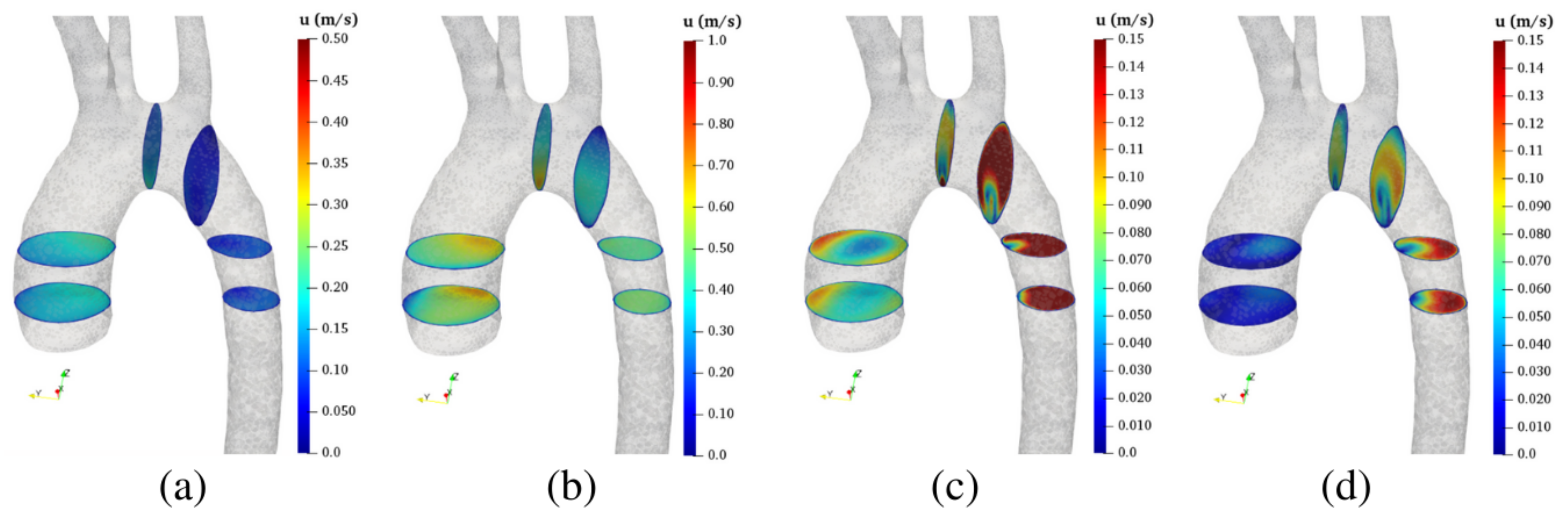
5.1. Flow Distribution
Each aortic dissection impacts the distribution of the blood volume flow through the different branches in a peculiar way (see
Table 8). In the following, we quantify such impacts by computing the percentage volume flow increase/decrease for each outlet with respect to HA. In FPA, the FL drains the TL flow [
38]. As a consequence, the abdominal branches (that, in this case, are connected to TL) undergo a significant flow reduction equal to
(O
),
(O
) and
(O
). Conversely, the volume flow increases by approximately
in the iliac branches (O
, O
) that are connected to the FL. For the PTA, the flow through the aortic arch FL reduces the flux through O
by approximately
, while it increases the flux at O
and O
by
and
, respectively. Note that the left renal artery (O
) is connected to the abdominal FL in both PTA and FTA and its flow is reduced by
and
, respectively. Finally, for the PTA, the right renal artery flow (O
) is reduced by
, as a consequence of the abdominal FL flow that originates from two tears in the abdominal aorta region. While these findings may be patient-specific since they depend on the number and location of the intimal tears, they are well in agreement with clinical evidence. In fact, the paravisceral segment FL thrombus (as in PTA and FTA) is a frequent cause of visceral blood flow reduction in the chronic phase of aortic dissection [
71].
5.2. Flow Kinematics
We analyse the flow kinematics for all the models at the systolic peak (
), where the velocity is maximum, and at diastole (
), where retrograde flow occurs in ascending aorta and the turbulence levels are higher. Further, we study in detail the streamlines patterns in the proximity of the tears, which are relevant flow regions in terms of aortic dissection outcome [
72]. Finally, we dissect
patterns at diastole. Enhanced-visualisation streamlines and
Q contours are available at
https://github.com/andrea-facci/Aorta (accessed on 23 January 2023) as supplementary movies.
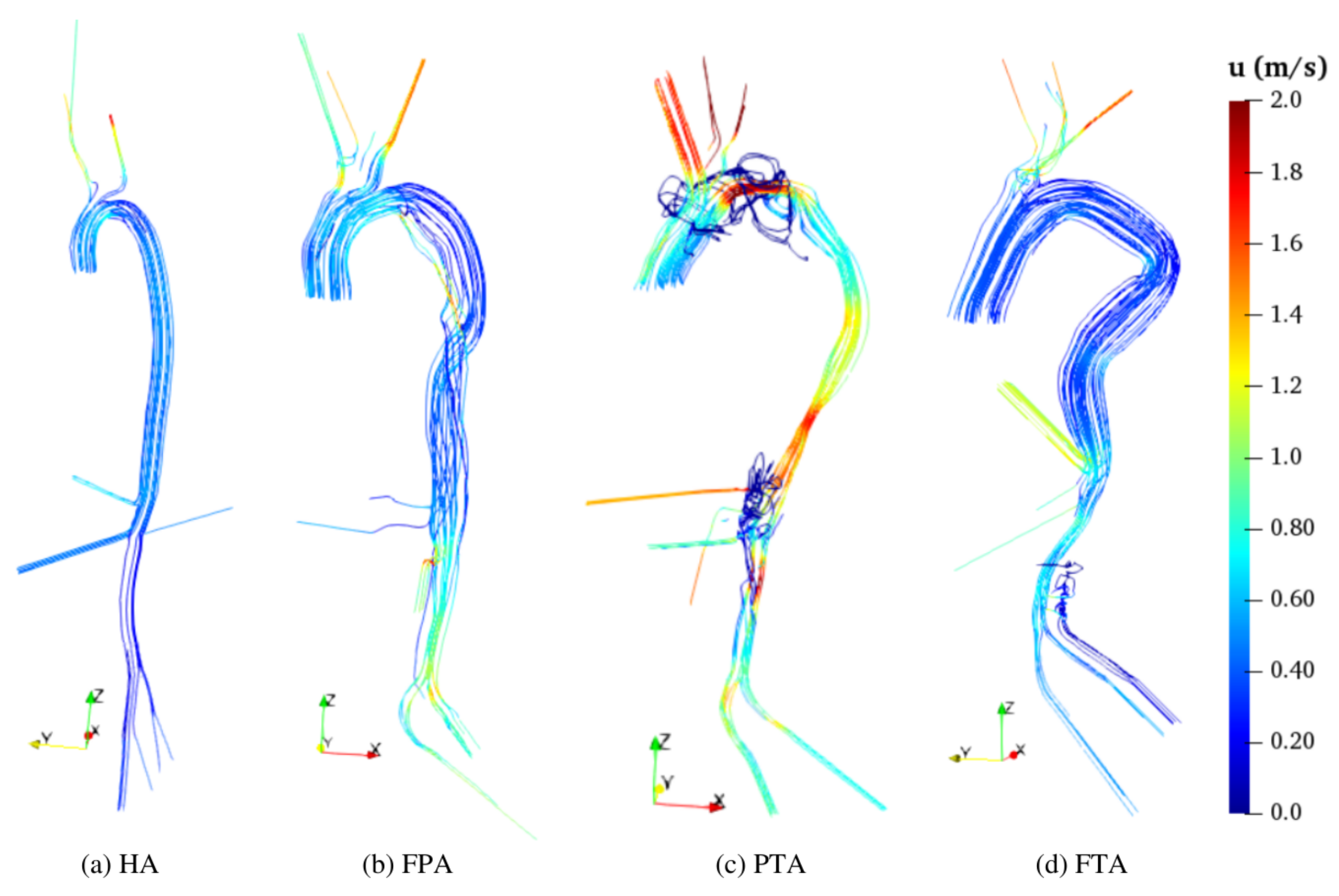
In all diseased aortas, the velocity pattern is less homogeneous than in HA (
Figure 10 and
Figure 11), and the flow accelerates along the abdominal aorta and the iliac branches (
Figure 10) due to abrupt diameter variations in the TL. Such a result is more prominent in the FPA TL and in both PTA TL and FL, while the flow in the FL of the FPA (
Figure 10b) and in the TL of the FTA (
Figure 10d) is ordered and similar to the pattern of the HA (see also [
48]). The highest velocity magnitude locates in the PTA TL that has the smallest cross-section, and three major restrictions identifiable in
Figure 10c as the regions at high velocity values. One of these restrictions is located in the arch and is followed by an abrupt section enlargement that yields recirculating flow (see
Figure 11c and the focused view in
Figure 12b).The other two restrictions are located in the abdominal aorta. Therein, blood flow in the FL is fed from the TL exclusively through five tears located below O
(
Figure 12c). As a consequence, the upper part of the abdominal FL is washed by an ascending and recirculating flow as displayed by low
u closed streamlines. Similar evidence can be found in [
18]. In the FTA, the tears are also located below O
(
Figure 12d), which receives its flux through an ascending flow. Streamlines show a helical flow pattern of very low velocity (
Figure 12c,d). This is consistent with reduced volume flows through O
(see
Table 8).
In HA, the
Q pattern is regular, with stable organized vortexes located close to the walls (
Figure 13a) that represent the helical flow developing along the entire aorta (similar results are found in [
60]). These structures are continuous, organized, and regular (see also [
73]), and the flow pattern does not show stagnation zones (black arrows in
Figure 13 and
Figure 14). Moreover, such vortexes are stable in time, as arguable by comparing
Q values for systole (
Figure 13a) and for diastole (
Figure 14a). However, at systole, coherent structures involve the whole domain, while, at diastole, they mainly develop along the descending and abdominal aorta. In fact, at diastole, vortex structures originate within the arch and proceed through the descending aorta [
73].
In dissected aortas, coherent structures are fragmented (in particular in FPA and PTA), and stagnation regions persist in FLs during both systole and diastole, as highlighted by the arrows in
Figure 13 and
Figure 14. These are consistent with the rotating streamlines visible in
Figure 10 and
Figure 11. Specifically, FPA exhibits a single significant stagnation point that involves the aortic arch FL (
Figure 13b and
Figure 14b). The PTA has three dominant stagnation regions (
Figure 13c and
Figure 14c): two in the aortic arch FL and one in the upper portion of the abdominal FL. The FTA exhibits 25 fragmented vortex structures and a single stagnation point located in proximity of O
(
Figure 13d and
Figure 14d).
The intensity of
T is generally higher at diastole than at systole in all geometries
Figure 15. In the HA,
mainly in the aortic arch (
Figure 16a), consistent with the streamlines pattern in
Figure 11 and the
Q contours in
Figure 14. At the systolic peak, maximum
T is approximately equal to
in the ascending aorta. Small regions at higher values are located at the roots of the upper arteries. In dissected aortas, the region at
is more extended than in HA, both at systole (
Figure 15) and diastole (
Figure 16). In the FPA, the region at higher
T is located downstream the tear in FL at systolic peak (
Figure 15b), and upstream the tear in the aortic arch at diastole (
Figure 16b). In the PTA, the high
T region is more distinctly observed at systolic peak (
Figure 15c) than in the other models.
Figure 15c also shows high
T regions (
) in proximity of the tears in the aortic arch and abdominal FLs. Such regions correspond to the abrupt TL restrictions. Unlike other geometries, the FTA displays high
T also below the abdominal branches (O
) (
Figure 15d and
Figure 16d).
Our results demonstrate that the tears are major sources of highly turbulent flow, as is also clearly visible in
Figure 12. For instance,
Figure 12(c.2,d.2) show that the volume flow rate through O
drastically drops due to the stagnating flow region. Stagnation is generated therein by the specific geometry of the FL (the top thrombosed FL is three to five FL diameters above O
) as well as by the relative position of O
and the tear (O
is approximately two FL diameters above the tear). In FPA and FTA, the flow through the tears is directed from the TL to the FL. Conversely, in the PTA, the FL bidirectionally communicates with the TL. Since the descending FL is thrombosed, the aortic arch FL feeds the TL upstream the thrombus (
Figure 12b). As in the other geometries, the abdominal FL is instead fed by the TL.
To further explore flow kinematics, we report results for
at diastole in
Figure 17. Such a flow pattern is generally more complicated than at systole due to the retrograde flow in ascending aorta (
Figure 11). Retrograde flow is well evidenced by the
contour plot in
Figure 17, whereby negative values in the ascending aorta represent backflow. In the HA (
Figure 17a) and FPA (
Figure 17b), the pattern is similar. As expected, in the FPA aortic arch,
is higher than in other models due to the TL restriction in proximity of the tear. In the PTA,
is also high in the ascending aorta (
Figure 17c), while it is null for a large part of the FL due to geometric constraints. Positive
values in the descending aorta are a consequence of the vortexes evidenced at diastole in
Figure 11.
5.3. Hemodynamic Forces
Hemodynamic forces are related to pressure and WSS. The pressure is higher when the flow acceleration is maximum (i.e., at
) for all considered cases. In the HA and the FTA, the pressure is higher at the inlet and reduces towards the outlets for
(i.e., when the blood flow accelerates), while it has an opposite pattern for
(when the flow decelerates). On the contrary, in the FPA and PTA, the pressure reduces through the aorta also for a large part of the flow deceleration (i.e., for
) due to the larger pressure drop, see the supplementary movies. In fact, in the FPA and PTA, restrictions increase the average velocity and, thus, the friction pressure losses. Moreover, vortexes identified in
Figure 11 for dissected aortas are additional sources of pressure drop. In general, these considerations highlight a different balance between fluid inertia and viscous forces among the different pathologies. Specifically, for HA and FTA, inertia dominates, whereas friction dominates for FPA and PTA.
Figure 18 shows that greater
p values locate in the ascending aorta and aortic arch (in agreement with [
63]). Moreover, in FPA and PTA,
p is significantly higher than in HA and FTA, reaching 150 mmHg for the PTA FL and 130 mmHg for the FPA. FPA and PTA have greater velocity at the iliac arteries (see
Figure 10). As a consequence, in these cases, the flow back-pressure is higher, increasing the outlet pressure also for all the other branches. Specifically, the average outlet pressure is approximately
and
higher compared to the HA, for all the outlets in FPA and PTA, respectively. Conversely, in the iliac branches of the FTA, the velocity is lower compared to the HA and, in turn, the flow back-pressure is reduced by
. In fact, the thrombosed FL acts as a stenosis on the TL, thus reducing the distal blood flow.
We compute the average pressure drop in the cardiac cycle as
for the HA,
for the FTA,
for the FPA, and
for the PTA. Note that the pressure variation for the PTA is about 13 times higher compared to the HA. This probably explains the fact that PTA FL is more prone to aneurysmal degeneration and, ultimately, to aortic rupture [
63]. In fact, in addition to greater pressure variations, diseased aortic walls have lower elasticity and, thus, more likely experience irreversible expansion. A preventive treatment of PTA FL may consist of surgically reaching complete FL thrombosis.
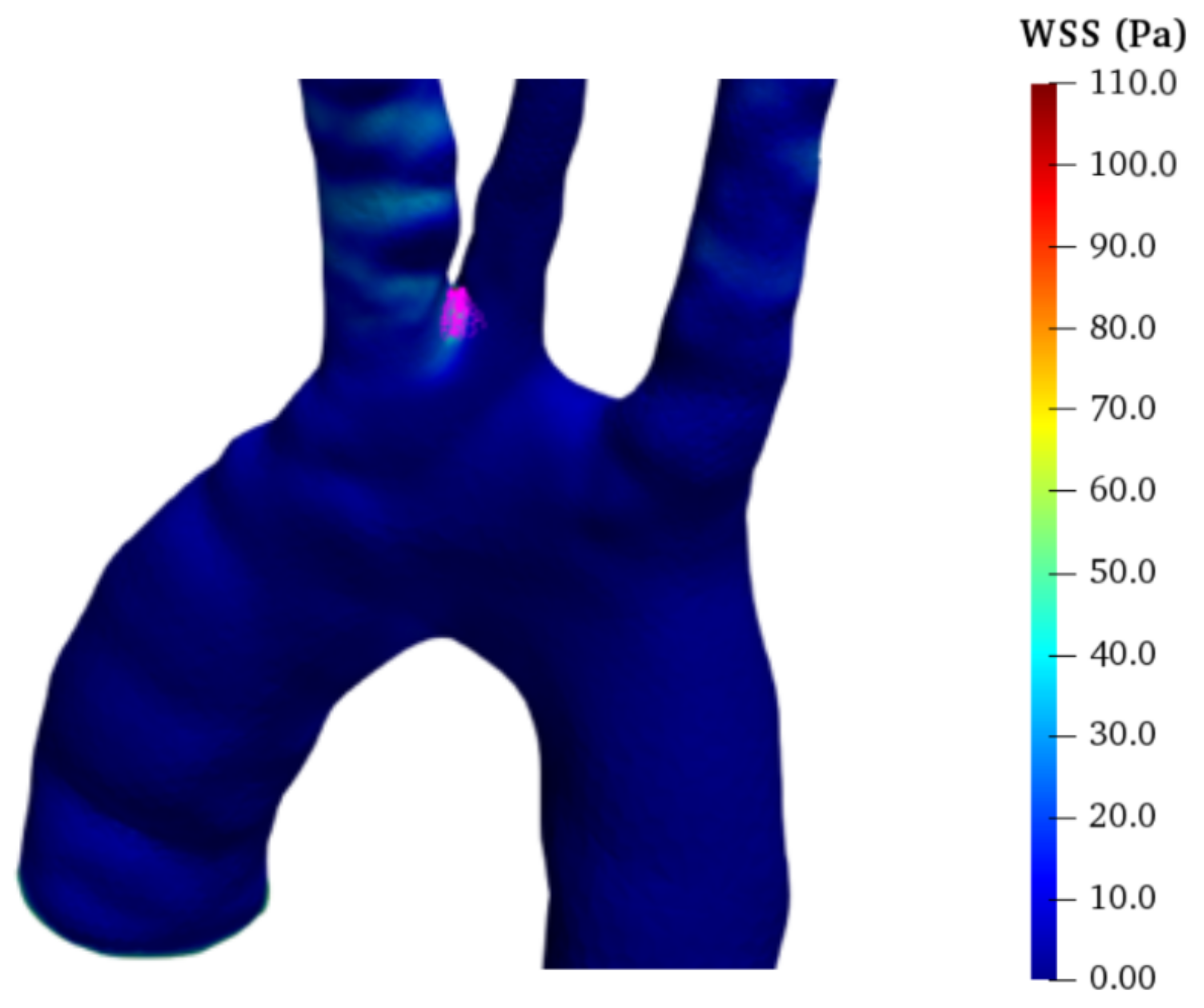
Pressure drop along the aorta derives from WSS and turbulent dissipation in vortex regions. In particular, according to [
23], we evaluate the time-averaged WSS (TAWSS):
as an indicator of the total stress on the aortic wall for a complete cardiac cycle. In all models, TAWSS is large at the carotids and subclavian roots (
Figure 19) where the flow section narrows, the velocity increases (
Figure 10 and
Figure 11), and the branch root acts as a flow obstacle. Except for such regions, in the HA,
is generally less than
Pa. Among dissected models, FTA has the lowest TAWSS pattern; however, moderate TAWSS values (i.e.,
Pa) are detected in the abdominal TL, and greater values (
Pa) are found close to the tears of the abdominal FL due to the high velocities of the jet-like flow (see
Figure 12 and
Figure 19d). Notably, the literature identifies such areas of communication between TL and FL as potentially problematic spots [
72].
In the PTA,
Pa for the majority of the TL (
Figure 19c), including the iliac arteries. Similar to the FTA, the jets through the communication tears between TL and FL generate high-TAWSS regions in the abdominal FL. However, the descending and aortic arch FLs exhibit the lowest TAWSS values due to the low velocity field and flow stagnation (see
Section 5.2). High TAWSS values can also be observed in the FPA. Namely, TAWSS in the descending TL of the FPA is large due to the reduced flow area and consequently large velocity (
Figure 19b). Similar values are detected close to the main tear in the TL aortic arch. Conversely, the FPA FL is characterized by relatively low TAWSS. However, its value increases along the abdominal FL and iliac arteries. In fact, as stated in
Section 5.1, in O
and O
, the volume flow is significantly larger compared with the other geometries.
Comprehension of the pathology evolution can further benefit from evaluation of WSS dynamics [
23,
35,
48]. Specifically, despite low TAWSS, vortex flow regions could be prone to aneurysm formation [
23] as a consequence of stress oscillation that elicits structural fatigue. The oscillatory shear index (OSI) evaluates such unsteady WSS effects [
23,
25]:
By definition,
, and extended regions where OSI is close to
identify possible aneurysm formation [
35].
In HA, high-OSI regions have limited extension and are generally disconnected along the abdominal aorta. Among all diseased models, the PTA exhibits the largest regions with high OSI (
Figure 20c). Specifically,
for the whole aortic arch and within the upper portion of the abdominal FL, in the ascending aorta and aortic arch TL as well as where the FL merges into the abdominal TL. This is due to the presence of the stable vortex structures and stagnating flow already discussed in Secion
Section 5.2. FPA shows the second most extended surface with
, as reported in
Figure 20b. In particular, critical regions comprise the descending and aortic arch FL and the whole TL. This can be attributed to the stable helical (vortex) flow and the irregular FL surface. Finally, in the FTA, critical regions are consistent with the locations of the vortex structures identified in
Section 5.2. Specifically, the high-OSI region in the upper portion of the abdominal FL corresponds to the stagnating flow. Furthermore, regions exhibiting periodic vortexes during diastole also have high OSI values. This is in agreement with findings in [
75].