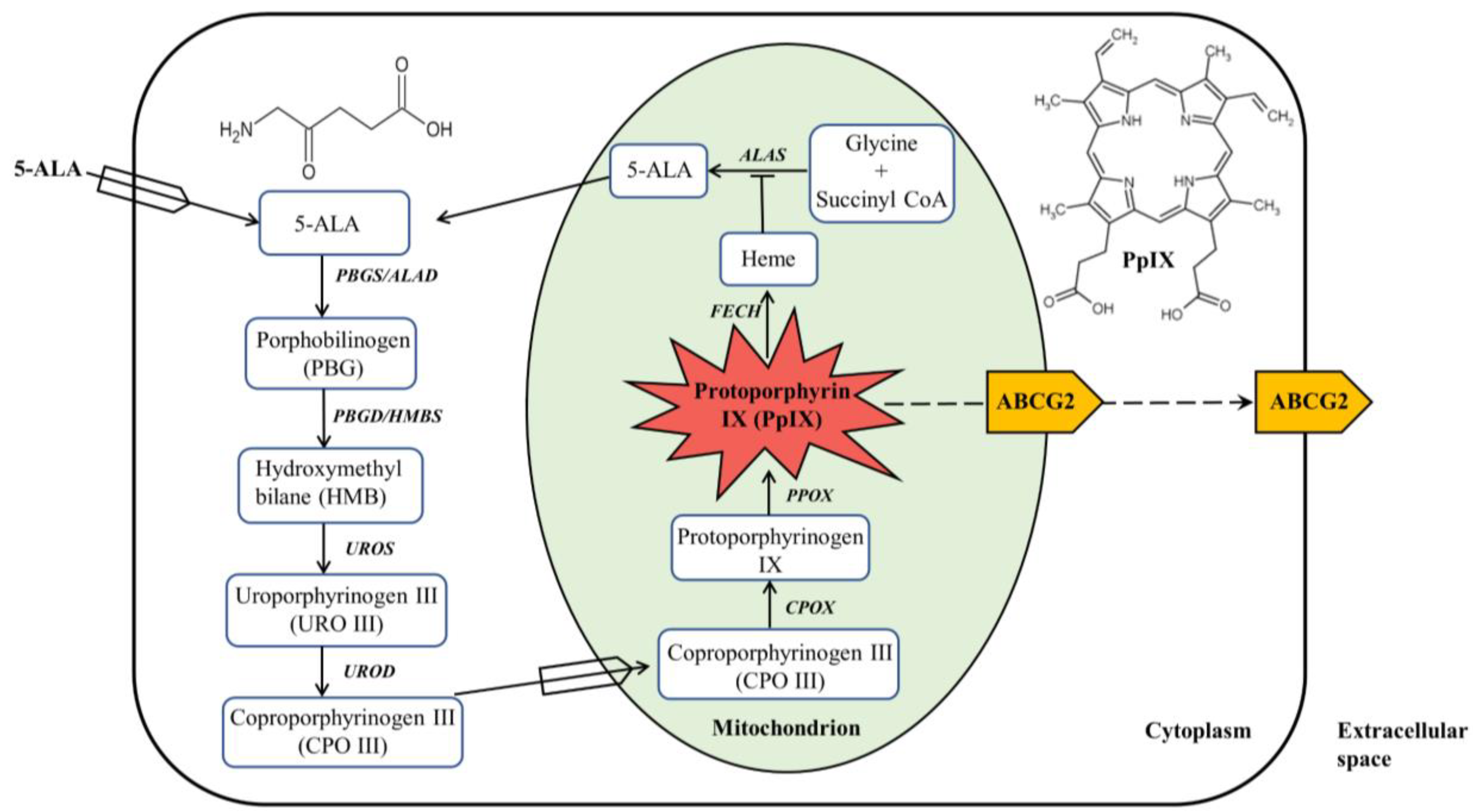
2. ALA-Mediated PpIX Biosynthesis in the Heme Biosynthesis Pathway
3. ALA as a Therapeutic Agent for PDT
4. Cell Death Mechanisms Induced by ALA-PDT
5. ALA as an Intraoperative Imaging Probe
5.1. Glioma
5.2. Bladder Cancer
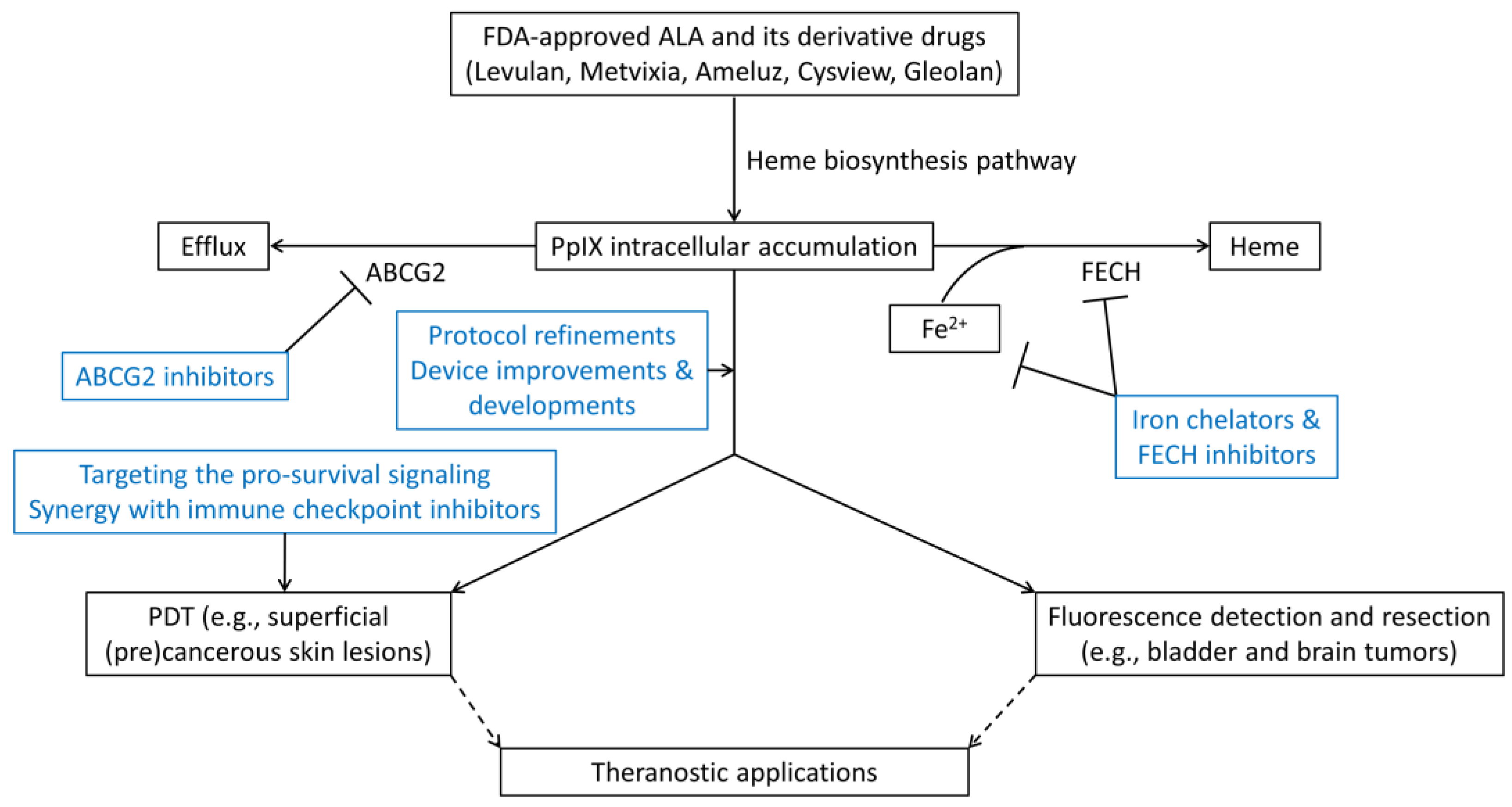
6. Strategies for Enhancing ALA-PpIX Fluorescence and ALA-PDT Response
6.1. Protocol Refinements
6.2. Device Improvements & Developments
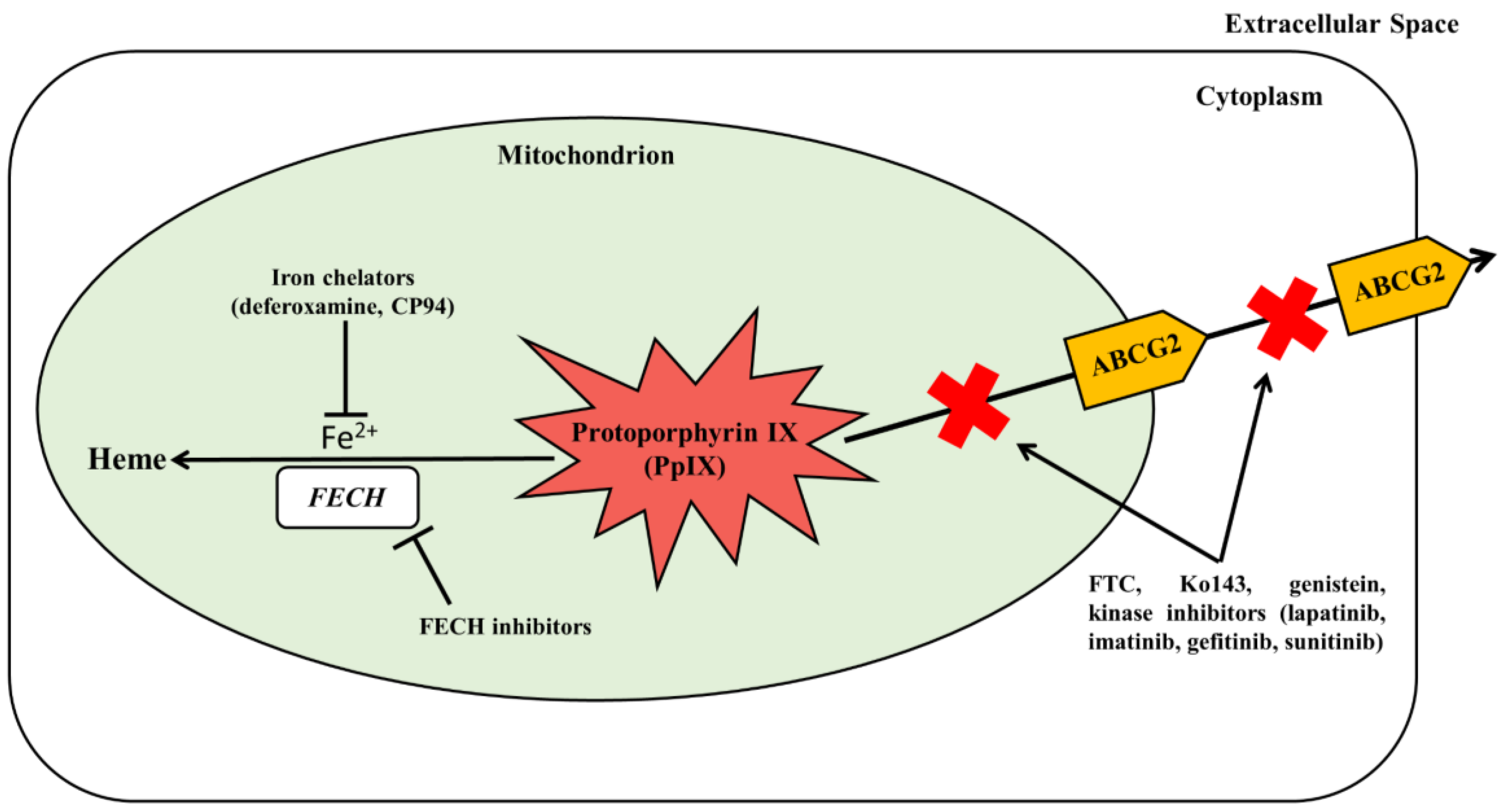
6.3. Inhibition of PpIX Bioconversion
6.4. Inhibition of PpIX Efflux
6.5. Targeting the Pro-Survival Signaling
6.6. Synergy with Immune Checkpoint Inhibitors
7. ALA-PpIX as a Fluorescent Theranostic Agent
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem. Photobiol. 2007, 74, 656–669. [Google Scholar] [CrossRef]
- Abdel-Kader, M.H. History of Photodynamic Therapy. In Photodynamic Therapy; Abdel-Kader, M.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–22. ISBN 978-3-642-39628-1. [Google Scholar]
- Daniell, M.D.; Hill, J.S. A history of photodynamic therapy. ANZ J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Battersby, A.R. Tetrapyrroles: The Pigments of Life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Lugaci, H. Destruction of Erythroleukaemic Cells by Photoactivation of Endogenous Porphyrins. Br. J. Cancer 1987, 56, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic Therapy with Endogenous Protoporphyrin: IX: Basic Principles and Present Clinical Experience. J. Photochem. Photobiol. B Biol. 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Casas, A. Clinical Uses of 5-Aminolaevulinic Acid in Photodynamic Treatment and Photodetection of Cancer: A Review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ajioka, R.S.; Phillips, J.D.; Kushner, J.P. Biosynthesis of Heme in Mammals. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 723–736. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the Management of Malignant Glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in Pathophysiology: A Matter of Scavenging, Metabolism and Trafficking across Cell Membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Ponka, P. Cell Biology of Heme. Am. J. Med. Sci. 1999, 318, 241–256. [Google Scholar] [CrossRef]
- Heinemann, I.U.; Jahn, M.; Jahn, D. The Biochemistry of Heme Biosynthesis. Arch. Biochem. Biophys. 2008, 474, 238–251. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Spatially Resolved Cellular Responses to Singlet Oxygen. Photochem. Photobiol. 2006, 82, 1178. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Sassa, S.; Schwartz, S.; Ruth, A. Accumulation of Protoporphyrin IX from delta-Aminolevulinic Acid in Bovine Skin Fibroblasts with Hereditary Erythropoietic Protoporphyria. A Gene-Dosage Effect. J. Exp. Med. 1981, 153, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Ormrod, D.; Jarvis, B. Topical Aminolevulinic Acid HCl Photodynamic Therapy. Am. J. Clin. Dermatol. 2000, 1, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Schulte, K.W.; Ruzicka, T.; Fritsch, C. Aminolevulinic Acid (Levulan) in Photodynamic Therapy of Actinic Keratoses. Ski. Ther. Lett. 2001, 6, 5. [Google Scholar]
- Gauffier, J.-M.; Berg, K.; Peng, Q.; Anholt, H.; Selbo, P.K.; Ma, L.-W.; Moan, J. Use of 5-Aminolevulinic Acid Esters to Improve Photodynamic Therapy on Cells in Culture. Cancer Res. 1997, 57, 1481–1486. [Google Scholar]
- Fritsch, C.; Homey, B.; Stahl, W.; Lehmann, P.; Ruzicka, T.; Sies, H. Preferential Relative Porphyrin Enrichment in Solar Keratoses upon Topical Application of ^-Aminolevulinic Acid Methylester. Photochem. Photobiol. 1998, 68, 218–221. [Google Scholar] [CrossRef]
- Peng, Q.; Soler, A.M.; Warloe, T.; Nesland, J.M.; Giercksky, K.E. Selective Distribution of Porphyrins in Skin Thick Basal Cell Carcinoma after Topical Application of Methyl 5-Aminolevulinate. J. Photochem. Photobiol. B 2001, 62, 140–145. [Google Scholar] [CrossRef]
- Siddiqui, M.A.A.; Perry, C.M.; Scott, L.J. Topical Methyl Aminolevulinate. Am. J. Clin. Derm. 2004, 5, 127–137. [Google Scholar] [CrossRef]
- Maisch, T.; Santarelli, F.; Schreml, S.; Babilas, P.; Szeimies, R.-M. Fluorescence Induction of Protoporphyrin IX by a New 5-Aminolevulinic Acid Nanoemulsion Used for Photodynamic Therapy in a Full-Thickness Ex Vivo Skin Model. Exp. Dermatol. 2010, 19, e302–e305. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, U. A Review of BF-200 ALA for the Photodynamic Treatment of Mild-to-Moderate Actinic Keratosis. Future Oncol. 2017, 13, 2413–2428. [Google Scholar] [CrossRef]
- Peng, Q.; Berg, K.; Moan, J.; Kongshaug, M.; Nesland, J.M. 5-Aminolevulinic Acid-Based Photodynamic Therapy: Principles and Experimental Research. Photochem. Photobiol. 1997, 65, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Grebeňová, D.; Kuželová, K.; Smetana, K.; Pluskalová, M.; Cajthamlová, H.; Marinov, I.; Fuchs, O.; Souček, J.; Jarolím, P.; Hrkal, Z. Mitochondrial and Endoplasmic Reticulum Stress-Induced Apoptotic Pathways Are Activated by 5-Aminolevulinic Acid-Based Photodynamic Therapy in HL60 Leukemia Cells. J. Photochem. Photobiol. B Biol. 2003, 69, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kajimoto, Y.; Shibata, M.-A.; Miyoshi, N.; Ogawa, N.; Miyatake, S.-I.; Otsuki, Y.; Kuroiwa, T. Massive Apoptotic Cell Death of Human Glioma Cells via a Mitochondrial Pathway Following 5-Aminolevulinic Acid-Mediated Photodynamic Therapy. J. Neurooncol. 2007, 83, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Lv, T.; Zhang, Y.; Huang, Z.; Wang, X.; Wang, H. Induction of Apoptosis in HPV16 E7 Transfected Human Keratinocyte by ALA-Mediated Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2016, 13, 205–210. [Google Scholar] [CrossRef]
- Zhou, B.-R.; Zhang, L.-C.; Permatasari, F.; Liu, J.; Xu, Y.; Luo, D. ALA-PDT Elicits Oxidative Damage and Apoptosis in UVB-Induced Premature Senescence of Human Skin Fibroblasts. Photodiagnosis Photodyn. Ther. 2016, 14, 47–56. [Google Scholar] [CrossRef]
- Furre, I.E.; Møller, M.T.N.; Shahzidi, S.; Nesland, J.M.; Peng, Q. Involvement of Both Caspase-Dependent and -Independent Pathways in Apoptotic Induction by Hexaminolevulinate-Mediated Photodynamic Therapy in Human Lymphoma Cells. Apoptosis 2006, 11, 2031–2042. [Google Scholar] [CrossRef]
- Noodt, B.B.; Berg, K.; Stokke, T.; Peng, Q.; Nesland, J.M. Apoptosis and Necrosis Induced with Light and 5-Aminolaevulinic Acid-Derived Protoporphyrin IX. Br. J. Cancer 1996, 74, 22–29. [Google Scholar] [CrossRef]
- Kriska, T.; Korytowski, W.; Girotti, A.W. Hyperresistance to Photosensitized Lipid Peroxidation and Apoptotic Killing in 5-Aminolevulinate-Treated Tumor Cells Overexpressing Mitochondrial GPX4. Free Radic. Biol. Med. 2002, 33, 1389–1402. [Google Scholar] [CrossRef]
- Bisland, S.K.; Lilge, L.; Lin, A.; Rusnov, R.; Wilson, B.C. Metronomic Photodynamic Therapy as a New Paradigm for Photodynamic Therapy: Rationale and Preclinical Evaluation of Technical Feasibility for Treating Malignant Brain Tumors. Photochem. Photobiol. 2004, 80, 22–30. [Google Scholar] [CrossRef]
- François, A.; Salvadori, A.; Bressenot, A.; Bezdetnaya, L.; Guillemin, F.; D’Hallewin, M.A. How to Avoid Local Side Effects of Bladder Photodynamic Therapy: Impact of the Fluence Rate. J. Urol. 2013, 190, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Kist, M.; Vucic, D. Cell Death Pathways: Intricate Connections and Disease Implications. EMBO J. 2021, 40, e106700. [Google Scholar] [CrossRef] [PubMed]
- Coupienne, I.; Fettweis, G.; Rubio, N.; Agostinis, P.; Piette, J. 5-ALA-PDT Induces RIP3-Dependent Necrosis in Glioblastoma. Photochem. Photobiol. Sci. 2011, 10, 1868. [Google Scholar] [CrossRef] [PubMed]
- Coupienne, I.; Fettweis, G.; Piette, J. RIP3 Expression Induces a Death Profile Change in U2OS Osteosarcoma Cells after 5-ALA-PDT. Lasers Surg. Med. 2011, 43, 557–564. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Coupienne, I.; Bontems, S.; Dewaele, M.; Rubio, N.; Habraken, Y.; Fulda, S.; Agostinis, P.; Piette, J. NF-KappaB Inhibition Improves the Sensitivity of Human Glioblastoma Cells to 5-Aminolevulinic Acid-Based Photodynamic Therapy. Biochem. Pharm. 2011, 81, 606–616. [Google Scholar] [CrossRef]
- Zeng, L.; Zou, Q.; Huang, P.; Xiong, L.; Cheng, Y.; Chen, Q.; Li, Y.; He, H.; Yi, W.; Wei, W. Inhibition of Autophagy with Chloroquine Enhanced Apoptosis Induced by 5-Aminolevulinic Acid-Photodynamic Therapy in Secondary Hyperparathyroidism Primary Cells and Organoids. Biomed. Pharm. 2021, 142, 111994. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, M.; Wang, W.; Hong, L.; Wu, Z.; Luo, G.; Lu, S.; Tang, Y.; Li, J.; Wang, J.; et al. 5-ALA Mediated Photodynamic Therapy with Combined Treatment Improves Anti-Tumor Efficacy of Immunotherapy through Boosting Immunogenic Cell Death. Cancer Lett. 2023, 554, 216032. [Google Scholar] [CrossRef]
- Fettweis, G.; Di Valentin, E.; L’homme, L.; Lassence, C.; Dequiedt, F.; Fillet, M.; Coupienne, I.; Piette, J. RIP3 Antagonizes a TSC2-Mediated pro-Survival Pathway in Glioblastoma Cell Death. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 113–124. [Google Scholar] [CrossRef]
- Wirth, D.J.; Sibai, M.; Wilson, B.C.; Roberts, D.W.; Paulsen, K. First Experience with Spatial Frequency Domain Imaging and Red-Light Excitation of Protoporphyrin IX Fluorescence during Tumor Resection. Biomed. Opt. Express. 2020, 11, 4306–4315. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA Approval for Glioma Surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical Implications of the 2021 Edition of the WHO Classification of Central Nervous System Tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA Fluorescence-Guided Resection of High-Grade Glioma Leads to Greater Extent of Resection with Better Outcomes: A Systematic Review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef]
- Walter, S.; Susanne, S.; Simon, W.; Herbert, S.; Clemens, F.; Claudia, G.; Alwin, E.G.; Rainer, K.; Hans, J.R. Intraoperative Detection of Malignant Gliomas by 5-Aminolevulinic Acid-Induced Porphyrin Fluorescence. Neurosurgery 1998, 42, 518–526. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Schupper, A.J.; Baron, R.B.; Cheung, W.; Rodriguez, J.; Kalkanis, S.N.; Chohan, M.O.; Andersen, B.J.; Chamoun, R.; Nahed, B.V.; Zacharia, B.E.; et al. 5-Aminolevulinic Acid for Enhanced Surgical Visualization of High-Grade Gliomas: A Prospective, Multicenter Study. J. Neurosurg. 2021, 136, 1525–1534. [Google Scholar] [CrossRef]
- Black, D.; Kaneko, S.; Walke, A.; König, S.; Stummer, W.; Suero Molina, E. Characterization of Autofluorescence and Quantitative Protoporphyrin IX Biomarkers for Optical Spectroscopy-Guided Glioma Surgery. Sci. Rep. 2021, 11, 20009. [Google Scholar] [CrossRef]
- Hosmann, A.; Millesi, M.; Wadiura, L.I.; Kiesel, B.; Mercea, P.A.; Mischkulnig, M.; Borkovec, M.; Furtner, J.; Roetzer, T.; Wolfsberger, S.; et al. 5-ALA Fluorescence Is a Powerful Prognostic Marker during Surgery of Low-Grade Gliomas (WHO Grade II)-Experience at Two Specialized Centers. Cancers 2021, 13, 2540. [Google Scholar] [CrossRef]
- Jaber, M.; Ewelt, C.; Wölfer, J.; Brokinkel, B.; Thomas, C.; Hasselblatt, M.; Grauer, O.; Stummer, W. Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas? Neurosurgery 2019, 84, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Molina, E.S.; Sporns, P.; Schipmann, S.; Black, D.; Stummer, W. Fluorescence Real-Time Kinetics of Protoporphyrin IX after 5-ALA Administration in Low-Grade Glioma. J. Neurosurg. 2021, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ennis, S.R.; Novotny, A.; Xiang, J.; Shakui, P.; Masada, T.; Stummer, W.; Smith, D.E.; Keep, R.F. Transport of 5-Aminolevulinic Acid between Blood and Brain. Brain Res. 2003, 959, 226–234. [Google Scholar] [CrossRef]
- Molina, E.S.; Black, D.; Kaneko, S.; Müther, M.; Stummer, W. Double Dose of 5-Aminolevulinic Acid and Its Effect on Protoporphyrin IX Accumulation in Low-Grade Glioma. J. Neurosurg. 2022, 137, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kriegmair, M.; Baumgartner, R.; Knuechel, R.; Steinbach, P.; Ehsan, A.; Lumper, W.; Hofstädter, F.; Hofstetter, A. Fluorescence Photodetection of Neoplastic Urothelial Lesions Following Intravesical Instillation of 5-Aminolevulinic Acid. Urology 1994, 44, 836–841. [Google Scholar] [CrossRef]
- Koenig, F.; McGovern, F.J.; Larne, R.; Enquist, H.; Schomacker, K.T.; Deutsch, T.F. Diagnosis of Bladder Carcinoma Using Protoporphyrin IX Fluorescence Induced by 5-Aminolaevulinic Acid. BJU Int. 1999, 83, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Uehlinger, P.; Zellweger, M.; Wagnières, G.; Juillerat-Jeanneret, L.; van den Bergh, H.; Lange, N. 5-Aminolevulinic Acid and Its Derivatives: Physical Chemical Properties and Protoporphyrin IX Formation in Cultured Cells. J. Photochem. Photobiol. B Biol. 2000, 54, 72–80. [Google Scholar] [CrossRef]
- Marti, A.; Jichlinski, P.; Lange, N.; Ballini, J.-P.; Guillou, L.; Leisinger, H.J.; Kucera, P. Comparison of Aminolevulinic Acid and Hexylester Aminolevulinate Induced Protoporphyrin IX Distribution in Human Bladder Cancer. J. Urol. 2003, 170, 428–432. [Google Scholar] [CrossRef]
- Grossman, H.B.; Gomella, L.; Fradet, Y.; Morales, A.; Presti, J.; Ritenour, C.; Nseyo, U.; Droller, M.J. PC B302/01 Study Group A Phase III, Multicenter Comparison of Hexaminolevulinate Fluorescence Cystoscopy and White Light Cystoscopy for the Detection of Superficial Papillary Lesions in Patients with Bladder Cancer. J. Urol. 2007, 178, 62–67. [Google Scholar] [CrossRef]
- Stenzl, A.; Burger, M.; Fradet, Y.; Mynderse, L.A.; Soloway, M.S.; Witjes, J.A.; Kriegmair, M.; Karl, A.; Shen, Y.; Grossman, H.B. Hexaminolevulinate Guided Fluorescence Cystoscopy Reduces Recurrence in Patients With Nonmuscle Invasive Bladder Cancer. J. Urol. 2010, 184, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Maytin, E.V.; Kaw, U.; Ilyas, M.; Mack, J.A.; Hu, B. Blue Light versus Red Light for Photodynamic Therapy of Basal Cell Carcinoma in Patients with Gorlin Syndrome: A Bilaterally Controlled Comparison Study. Photodiagnosis Photodyn. Ther. 2018, 22, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kaw, U.; Ilyas, M.; Bullock, T.; Rittwage, L.; Riha, M.; Vidimos, A.; Hu, B.; Warren, C.B.; Maytin, E.V. A Regimen to Minimize Pain during Blue Light Photodynamic Therapy of Actinic Keratoses: Bilaterally Controlled, Randomized Trial of Simultaneous versus Conventional Illumination. J. Am. Acad. Derm. 2020, 82, 862–868. [Google Scholar] [CrossRef] [PubMed]
- De Haas, E.R.M.; Kruijt, B.; Sterenborg, H.J.C.M.; Neumann, H.A.M.; Robinson, D.J. Fractionated Illumination Significantly Improves the Response of Superficial Basal Cell Carcinoma to Aminolevulinic Acid Photodynamic Therapy. J. Investig. Derm. 2006, 126, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, E.; Apalla, Z.; Chovarda, E.; Goussi, C.; Trigoni, A.; Ioannides, D. Single vs. Fractionated Photodynamic Therapy for Face and Scalp Actinic Keratoses: A Randomized, Intraindividual Comparison Trial with 12-Month Follow-Up. J. Eur. Acad. Derm. Venereol. 2012, 26, 36–40. [Google Scholar] [CrossRef]
- de Vijlder, H.C.; Sterenborg, H.J.C.M.; Neumann, H.A.M.; Robinson, D.J.; de Haas, E.R.M. Light Fractionation Significantly Improves the Response of Superficial Basal Cell Carcinoma to Aminolaevulinic Acid Photodynamic Therapy: Five-Year Follow-up of a Randomized, Prospective Trial. Acta. Derm. Venereol. 2012, 92, 641–647. [Google Scholar] [CrossRef]
- de Bruijn, H.S.; Casas, A.G.; Di Venosa, G.; Gandara, L.; Sterenborg, H.J.C.M.; Batlle, A.; Robinson, D.J. Light Fractionated ALA-PDT Enhances Therapeutic Efficacy in Vitro; the Influence of PpIX Concentration and Illumination Parameters. Photochem. Photobiol. Sci. 2013, 12, 241–245. [Google Scholar] [CrossRef]
- de Souza, A.L.R.; Marra, K.; Gunn, J.; Samkoe, K.S.; Kanick, S.C.; Davis, S.C.; Chapman, M.S.; Maytin, E.V.; Hasan, T.; Pogue, B.W. Comparing Desferrioxamine and Light Fractionation Enhancement of ALA-PpIX Photodynamic Therapy in Skin Cancer. Br. J. Cancer 2016, 115, 805–813. [Google Scholar] [CrossRef]
- Kaneko, S.; Suero Molina, E.; Ewelt, C.; Warneke, N.; Stummer, W. Fluorescence-Based Measurement of Real-Time Kinetics of Protoporphyrin IX After 5-Aminolevulinic Acid Administration in Human In Situ Malignant Gliomas. Neurosurgery 2019, 85, E739–E746. [Google Scholar] [CrossRef]
- Kaneko, S.; Brokinkel, B.; Suero Molina, E.; Warneke, N.; Holling, M.; Bunk, E.C.; Hess, K.; Senner, V.; Paulus, W.; Stummer, W. Real-Time in Vivo Kinetics of Protoporphyrin IX after Administration of 5-Aminolevulinic Acid in Meningiomas and Comparative Analyses with Glioblastomas. Acta Neurochir. 2020, 162, 2197–2202. [Google Scholar] [CrossRef]
- Beiki, D.; Eggleston, I.M.; Pourzand, C. Daylight-PDT: Everything under the Sun. Biochem. Soc. Trans. 2022, 50, 975–985. [Google Scholar] [CrossRef]
- Bai-Habelski, J.C.; Medrano, K.; Palacio, A.; Reinhold, U. No Room for Pain: A Prospective Study Showing Effective and Nearly Pain-Free Treatment of Actinic Keratosis with Simulated Daylight Photodynamic Therapy (SDL-PDT) Using the IndoorLux® System in Combination with BF-200 ALA (Ameluz®). Photodiagnosis Photodyn. Ther. 2022, 37, 102692. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Stepp, H.; Möller, G.; Ehrhardt, A.; Leonhard, M.; Reulen, H.J. Technical Principles for Protoporphyrin-IX-Fluorescence Guided Microsurgical Resection of Malignant Glioma Tissue. Acta. Neurochir. 1998, 140, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Suero Molina, E.; Ewelt, C.; Warneke, N.; Schwake, M.; Müther, M.; Schipmann, S.; Stummer, W. Dual Labeling with 5-Aminolevulinic Acid and Fluorescein in High-Grade Glioma Surgery with a Prototype Filter System Built into a Neurosurgical Microscope: Technical Note. J. Neurosurg. 2019, 132, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Suero Molina, E.; Stögbauer, L.; Jeibmann, A.; Warneke, N.; Stummer, W. Validating a New Generation Filter System for Visualizing 5-ALA-Induced PpIX Fluorescence in Malignant Glioma Surgery: A Proof of Principle Study. Acta. Neurochir. 2020, 162, 785–793. [Google Scholar] [CrossRef]
- Strickland, B.A.; Wedemeyer, M.; Ruzevick, J.; Micko, A.; Shahrestani, S.; Daneshmand, S.; Shiroishi, M.S.; Hwang, D.H.; Attenello, F.; Chen, T.; et al. 5-Aminolevulinic Acid-Enhanced Fluorescence-Guided Treatment of High-Grade Glioma Using Angled Endoscopic Blue Light Visualization: Technical Case Series with Preliminary Follow-Up. J. Neurosurg. 2022, 137, 1–9. [Google Scholar] [CrossRef]
- Suero Molina, E.; Hellwig, S.J.; Walke, A.; Jeibmann, A.; Stepp, H.; Stummer, W. Development and Validation of a Triple-LED Surgical Loupe Device for Fluorescence-Guided Resections with 5-ALA. J. Neurosurg. 2021, 137, 582–590. [Google Scholar] [CrossRef]
- Erkkilä, M.T.; Bauer, B.; Hecker-Denschlag, N.; Madera Medina, M.J.; Leitgeb, R.A.; Unterhuber, A.; Gesperger, J.; Roetzer, T.; Hauger, C.; Drexler, W.; et al. Widefield Fluorescence Lifetime Imaging of Protoporphyrin IX for Fluorescence-Guided Neurosurgery: An Ex Vivo Feasibility Study. J. Biophotonics. 2019, 12, e201800378. [Google Scholar] [CrossRef]
- Bravo, J.J.; Olson, J.D.; Davis, S.C.; Roberts, D.W.; Paulsen, K.D.; Kanick, S.C. Hyperspectral Data Processing Improves PpIX Contrast during Fluorescence Guided Surgery of Human Brain Tumors. Sci. Rep. 2017, 7, 9455. [Google Scholar] [CrossRef]
- Wei, L.; Roberts, D.W.; Sanai, N.; Liu, J.T.C. Visualization Technologies for 5-ALA-Based Fluorescence-Guided Surgeries. J. Neurooncol. 2019, 141, 495–505. [Google Scholar] [CrossRef]
- Herta, J.; Cho, A.; Roetzer-Pejrimovsky, T.; Höftberger, R.; Marik, W.; Kronreif, G.; Peilnsteiner, T.; Rössler, K.; Wolfsberger, S. Optimizing Maximum Resection of Glioblastoma: Raman Spectroscopy versus 5-Aminolevulinic Acid. J. Neurosurg. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sassa, S.; Zalar, G.L.; Poh-Fitzpatrick, M.B.; Kappas, A. Studies in Porphyria IX: Detection of the Gene Defect of Erythropoietic Protoporphyria in Mitogen-Stimulated Human Lymphocytes. Trans. Assoc. Am. Physicians 1979, 92, 268–276. [Google Scholar] [PubMed]
- Hanania, J.; Malik, Z. The Effect of EDTA and Serum on Endogenous Porphyrin Accumulation and Photodynamic Sensitization of Human K562 Leukemic Cells. Cancer Lett. 1992, 65, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Anholt, H.; Bech, O.; Moan, J. The Influence of Iron Chelators on the Accumulation of Protoporphyrin IX in 5-Aminolaevulinic Acid-Treated Cells. Br. J. Cancer 1996, 74, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, S.; Farshi, S.S.; Ortel, B.; Hasan, T. A Mechanistic Study of Cellular Photodestruction with 5-Aminolaevulinic Acid-Induced Porphyrin. Br. J. Cancer 1994, 70, 21–28. [Google Scholar] [CrossRef]
- Tan, W.C.; Krasner, N.; O’Toole, P.; Lombard, M. Enhancement of Photodynamic Therapy in Gastric Cancer Cells by Removal of Iron. Gut 1997, 41, 14–18. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.; Liu, X.; Jiang, S.; Xiong, L. Desferrioxamine Shows Different Potentials for Enhancing 5-Aminolaevulinic Acid-Based Photodynamic Therapy in Several Cutaneous Cell Lines. Lasers Med. Sci. 2010, 25, 251–257. [Google Scholar] [CrossRef]
- Valdés, P.A.; Samkoe, K.; O’Hara, J.A.; Roberts, D.W.; Paulsen, K.D.; Pogue, B.W. Deferoxamine Iron Chelation Increases δ-Aminolevulinic Acid Induced Protoporphyrin IX in Xenograft Glioma Model. Photochem. Photobiol. 2010, 86, 471–475. [Google Scholar] [CrossRef]
- Reinert, M.; Piffaretti, D.; Wilzbach, M.; Hauger, C.; Guckler, R.; Marchi, F.; D’Angelo, M.L. Quantitative Modulation of PpIX Fluorescence and Improved Glioma Visualization. Front. Surg. 2019, 6, 41. [Google Scholar] [CrossRef]
- Qin, J.; Zhou, C.; Zhu, M.; Shi, S.; Zhang, L.; Zhao, Y.; Li, C.; Wang, Y.; Wang, Y. Iron Chelation Promotes 5-Aminolaevulinic Acid-Based Photodynamic Therapy against Oral Tongue Squamous Cell Carcinoma. Photodiagnosis Photodyn. Ther. 2020, 31, 101907. [Google Scholar] [CrossRef]
- Pye, A.; Curnow, A. Direct Comparison of Delta-Aminolevulinic Acid and Methyl-Aminolevulinate-Derived Protoporphyrin IX Accumulations Potentiated by Desferrioxamine or the Novel Hydroxypyridinone Iron Chelator CP94 in Cultured Human Cells. Photochem. Photobiol. 2007, 83, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Blake, E.; Allen, J.; Curnow, A. An in Vitro Comparison of the Effects of the Iron-Chelating Agents, CP94 and Dexrazoxane, on Protoporphyrin IX Accumulation for Photodynamic Therapy and/or Fluorescence Guided Resection. Photochem. Photobiol. 2011, 87, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Blake, E.; Curnow, A. The Hydroxypyridinone Iron Chelator CP94 Can Enhance PpIX-Induced PDT of Cultured Human Glioma Cells. Photochem. Photobiol. 2010, 86, 1154–1160. [Google Scholar] [CrossRef]
- Bech, O.; Phillips, D.; Moan, J.; MacRobert, A.J. A Hydroxypyridinone (CP94) Enhances Protoporphyrin IX Formation in 5-Aminolaevulinic Acid Treated Cells. J. Photochem. Photobiol. B 1997, 41, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; MacRobert, A.J.; Porter, J.B.; Bown, S.G. The Efficacy of an Iron Chelator (CP94) in Increasing Cellular Protoporphyrin IX Following Intravesical 5-Aminolaevulinic Acid Administration: An in Vivo Study. J. Photochem. Photobiol. B 1997, 38, 114–122. [Google Scholar] [CrossRef]
- Curnow, A.; McIlroy, B.W.; Postle-Hacon, M.J.; Porter, J.B.; MacRobert, A.J.; Bown, S.G. Enhancement of 5-Aminolaevulinic Acid-Induced Photodynamic Therapy in Normal Rat Colon Using Hydroxypyridinone Iron-Chelating Agents. Br. J. Cancer 1998, 78, 1278–1282. [Google Scholar] [CrossRef]
- Pye, A.; Campbell, S.; Curnow, A. Enhancement of Methyl-Aminolevulinate Photodynamic Therapy by Iron Chelation with CP94: An in Vitro Investigation and Clinical Dose-Escalating Safety Study for the Treatment of Nodular Basal Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2008, 134, 841–849. [Google Scholar] [CrossRef]
- Anayo, L.; Magnussen, A.; Perry, A.; Wood, M.; Curnow, A. An Experimental Investigation of a Novel Iron Chelating Protoporphyrin IX Prodrug for the Enhancement of Photodynamic Therapy. Lasers Surg. Med. 2018, 50, 552–565. [Google Scholar] [CrossRef]
- Curnow, A.; Perry, A.; Wood, M. Improving in Vitro Photodynamic Therapy through the Development of a Novel Iron Chelating Aminolaevulinic Acid Prodrug. Photodiagnosis Photodyn. Ther. 2019, 25, 157–165. [Google Scholar] [CrossRef]
- Magnussen, A.; Reburn, C.; Perry, A.; Wood, M.; Curnow, A. Experimental Investigation of a Combinational Iron Chelating Protoporphyrin IX Prodrug for Fluorescence Detection and Photodynamic Therapy. Lasers Med. Sci. 2022, 37, 1155–1166. [Google Scholar] [CrossRef]
- Malik, Z.; Kostenich, G.; Roitman, L.; Ehrenberg, B.; Orenstein, A. Topical Application of 5-Aminolevulinic Acid, DMSO and EDTA: Protoporphyrin IX Accumulation in Skin and Tumours of Mice. J. Photochem. Photobiol. B 1995, 28, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Warloe, T.; Moan, J.; Heyerdahl, H.; Steen, H.B.; Nesland, J.M.; Giercksky, K.E. Distribution of 5-Aminolevulinic Acid-Induced Porphyrins in Noduloulcerative Basal Cell Carcinoma. Photochem. Photobiol. 1995, 62, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Choudry, K.; Brooke, R.C.C.; Farrar, W.; Rhodes, L.E. The Effect of an Iron Chelating Agent on Protoporphyrin IX Levels and Phototoxicity in Topical 5-Aminolaevulinic Acid Photodynamic Therapy. Br. J. Derm. 2003, 149, 124–130. [Google Scholar] [CrossRef]
- Mansi, M.; Howley, R.; Chandratre, S.; Chen, B. Inhibition of ABCG2 Transporter by Lapatinib Enhances 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Fluorescence and Photodynamic Therapy Response in Human Glioma Cell Lines. Biochem. Pharmacol. 2022, 200, 115031. [Google Scholar] [CrossRef] [PubMed]
- Palasuberniam, P.; Kraus, D.; Mansi, M.; Braun, A.; Howley, R.; Myers, K.A.; Chen, B. Ferrochelatase Deficiency Abrogated the Enhancement of Aminolevulinic Acid-Mediated Protoporphyrin IX by Iron Chelator Deferoxamine. Photochem. Photobiol. 2019, 95, 1052–1059. [Google Scholar] [CrossRef]
- Howley, R.; Mansi, M.; Shinde, J.; Restrepo, J.; Chen, B. Analysis of Renal Cell Carcinoma Cell Response to the Enhancement of 5-aminolevulinic Acid-mediated Protoporphyrin IX Fluorescence by Iron Chelator Deferoxamine. Photochem. Photobiol. 2022, 99, 787–792. [Google Scholar] [CrossRef]
- Kemmner, W.; Wan, K.; Rüttinger, S.; Ebert, B.; Macdonald, R.; Klamm, U.; Moesta, K.T. Silencing of Human Ferrochelatase Causes Abundant Protoporphyrin-IX Accumulation in Colon Cancer. FASEB J. 2008, 22, 500–509. [Google Scholar] [CrossRef]
- Miyake, M.; Ishii, M.; Kawashima, K.; Kodama, T.; Sugano, K.; Fujimoto, K.; Hirao, Y. SiRNA-Mediated Knockdown of the Heme Synthesis and Degradation Pathways: Modulation of Treatment Effect of 5-Aminolevulinic Acid-Based Photodynamic Therapy in Urothelial Cancer Cell Lines. Photochem. Photobiol. 2009, 85, 1020–1027. [Google Scholar] [CrossRef]
- Teng, L.; Nakada, M.; Zhao, S.-G.; Endo, Y.; Furuyama, N.; Nambu, E.; Pyko, I.V.; Hayashi, Y.; Hamada, J.-I. Silencing of Ferrochelatase Enhances 5-Aminolevulinic Acid-Based Fluorescence and Photodynamic Therapy Efficacy. Br. J. Cancer 2011, 104, 798–807. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Palasuberniam, P.; Myers, K.A.; Wang, C.; Chen, B. Effects of Silencing Heme Biosynthesis Enzymes on 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Fluorescence and Photodynamic Therapy. Photochem. Photobiol. 2015, 91, 923–930. [Google Scholar] [CrossRef]
- Wan, K.; Ebert, B.; Voigt, J.; Wang, Q.; Dai, Y.; Haag, R.; Kemmner, W. In Vivo Tumor Imaging Using a Novel RNAi-Based Detection Mechanism. Nanomedicine 2012, 8, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Karashima, T.; Kamada, M.; Shuin, T.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J. Regulation of 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Accumulation in Human Urothelial Carcinomas. Pathobiology 2009, 76, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Kobuchi, H.; Munetomo, K.; Fujita, H.; Yamamoto, M.; Utsumi, T.; Inoue, K.; Shuin, T.; Sasaki, J.; Inoue, M.; et al. Serum-Dependent Export of Protoporphyrin IX by ATP-Binding Cassette Transporter G2 in T24 Cells. Mol. Cell. Biochem. 2011, 358, 297. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Inoue, K.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J.; Shuin, T. The Inhibition of Ferrochelatase Enhances 5-Aminolevulinic Acid-Based Photodynamic Action for Prostate Cancer. Photodiagnosis Photodyn. Ther. 2013, 10, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Wilson, C.; Hasan, T.; Maytin, E.V. Vitamin D3 Enhances the Apoptotic Response of Epithelial Tumors to Aminolevulinate-Based Photodynamic Therapy. Cancer Res. 2011, 71, 6040–6050. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ishii, T.; Kohda, T.; Ishizuka, M.; Yamazaki, K.; Nishimura, Y.; Tanaka, T.; Dan, S.; Nakajima, M. Mechanistic Study of PpIX Accumulation Using the JFCR39 Cell Panel Revealed a Role for Dynamin 2-Mediated Exocytosis. Sci. Rep. 2019, 9, 8666. [Google Scholar] [CrossRef]
- Jonker, J.W.; Buitelaar, M.; Wagenaar, E.; van der Valk, M.A.; Scheffer, G.L.; Scheper, R.J.; Plösch, T.; Kuipers, F.; Elferink, R.P.J.O.; Rosing, H.; et al. The Breast Cancer Resistance Protein Protects against a Major Chlorophyll-Derived Dietary Phototoxin and Protoporphyria. Proc. Natl. Acad. Sci. USA 2002, 99, 15649–15654. [Google Scholar] [CrossRef]
- Zhou, S.; Zong, Y.; Ney, P.A.; Nair, G.; Stewart, C.F.; Sorrentino, B.P. Increased Expression of the Abcg2 Transporter during Erythroid Maturation Plays a Role in Decreasing Cellular Protoporphyrin IX Levels. Blood 2005, 105, 2571–2576. [Google Scholar] [CrossRef]
- Robey, R.W.; Steadman, K.; Polgar, O.; Bates, S.E. ABCG2-Mediated Transport of Photosensitizers: Potential Impact on Photodynamic Therapy. Cancer Biol. Ther. 2005, 4, 195–202. [Google Scholar] [CrossRef]
- Allen, J.D.; van Loevezijn, A.; Lakhai, J.M.; van der Valk, M.; van Tellingen, O.; Reid, G.; Schellens, J.H.M.; Koomen, G.-J.; Schinkel, A.H. Potent and Specific Inhibition of the Breast Cancer Resistance Protein Multidrug Transporter in Vitro and in Mouse Intestine by a Novel Analogue of Fumitremorgin C. Mol. Cancer Ther. 2002, 1, 417–425. [Google Scholar]
- Bebes, A.; Nagy, T.; Bata-Csörgő, Z.; Kemény, L.; Dobozy, A.; Széll, M. Specific Inhibition of the ABCG2 Transporter Could Improve the Efficacy of Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2011, 105, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, H.; Moriya, K.; Ogino, T.; Fujita, H.; Inoue, K.; Shuin, T.; Yasuda, T.; Utsumi, K.; Utsumi, T. Mitochondrial Localization of ABC Transporter ABCG2 and Its Function in 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Accumulation. PLoS ONE 2012, 7, e50082. [Google Scholar] [CrossRef] [PubMed]
- Barron, G.A.; Moseley, H.; Woods, J.A. Differential Sensitivity in Cell Lines to Photodynamic Therapy in Combination with ABCG2 Inhibition. J. Photochem. Photobiol. B Biol. 2013, 126, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Palasuberniam, P.; Yang, X.; Kraus, D.; Jones, P.; Myers, K.A.; Chen, B. ABCG2 Transporter Inhibitor Restores the Sensitivity of Triple Negative Breast Cancer Cells to Aminolevulinic Acid-Mediated Photodynamic Therapy. Sci. Rep. 2015, 5, 13298. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Abdel Gaber, S.A.; Zimmermann, W.; Wittig, R.; Stepp, H. ABCG2 Influence on the Efficiency of Photodynamic Therapy in Glioblastoma Cells. J. Photochem. Photobiol. B Biol. 2020, 210, 111963. [Google Scholar] [CrossRef]
- Howley, R.; Mansi, M.; Shinde, J.; Restrepo, J.; Chen, B. Evaluation of Aminolevulinic Acid-Mediated Protoporphyrin IX Fluorescence and Enhancement by ABCG2 Inhibitors in Renal Cell Carcinoma Cells. J. Photochem. Photobiol. B Biol. 2020, 211, 112017. [Google Scholar] [CrossRef]
- Palasuberniam, P.; Kraus, D.; Mansi, M.; Howley, R.; Braun, A.; Myers, K.A.; Chen, B. Small Molecule Kinase Inhibitors Enhance Aminolevulinic Acid-Mediated Protoporphyrin IX Fluorescence and PDT Response in Triple Negative Breast Cancer Cell Lines. J. Biomed. Opt. 2021, 26, 098002. [Google Scholar] [CrossRef]
- Weidner, L.D.; Zoghbi, S.S.; Lu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Mulder, J.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The Inhibitor Ko143 Is Not Specific for ABCG2. J. Pharm. Exp. Ther. 2015, 354, 384–393. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, J.; Huang, Y.; Li, C.; Lu, J.; Sachar, M.; Li, S.; Ma, X. Metabolism of KO143, an ABCG2 Inhibitor. Drug. Metab. Pharm. 2017, 32, 193–200. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Özvegy-Laczka, C.; Hegedűs, T.; Várady, G.; Ujhelly, O.; Schuetz, J.D.; Váradi, A.; Kéri, G.; Őrfi, L.; Német, K.; Sarkadi, B. High-Affinity Interaction of Tyrosine Kinase Inhibitors with the ABCG2 Multidrug Transporter. Mol. Pharm. 2004, 65, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Unadkat, J.D. Role of the Breast Cancer Resistance Protein (BCRP/ABCG2) in Drug Transport—An Update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Liu, W.; Baer, M.R.; Bowman, M.J.; Pera, P.; Zheng, X.; Morgan, J.; Pandey, R.A.; Oseroff, A.R. The Tyrosine Kinase Inhibitor Imatinib Mesylate Enhances the Efficacy of Photodynamic Therapy by Inhibiting ABCG2. Clin. Cancer Res. 2007, 13, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Kajimoto, Y.; Inoue, H.; Miyatake, S.-I.; Ishikawa, T.; Kuroiwa, T. Gefitinib Enhances the Efficacy of Photodynamic Therapy Using 5-Aminolevulinic Acid in Malignant Brain Tumor Cells. Photodiagnosis Photodyn. Ther. 2013, 10, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Piffaretti, D.; Burgio, F.; Thelen, M.; Kaelin-Lang, A.; Paganetti, P.; Reinert, M.; D’Angelo, M.L. Protoporphyrin IX Tracer Fluorescence Modulation for Improved Brain Tumor Cell Lines Visualization. J. Photochem. Photobiol. B 2019, 201, 111640. [Google Scholar] [CrossRef]
- Fang, S.; Wu, Y.; Zhang, H.; Zeng, Q.; Wang, P.; Zhang, L.; Yan, G.; Zhang, G.; Wang, X. Molecular Characterization of Gene Expression Changes in Murine Cutaneous Squamous Cell Carcinoma after 5-Aminolevulinic Acid Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2022, 39, 102907. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; He, J.; Li, X.; Li, L.; Chen, X.; Lan, P. Influence and Mechanism of 5-Aminolevulinic Acid-Photodynamic Therapy on the Metastasis of Esophageal Carcinoma. Photodiagnosis Photodyn. Ther. 2017, 20, 78–85. [Google Scholar] [CrossRef]
- Ge, X.; Liu, J.; Shi, Z.; Jing, L.; Yu, N.; Zhang, X.; Jiao, Y.; Wang, Y.; Li, P.A. Inhibition of MAPK Signaling Pathways Enhances Cell Death Induced by 5-Aminolevulinic Acid-Photodynamic Therapy in Skin Squamous Carcinoma Cells. Eur. J. Derm. 2016, 26, 164–172. [Google Scholar] [CrossRef]
- Lv, T.; Huang, J.; Wu, M.; Wang, H.; Zeng, Q.; Wang, X. Halofuginone Enhances the Anti-Tumor Effect of ALA-PDT by Suppressing NRF2 Signaling in CSCC. Photodiagnosis Photodyn. Ther. 2022, 37, 102572. [Google Scholar] [CrossRef]
- Girotti, A.W.; Fahey, J.M. Upregulation of Pro-Tumor Nitric Oxide by Anti-Tumor Photodynamic Therapy. Biochem. Pharm. 2020, 176, 113750. [Google Scholar] [CrossRef]
- Bhowmick, R.; Girotti, A.W. Cytoprotective Induction of Nitric Oxide Synthase in a Cellular Model of 5-Aminolevulinic Acid-Based Photodynamic Therapy. Free Radic. Biol. Med. 2010, 48, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Girotti, A.W. Cytoprotective Signaling Associated with Nitric Oxide Upregulation in Tumor Cells Subjected to Photodynamic Therapy-like Oxidative Stress. Free Radic. Biol. Med. 2013, 57, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Girotti, A.W. Pro-Survival and pro-Growth Effects of Stress-Induced Nitric Oxide in a Prostate Cancer Photodynamic Therapy Model. Cancer Lett. 2014, 343, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.M.; Emmer, J.V.; Korytowski, W.; Hogg, N.; Girotti, A.W. Antagonistic Effects of Endogenous Nitric Oxide in a Glioblastoma Photodynamic Therapy Model. Photochem. Photobiol. 2016, 92, 842–853. [Google Scholar] [CrossRef]
- Fahey, J.M.; Girotti, A.W. Accelerated Migration and Invasion of Prostate Cancer Cells after a Photodynamic Therapy-like Challenge: Role of Nitric Oxide. Nitric. Oxide. 2015, 49, 47–55. [Google Scholar] [CrossRef]
- Fahey, J.M.; Stancill, J.S.; Smith, B.C.; Girotti, A.W. Nitric Oxide Antagonism to Glioblastoma Photodynamic Therapy and Mitigation Thereof by BET Bromodomain Inhibitor JQ1. J. Biol. Chem. 2018, 293, 5345–5359. [Google Scholar] [CrossRef]
- Fahey, J.M.; Korytowski, W.; Girotti, A.W. Upstream Signaling Events Leading to Elevated Production of Pro-Survival Nitric Oxide in Photodynamically-Challenged Glioblastoma Cells. Free Radic. Biol. Med. 2019, 137, 37–45. [Google Scholar] [CrossRef]
- Skivka, L.M.; Gorobets, O.B.; Kutsenok, V.V.; Lozinsky, M.O.; Borisevich, A.N.; Fedorchuk, A.G.; Kholin, V.V.; Gamaleya, N.F. 5-Aminolevulinic Acid Mediated Photodynamic Therapy of Lewis Lung Carcinoma: A Role of Tumor Infiltration with Different Cells of Immune System. Exp. Oncol. 2004, 26, 312–315. [Google Scholar]
- Adamek, M.; Kawczyk-Krupka, A.; Mostowy, A.; Czuba, Z.; Krol, W.; Kasperczyk, S.; Jakobisiak, M.; Golab, J.; Sieron, A. Topical ALA-PDT Modifies Neutrophils’ Chemiluminescence, Lymphocytes’ Interleukin-1beta Secretion and Serum Level of Transforming Growth Factor Beta1 in Patients with Nonmelanoma Skin Malignancies A Clinical Study. Photodiagnosis Photodyn. Ther. 2005, 2, 65–72. [Google Scholar] [CrossRef]
- Evangelou, G.; Farrar, M.D.; White, R.D.; Sorefan, N.B.; Wright, K.P.; McLean, K.; Andrew, S.; Watson, R.E.B.; Rhodes, L.E. Topical Aminolaevulinic Acid-Photodynamic Therapy Produces an Inflammatory Infiltrate but Reduces Langerhans Cells in Healthy Human Skin in Vivo. Br. J. Derm. 2011, 165, 513–519. [Google Scholar] [CrossRef]
- Etminan, N.; Peters, C.; Lakbir, D.; Bünemann, E.; Börger, V.; Sabel, M.C.; Hänggi, D.; Steiger, H.-J.; Stummer, W.; Sorg, R.V. Heat-Shock Protein 70-Dependent Dendritic Cell Activation by 5-Aminolevulinic Acid-Mediated Photodynamic Treatment of Human Glioblastoma Spheroids in Vitro. Br. J. Cancer 2011, 105, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, J.; Zhang, H.; Fan, Z.; Zhang, L.; Shi, L.; Zhou, F.; Chen, W.R.; Wang, H.; Wang, X. Stimulation of Dendritic Cells by DAMPs in ALA-PDT Treated SCC Tumor Cells. Oncotarget 2015, 6, 44688–44702. [Google Scholar] [CrossRef] [PubMed]
- Hayami, J.; Okamoto, H.; Sugihara, A.; Horio, T. Immunosuppressive Effects of Photodynamic Therapy by Topical Aminolevulinic Acid. J. Derm. 2007, 34, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Matthews, Y.J.; Damian, D.L. Topical Photodynamic Therapy Is Immunosuppressive in Humans. Br. J. Derm. 2010, 162, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Yang, J.; Ji, J.; Wang, P.; Zhang, L.; Yan, G.; Wu, Y.; Chen, Q.; Liu, J.; Zhang, G.; et al. PD-L1 Blockade Potentiates the Antitumor Effects of ALA-PDT and Optimizes the Tumor Microenvironment in Cutaneous Squamous Cell Carcinoma. Oncoimmunology 2022, 11, 2061396. [Google Scholar] [CrossRef] [PubMed]
- Grandi, V.; Bacci, S.; Corsi, A.; Sessa, M.; Puliti, E.; Murciano, N.; Scavone, F.; Cappugi, P.; Pimpinelli, N. ALA-PDT Exerts Beneficial Effects on Chronic Venous Ulcers by Inducing Changes in Inflammatory Microenvironment, Especially through Increased TGF-Beta Release: A Pilot Clinical and Translational Study. Photodiagnosis Photodyn. Ther. 2018, 21, 252–256. [Google Scholar] [CrossRef]
- Evangelou, G.; Farrar, M.D.; Cotterell, L.; Andrew, S.; Tosca, A.D.; Watson, R.E.B.; Rhodes, L.E. Topical Photodynamic Therapy Significantly Reduces Epidermal Langerhans Cells during Clinical Treatment of Basal Cell Carcinoma. Br. J. Derm. 2012, 166, 1112–1115. [Google Scholar] [CrossRef]
- Rittenhouse-Diakun, K.; Van Leengoed, H.; Morgan, J.; Hryhorenko, E.; Paszkiewicz, G.; Whitaker, J.E.; Oseroff, A.R. The Role of Transferrin Receptor (CD71) in Photodynamic Therapy of Activated and Malignant Lymphocytes Using the Heme Precursor Delta-Aminolevulinic Acid (ALA). Photochem. Photobiol. 1995, 61, 523–528. [Google Scholar] [CrossRef]
- Hryhorenko, E.A.; Rittenhouse-Diakun, K.; Harvey, N.S.; Morgan, J.; Stewart, C.C.; Oseroff, A.R. Characterization of Endogenous Protoporphyrin IX Induced by Delta-Aminolevulinic Acid in Resting and Activated Peripheral Blood Lymphocytes by Four-Color Flow Cytometry. Photochem. Photobiol. 1998, 67, 565–572. [Google Scholar] [CrossRef]
- Hryhorenko, E.A.; Oseroff, A.R.; Morgan, J.; Rittenhouse-Diakun, K. Antigen Specific and Nonspecific Modulation of the Immune Response by Aminolevulinic Acid Based Photodynamic Therapy. Immunopharmacology 1998, 40, 231–240. [Google Scholar] [CrossRef]
- Darvekar, S.; Juzenas, P.; Oksvold, M.; Kleinauskas, A.; Holien, T.; Christensen, E.; Stokke, T.; Sioud, M.; Peng, Q. Selective Killing of Activated T Cells by 5-Aminolevulinic Acid Mediated Photodynamic Effect: Potential Improvement of Extracorporeal Photopheresis. Cancers 2020, 12, 377. [Google Scholar] [CrossRef]
- Christensen, E.; Foss, O.A.; Quist-Paulsen, P.; Staur, I.; Pettersen, F.; Holien, T.; Juzenas, P.; Peng, Q. Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Patients with Chronic Graft-versus-Host Disease: A First-in-Human Study. Pharmaceutics 2021, 13, 1558. [Google Scholar] [CrossRef]
- Gomes Marin, J.F.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-Emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef] [PubMed]
- Schipmann, S.; Müther, M.; Stögbauer, L.; Zimmer, S.; Brokinkel, B.; Holling, M.; Grauer, O.; Suero Molina, E.; Warneke, N.; Stummer, W. Combination of ALA-Induced Fluorescence-Guided Resection and Intraoperative Open Photodynamic Therapy for Recurrent Glioblastoma: Case Series on a Promising Dual Strategy for Local Tumor Control. J. Neurosurg. 2020, 134, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Bunk, E.C.; Wagner, A.; Stummer, W.; Senner, V.; Brokinkel, B. 5-ALA Kinetics in Meningiomas: Analysis of Tumor Fluorescence and PpIX Metabolism in Vitro and Comparative Analyses with High-Grade Gliomas. J. Neurooncol. 2021, 152, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Palasuberniam, P.; Kraus, D.; Chen, B. Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement. IJMS 2015, 16, 25865–25880. [Google Scholar] [CrossRef]
| Drug Name | Drug Formulation | Approval Time | Indications |
|---|---|---|---|
| Levulan | 20% ALA hydrochloride solution | 1999 | Topical application for PDT of actinic keratoses |
| Metvixia | 16.8% methyl aminolevulinate cream | 2004 | Topical application for PDT of actinic keratoses |
| Ameluz | 10% ALA hydrochloride nano-emulsion gel | 2016 | Topical application for lesion-directed and field-directed PDT of actinic keratoses |
| Cysview | 2 mg/mL hexaminolevulinate hydrochloride solution | 2010 | Intravesical instillation for the detection of non-muscle invasive papillary bladder cancer |
| Gleolan | ALA hydrochloride powder | 2017 | Oral administration for fluorescence-guided surgery of high-grade gliomas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howley, R.; Chandratre, S.; Chen, B. 5-Aminolevulinic Acid as a Theranostic Agent for Tumor Fluorescence Imaging and Photodynamic Therapy. Bioengineering 2023, 10, 496. https://doi.org/10.3390/bioengineering10040496
Howley R, Chandratre S, Chen B. 5-Aminolevulinic Acid as a Theranostic Agent for Tumor Fluorescence Imaging and Photodynamic Therapy. Bioengineering. 2023; 10(4):496. https://doi.org/10.3390/bioengineering10040496
Chicago/Turabian StyleHowley, Richard, Sharayu Chandratre, and Bin Chen. 2023. "5-Aminolevulinic Acid as a Theranostic Agent for Tumor Fluorescence Imaging and Photodynamic Therapy" Bioengineering 10, no. 4: 496. https://doi.org/10.3390/bioengineering10040496
APA StyleHowley, R., Chandratre, S., & Chen, B. (2023). 5-Aminolevulinic Acid as a Theranostic Agent for Tumor Fluorescence Imaging and Photodynamic Therapy. Bioengineering, 10(4), 496. https://doi.org/10.3390/bioengineering10040496






