Enhancing the Anaerobic Biodegradation of Petroleum Hydrocarbons in Soils with Electrically Conductive Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Characteristics
2.2. Electrically Conductive Materials
2.2.1. Biochar
2.2.2. Magnetite Nanoparticles
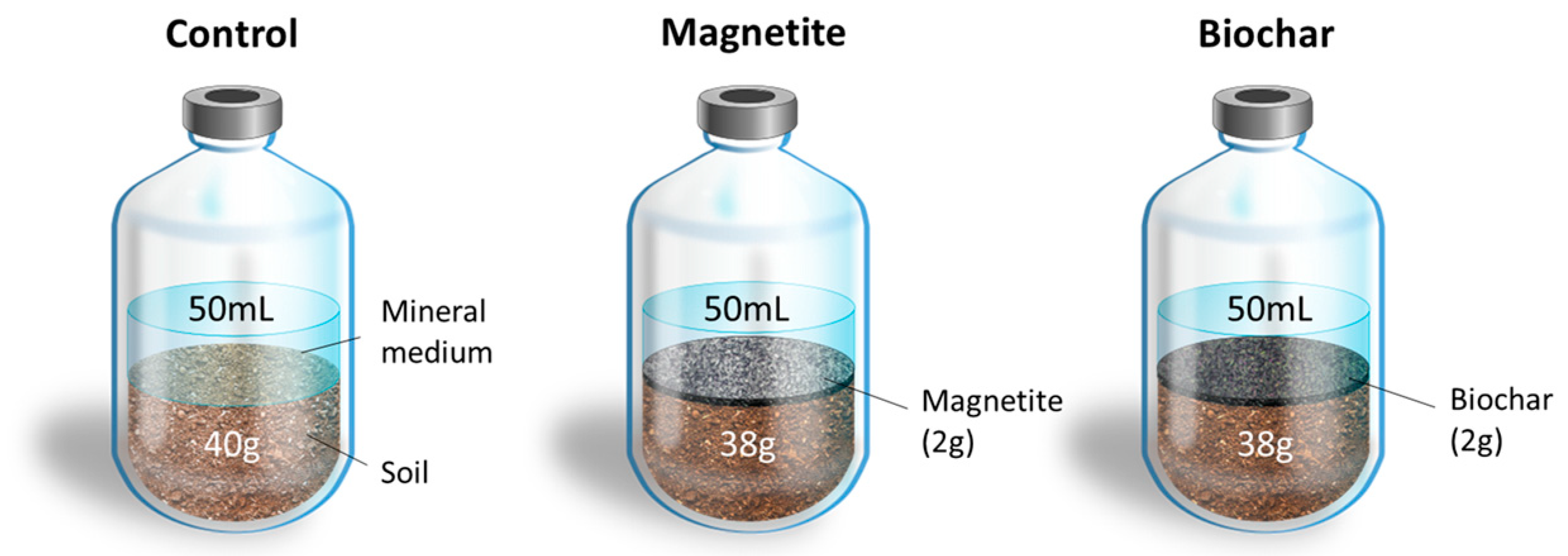
2.3. Experimental Setup
2.4. Analytical Methods
2.4.1. Gas Analyses
2.4.2. Hydrocarbons Determination
2.5. DNA Extraction, Quantification of Key-Functional Genes and High-Throughput 16S rRNA Gene Sequencing
3. Results
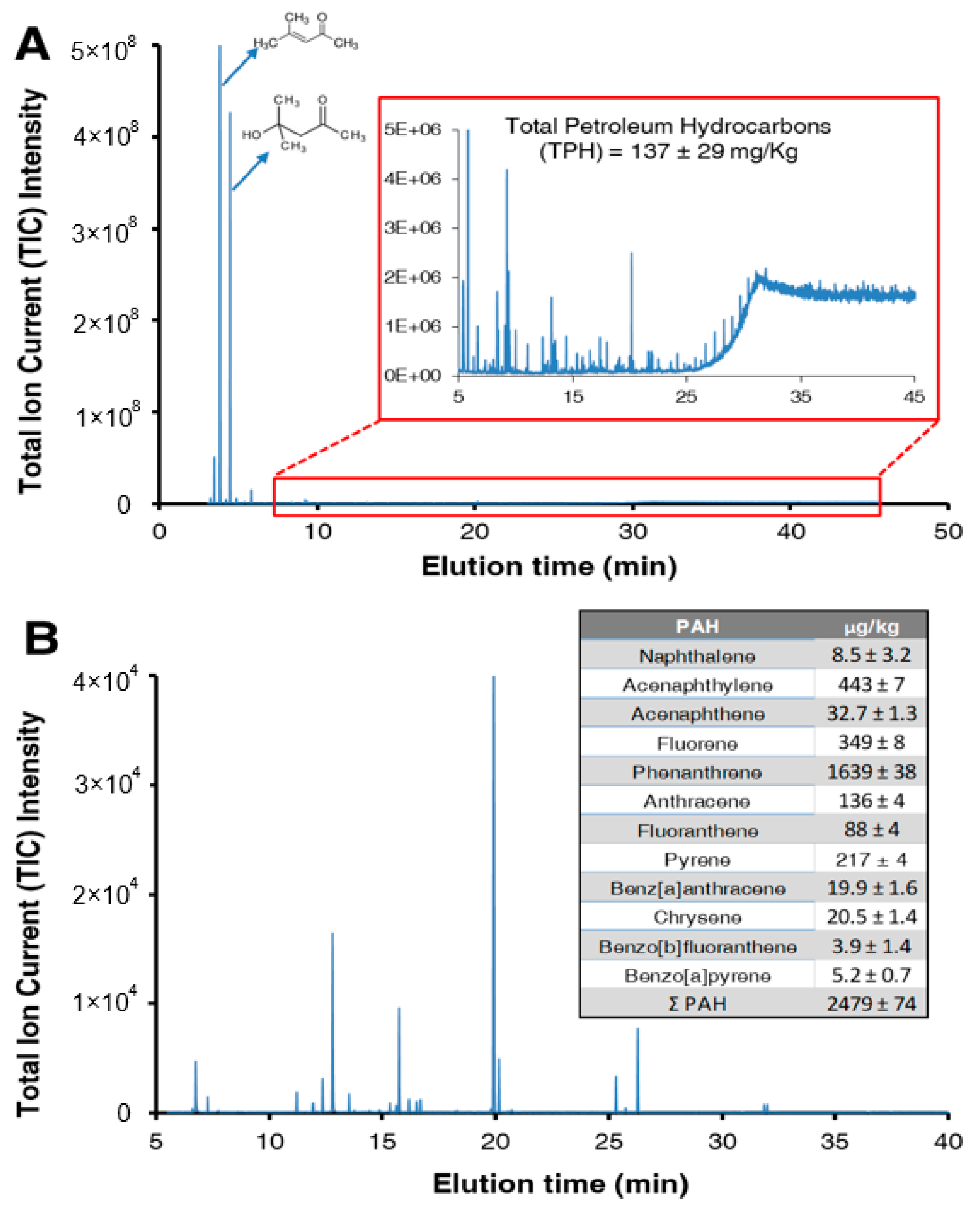
3.1. Soil Characterization
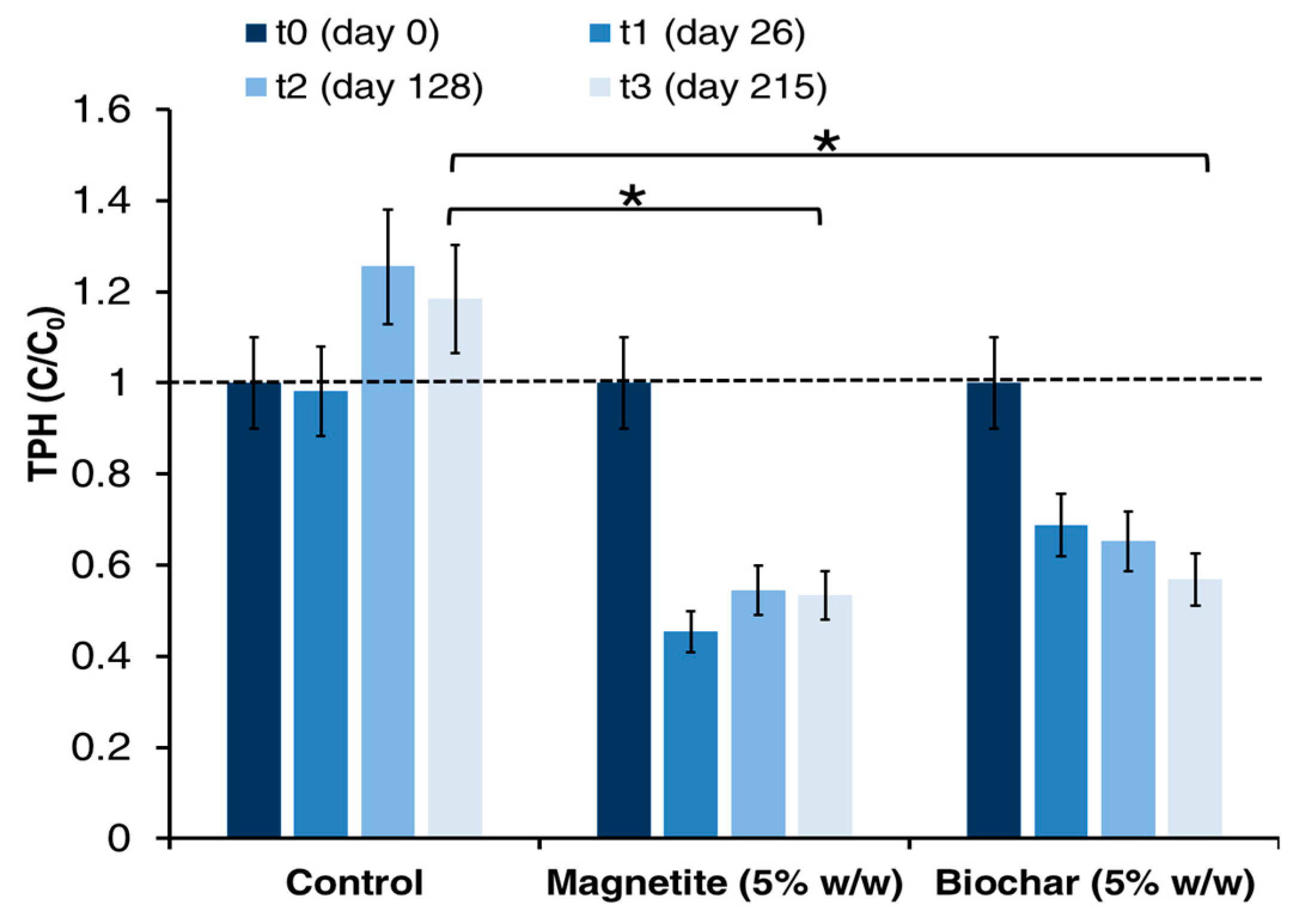
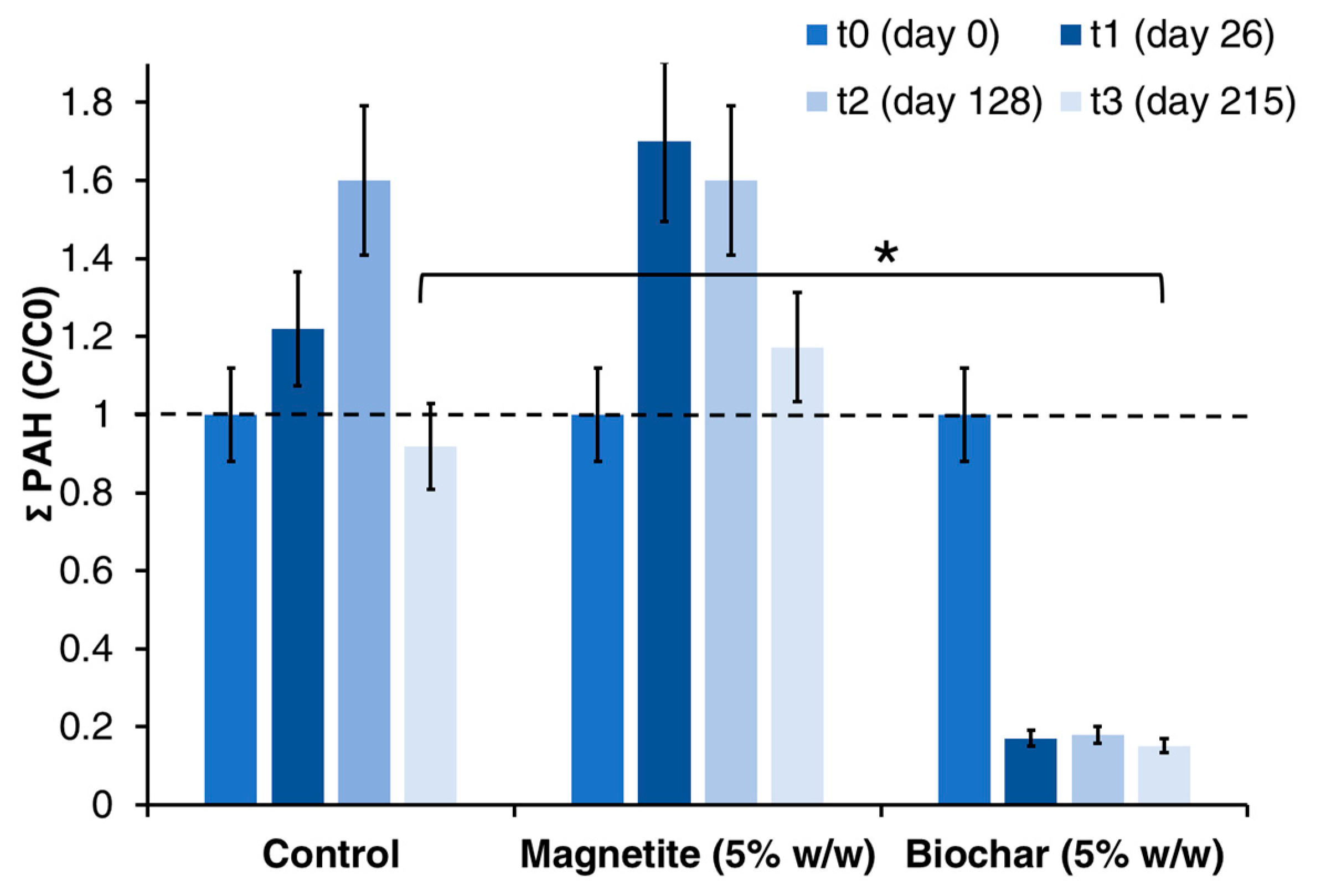
3.2. Effect of Electrically Conductive Particles on Hydrocarbons Degradation
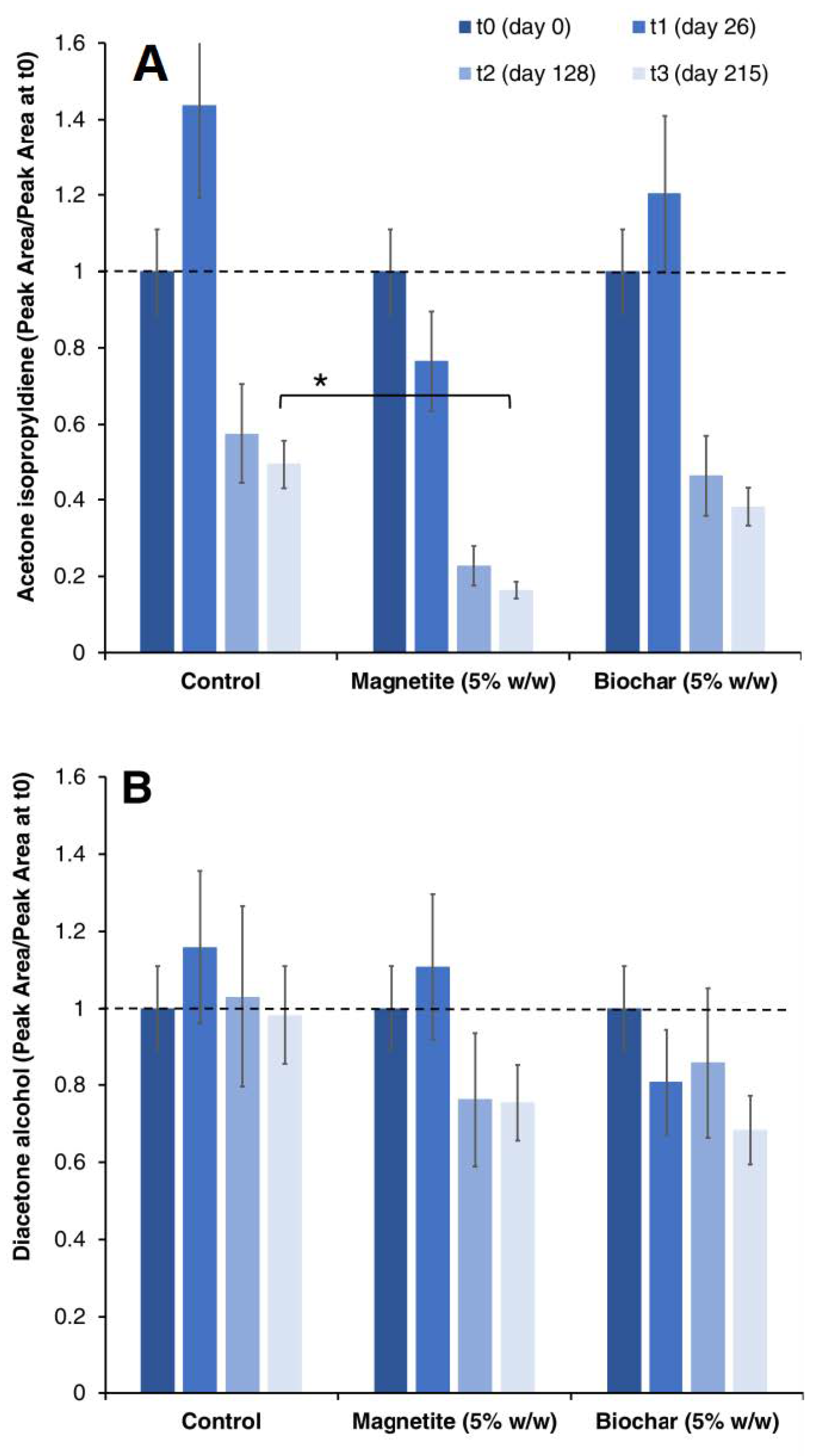
3.3. Biogas Production
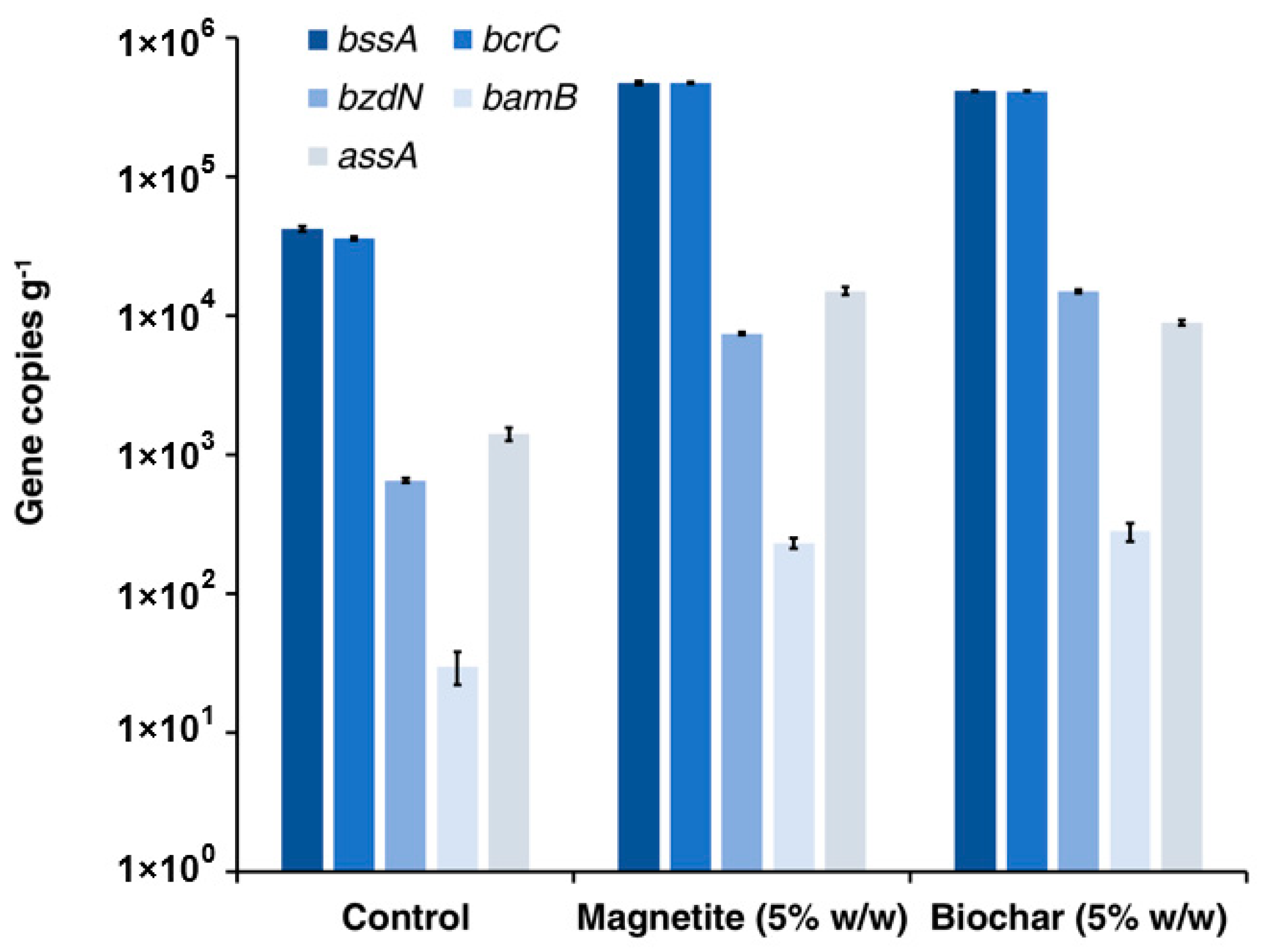
3.4. Microbial Community Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adedeji, J.A.; Tetteh, E.K.; Amankwa, M.O.; Asante-Sackey, D.; Ofori-Frimpong, S.; Armah, E.K.; Rathilal, S.; Mohammadi, A.H.; Chetty, M. Microbial Bioremediation and Biodegradation of Petroleum Products—A Mini Review. Appl. Sci. 2022, 12, 12212. [Google Scholar] [CrossRef]
- Tucci, M.; Viggi, C.C.; Núñez, A.E.; Schievano, A.; Rabaey, K.; Aulenta, F. Empowering electroactive microorganisms for soil remediation: Challenges in the bioelectrochemical removal of petroleum hydrocarbons. Chem. Eng. J. 2021, 419, 130008. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2019, 17, 100526. [Google Scholar] [CrossRef]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Viggi, C.C.; Tucci, M.; Resitano, M.; Matturro, B.; Crognale, S.; Feigl, V.; Molnár, M.; Rossetti, S.; Aulenta, F. Passive electrobioremediation approaches for enhancing hydrocarbons biodegradation in contaminated soils. Sci. Total. Environ. 2022, 845, 157325. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Tucci, M.; Viggi, C.C.; Dolfing, J.; Head, I.M.; Rotaru, A. An underappreciated DIET for anaerobic petroleum hydrocarbon-degrading microbial communities. Microb. Biotechnol. 2020, 14, 2–7. [Google Scholar] [CrossRef]
- Bellagamba, M.; Cruz Viggi, C.; Ademollo, N.; Rossetti, S.; Aulenta, F. Electrolysis-driven bioremediation of crude oil-contaminated marine sediments. New Biotechnol. 2017, 38, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Daghio, M.; Aulenta, F.; Vaiopoulou, E.; Franzetti, A.; Arends, J.B.; Sherry, A.; Suárez-Suárez, A.; Head, I.M.; Bestetti, G.; Rabaey, K. Electrobioremediation of oil spills. Water Res. 2017, 114, 351–370. [Google Scholar] [CrossRef]
- Wartell, B.; Boufadel, M.; Rodriguez-Freire, L. An effort to understand and improve the anaerobic biodegradation of petroleum hydrocarbons: A literature review. Int. Biodeterior. Biodegrad. 2020, 157, 105156. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Genet. 2009, 7, 568–577. [Google Scholar] [CrossRef]
- Gieg, L.M.; Fowler, S.J.; Berdugo-Clavijo, C. Syntrophic biodegradation of hydrocarbon contaminants. Curr. Opin. Biotechnol. 2014, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.D.; Sherry, A.; Hubert, C.; Dolfing, J.; Head, I.M. Methanogenic Degradation of Petroleum Hydrocarbons in Subsurface Environments: Remediation, Heavy Oil Formation, and Energy Recovery. Adv. Appl. Microbiol. 2010, 72, 137–161. [Google Scholar] [PubMed]
- Mbadinga, S.M.; Wang, L.-Y.; Zhou, L.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Microbial communities involved in anaerobic degradation of alkanes. Int. Biodeterior. Biodegrad. 2011, 65, 1–13. [Google Scholar] [CrossRef]
- Quéméner, E.D.-L.; Moscoviz, R.; Bernet, N.; Marcus, A. Modeling of interspecies electron transfer in anaerobic microbial communities. Curr. Opin. Biotechnol. 2021, 67, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Syntrophy Goes Electric: Direct Interspecies Electron Transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Viggi, C.C.; Simonetti, S.; Palma, E.; Pagliaccia, P.; Braguglia, C.; Fazi, S.; Baronti, S.; Navarra, M.A.; Pettiti, I.; Koch, C.; et al. Enhancing methane production from food waste fermentate using biochar: The added value of electrochemical testing in pre-selecting the most effective type of biochar. Biotechnol. Biofuels 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef]
- Bonaglia, S.; Broman, E.; Brindefalk, B.; Hedlund, E.; Hjorth, T.; Rolff, C.; Nascimento, F.J.; Udekwu, K.; Gunnarsson, J.S. Activated carbon stimulates microbial diversity and PAH biodegradation under anaerobic conditions in oil-polluted sediments. Chemosphere 2020, 248, 126023. [Google Scholar] [CrossRef]
- Chen, S.-C.; Musat, N.; Lechtenfeld, O.J.; Paschke, H.; Schmidt, M.; Said, N.; Popp, D.; Calabrese, F.; Stryhanyuk, H.; Jaekel, U.; et al. Anaerobic oxidation of ethane by archaea from a marine hydrocarbon seep. Nature 2019, 568, 108–111. [Google Scholar] [CrossRef]
- Müller, H.; Bosch, J.; Griebler, C.; Damgaard, L.R.; Nielsen, L.P.; Lueders, T.; Meckenstock, R.U. Long-distance electron transfer by cable bacteria in aquifer sediments. ISME J. 2016, 10, 2010–2019. [Google Scholar] [CrossRef]
- Nielsen, L.P.; Risgaard-Petersen, N.; Fossing, H.; Christensen, P.B.; Sayama, M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 2010, 463, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Pagnozzi, G.; Carroll, S.; Reible, D.D.; Millerick, K. Powdered activated carbon (PAC) amendment enhances naphthalene biodegradation under strictly sulfate-reducing conditions. Environ. Pollut. 2020, 268, 115641. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Posth, N.R.; Löscher, C.R.; Miracle, M.R.; Vicente, E.; Cox, R.P.; Thompson, J.; Poulton, S.W.; Thamdrup, B. Interspecies interactions mediated by conductive minerals in the sediments of the Iron rich Meromictic Lake La Cruz, Spain. Limnetica 2019, 38, 21–40. [Google Scholar] [CrossRef]
- Rossi, M.; Silvani, L.; Amanat, N.; Papini, M.P. Biochar from Pine Wood, Rice Husks and Iron-Eupatorium Shrubs for Remediation Applications: Surface Characterization and Experimental Tests for Trichloroethylene Removal. Materials 2021, 14, 1776. [Google Scholar] [CrossRef] [PubMed]
- Silvani, L.; Vrchotova, B.; Kastanek, P.; Demnerova, K.; Pettiti, I.; Papini, M.P. Characterizing Biochar as Alternative Sorbent for Oil Spill Remediation. Sci. Rep. 2017, 7, srep43912. [Google Scholar] [CrossRef]
- Viggi, C.C.; Colantoni, S.; Falzetti, F.; Bacaloni, A.; Montecchio, D.; Aulenta, F. Conductive Magnetite Nanoparticles Enhance the Microbial Electrosynthesis of Acetate from CO2 while Diverting Electrons away from Methanogenesis. Fuel Cells 2020, 20, 98–106. [Google Scholar] [CrossRef]
- Aulenta, F.; Tocca, L.; Verdini, R.; Reale, P.; Majone, M. Dechlorination of Trichloroethene in a Continuous-Flow Bioelectrochemical Reactor: Effect of Cathode Potential on Rate, Selectivity, and Electron Transfer Mechanisms. Environ. Sci. Technol. 2011, 45, 8444–8451. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, Y.; Yu, L.; Zhang, B.; Li, J.; Liu, T.; Yu, Z.; Zhou, S. Biochar enhances bioelectrochemical remediation of pentachlorophenol-contaminated soils via long-distance electron transfer. J. Hazard. Mater. 2020, 391, 122213. [Google Scholar] [CrossRef]
- Guo, J.; Yang, S.; He, Q.; Chen, Y.; Zheng, F.; Zhou, H.; Hou, C.; Du, B.; Jiang, S.; Li, H. Improving benzo(a)pyrene biodegradation in soil with wheat straw-derived biochar amendment: Performance, microbial quantity, CO2 emission, and soil properties. J. Anal. Appl. Pyrolysis 2021, 156, 105132. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, X.; Zhao, X.; Sun, Y.; Weng, L.; Li, Y. Long-term effect of biochar amendment on the biodegradation of petroleum hydrocarbons in soil microbial fuel cells. Sci. Total. Environ. 2018, 651, 796–806. [Google Scholar] [CrossRef]
- Liu, L.; Chen, P.; Sun, M.; Shen, G.; Shang, G. Effect of biochar amendment on PAH dissipation and indigenous degradation bacteria in contaminated soil. J. Soils Sediments 2014, 15, 313–322. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Wang, J.; Liao, C.; Zhou, L.; An, J.; Li, T.; Wang, X.; Zhou, Q. Construction of conductive network using magnetite to enhance microflora interaction and petroleum hydrocarbons removal in plant-rhizosphere microbial electrochemical system. Chem. Eng. J. 2022, 433, 133600. [Google Scholar] [CrossRef]
- Aitken, C.; Jones, D.; Maguire, M.; Gray, N.; Sherry, A.; Bowler, B.; Ditchfield, A.; Larter, S.; Head, I. Evidence that crude oil alkane activation proceeds by different mechanisms under sulfate-reducing and methanogenic conditions. Geochim. Cosmochim. Acta 2013, 109, 162–174. [Google Scholar] [CrossRef]
- Kuntze, K.; Vogt, C.; Richnow, H.-H.; Boll, M. Combined Application of PCR-Based Functional Assays for the Detection of Aromatic-Compound-Degrading Anaerobes. Appl. Environ. Microbiol. 2011, 77, 5056–5061. [Google Scholar] [CrossRef] [PubMed]
- Löffler, C.; Kuntze, K.; Vazquez, J.R.; Rugor, A.; Kung, J.W.; Böttcher, A.; Boll, M. Occurrence, genes and expression of the W/Se-containing class II benzoyl-coenzyme A reductases in anaerobic bacteria. Environ. Microbiol. 2010, 13, 696–709. [Google Scholar] [CrossRef]
- Winderl, C.; Schaefer, S.; Lueders, T. Detection of anaerobic toluene and hydrocarbon degraders in contaminated aquifers using benzylsuccinate synthase (bssA) genes as a functional marker. Environ. Microbiol. 2007, 9, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Matturro, B.; Heavner, G.L.; Richardson, R.E.; Rossetti, S. Quantitative estimation of Dehalococcoides mccartyi at laboratory and field scale: Comparative study between CARD-FISH and Real Time PCR. J. Microbiol. Methods 2013, 93, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; Casentini, B.; Amalfitano, S.; Fazi, S.; Petruccioli, M.; Rossetti, S. Biological As(III) oxidation in biofilters by using native groundwater microorganisms. Sci. Total. Environ. 2018, 651, 93–102. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Crognale, S.; Braguglia, C.; Gallipoli, A.; Gianico, A.; Rossetti, S.; Montecchio, D. Direct Conversion of Food Waste Extract into Caproate: Metagenomics Assessment of Chain Elongation Process. Microorganisms 2021, 9, 327. [Google Scholar] [CrossRef]
- Mukome, F.N.; Buelow, M.C.; Shang, J.; Peng, J.; Rodriguez, M.; Mackay, D.M.; Pignatello, J.J.; Sihota, N.; Hoelen, T.P.; Parikh, S.J. Biochar amendment as a remediation strategy for surface soils impacted by crude oil. Environ. Pollut. 2020, 265, 115006. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Gong, D.; Fan, M.-Y. Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int. Biodeterior. Biodegrad. 2013, 85, 150–155. [Google Scholar] [CrossRef]
- Tazangi, M.H.; Ebrahimi, S.; Nasrabadi, R.G.; Naeeni, S.A.M. Kinetic Monitoring of Bioremediators for Biodegradation of Gasoil-Polluted Soil. Water, Air, Soil Pollut. 2020, 231, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Rong, X.; Song, H.; Chen, J. Remediation of Petroleum-contaminated Soil Using Bulrush Straw Powder, Biochar and Nutrients. Bull. Environ. Contam. Toxicol. 2017, 98, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, L.; Guo, X.; Han, Z.; Ji, L.; He, Q.; Han, L.; Sun, K. Mechanism of biochar as a biostimulation strategy to remove polycyclic aromatic hydrocarbons from heavily contaminated soil in a coking plant. Geoderma 2020, 375, 114497. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Biochar Impacts on Soil Physical Properties and Greenhouse Gas Emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, J.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kato, S.; Watanabe, K. Microbial interspecies interactions: Recent findings in syntrophic consortia. Front. Microbiol. 2015, 6, 477. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, Z.; Liu, L.; Yang, W.; Wang, L.; Li, J.; Zhang, D. Soil Respiration and Organic Carbon Response to Biochar and Their Influencing Factors. Atmosphere 2022, 13, 2038. [Google Scholar] [CrossRef]
- Crampon, M.; Bodilis, J.; Portet-Koltalo, F.; Crampon, M.; Portet-Koltalo, F. Linking initial soil bacterial diversity and polycyclic aromatic hydrocarbons (PAHs) degradation potential. J. Hazard. Mater. 2018, 359, 500–509. [Google Scholar] [CrossRef]
- Hidalgo, K.J.; Sierra-Garcia, I.N.; Dellagnezze, B.M.; De Oliveira, V.M. Metagenomic Insights into the Mechanisms for Biodegradation of Polycyclic Aromatic Hydrocarbons in the Oil Supply Chain. Front. Microbiol. 2020, 11, 561506. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, Y.; Jeon, C.O. Biodegradation of naphthalene, BTEX, and aliphatic hydrocarbons by Paraburkholderia aromaticivorans BN5 isolated from petroleum-contaminated soil. Sci. Rep. 2019, 9, 860. [Google Scholar] [CrossRef]
- Weelink, S.A.B.; van Eekert, M.H.A.; Stams, A.J.M. Degradation of BTEX by anaerobic bacteria: Physiology and application. Rev. Environ. Sci. Bio/Technology 2010, 9, 359–385. [Google Scholar] [CrossRef]
- Widada, J.; Nojiri, H.; Kasuga, K.; Yoshida, T.; Habe, H.; Omori, T. Molecular detection and diversity of polycyclic aromatic hydrocarbon-degrading bacteria isolated from geographically diverse sites. Appl. Microbiol. Biotechnol. 2002, 58, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Singleton, D.R.; Aitken, M.D. Effects of Nonionic Surfactant Addition on Populations of Polycyclic Aromatic Hydrocarbon-Degrading Bacteria in a Bioreactor Treating Contaminated Soil. Environ. Sci. Technol. 2010, 44, 7266–7271. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, L.; Guo, X.; Dai, X.; Liu, L.; Xi, L.; Wang, J.; Song, L.; Wang, Y.; Zhu, Y.; et al. Diversity, Biogeography, and Biodegradation Potential of Actinobacteria in the Deep-Sea Sediments along the Southwest Indian Ridge. Front. Microbiol. 2016, 7, 1340. [Google Scholar] [CrossRef]
- Laso-Pérez, R.; Hahn, C.; van Vliet, D.M.; Tegetmeyer, H.E.; Schubotz, F.; Smit, N.T.; Pape, T.; Sahling, H.; Bohrmann, G.; Boetius, A.; et al. Anaerobic Degradation of Non-Methane Alkanes by “ Candidatus Methanoliparia” in Hydrocarbon Seeps of the Gulf of Mexico. Mbio 2019, 10, e01814-19. [Google Scholar] [CrossRef]
- Von Netzer, F.; Kuntze, K.; Vogt, C.; Richnow, H.H.; Boll, M.; Lueders, T. Functional Gene Markers for Fumarate-Adding and Dearomatizing Key Enzymes in Anaerobic Aromatic Hydrocarbon Degradation in Terrestrial Environments. J. Mol. Microbiol. Biotechnol. 2016, 26, 180–194. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Viggi, C.; Tucci, M.; Resitano, M.; Palushi, V.; Crognale, S.; Matturro, B.; Petrangeli Papini, M.; Rossetti, S.; Aulenta, F. Enhancing the Anaerobic Biodegradation of Petroleum Hydrocarbons in Soils with Electrically Conductive Materials. Bioengineering 2023, 10, 441. https://doi.org/10.3390/bioengineering10040441
Cruz Viggi C, Tucci M, Resitano M, Palushi V, Crognale S, Matturro B, Petrangeli Papini M, Rossetti S, Aulenta F. Enhancing the Anaerobic Biodegradation of Petroleum Hydrocarbons in Soils with Electrically Conductive Materials. Bioengineering. 2023; 10(4):441. https://doi.org/10.3390/bioengineering10040441
Chicago/Turabian StyleCruz Viggi, Carolina, Matteo Tucci, Marco Resitano, Valentina Palushi, Simona Crognale, Bruna Matturro, Marco Petrangeli Papini, Simona Rossetti, and Federico Aulenta. 2023. "Enhancing the Anaerobic Biodegradation of Petroleum Hydrocarbons in Soils with Electrically Conductive Materials" Bioengineering 10, no. 4: 441. https://doi.org/10.3390/bioengineering10040441
APA StyleCruz Viggi, C., Tucci, M., Resitano, M., Palushi, V., Crognale, S., Matturro, B., Petrangeli Papini, M., Rossetti, S., & Aulenta, F. (2023). Enhancing the Anaerobic Biodegradation of Petroleum Hydrocarbons in Soils with Electrically Conductive Materials. Bioengineering, 10(4), 441. https://doi.org/10.3390/bioengineering10040441











