Adjustment of Micro- and Macroporosity of ß-TCP Scaffolds Using Solid-Stabilized Foams as Bone Replacement
Abstract
1. Introduction
2. Materials and Methods
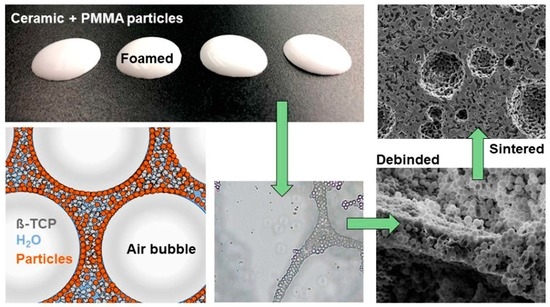
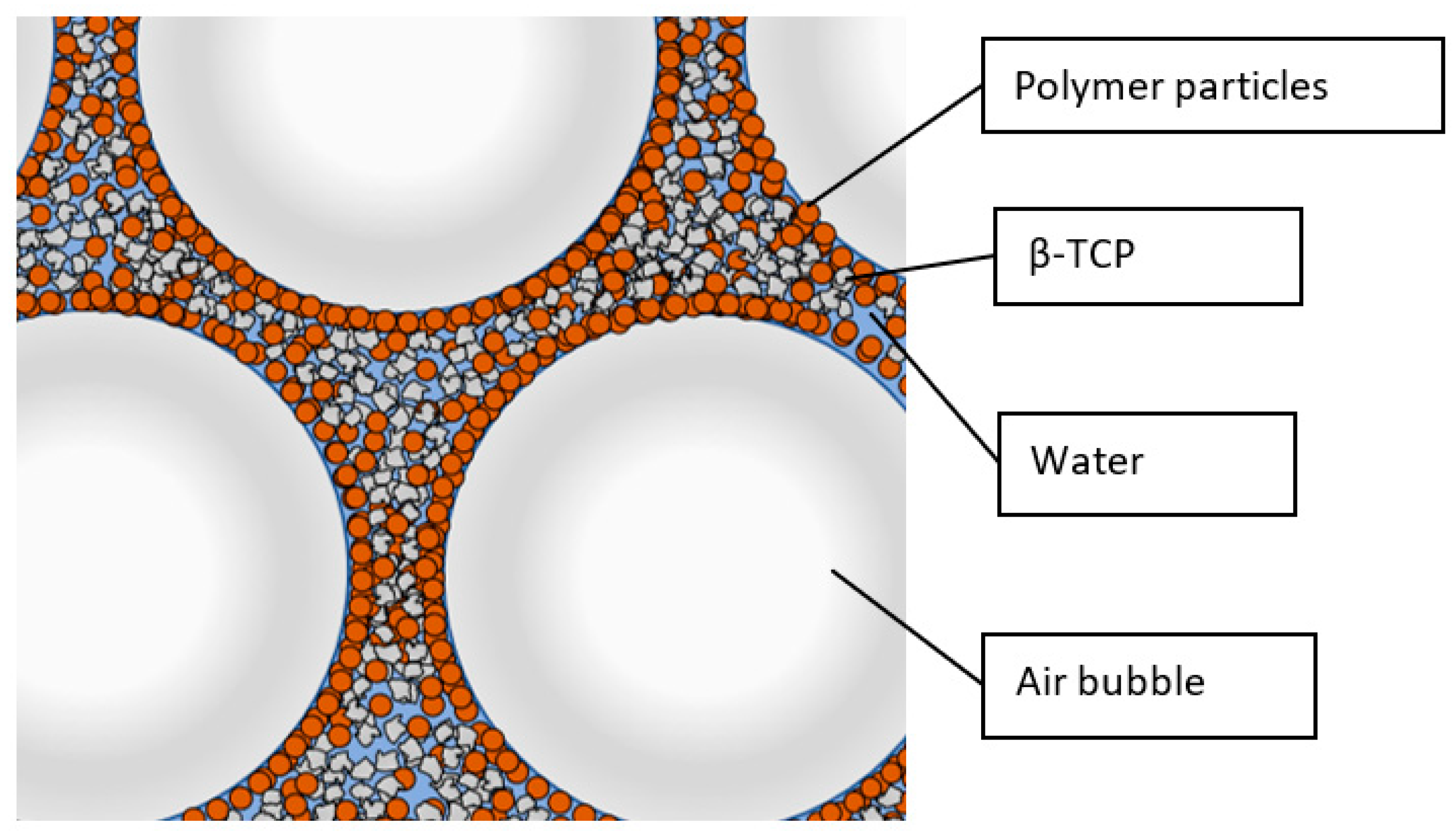
2.1. Production of the Micro- and Macroporous ß-TCP Scaffolds
2.2. Characterisation
2.3. Biocompatibility
2.3.1. Live Dead and Hoechst Assay
2.3.2. Cell Proliferation (WST-I)
2.4. Statistics
3. Results
3.1. Foaming Behaviour
3.2. Drying, Debinding and Sintering
3.3. Mechanical Characterisation
3.4. Biocompatibility
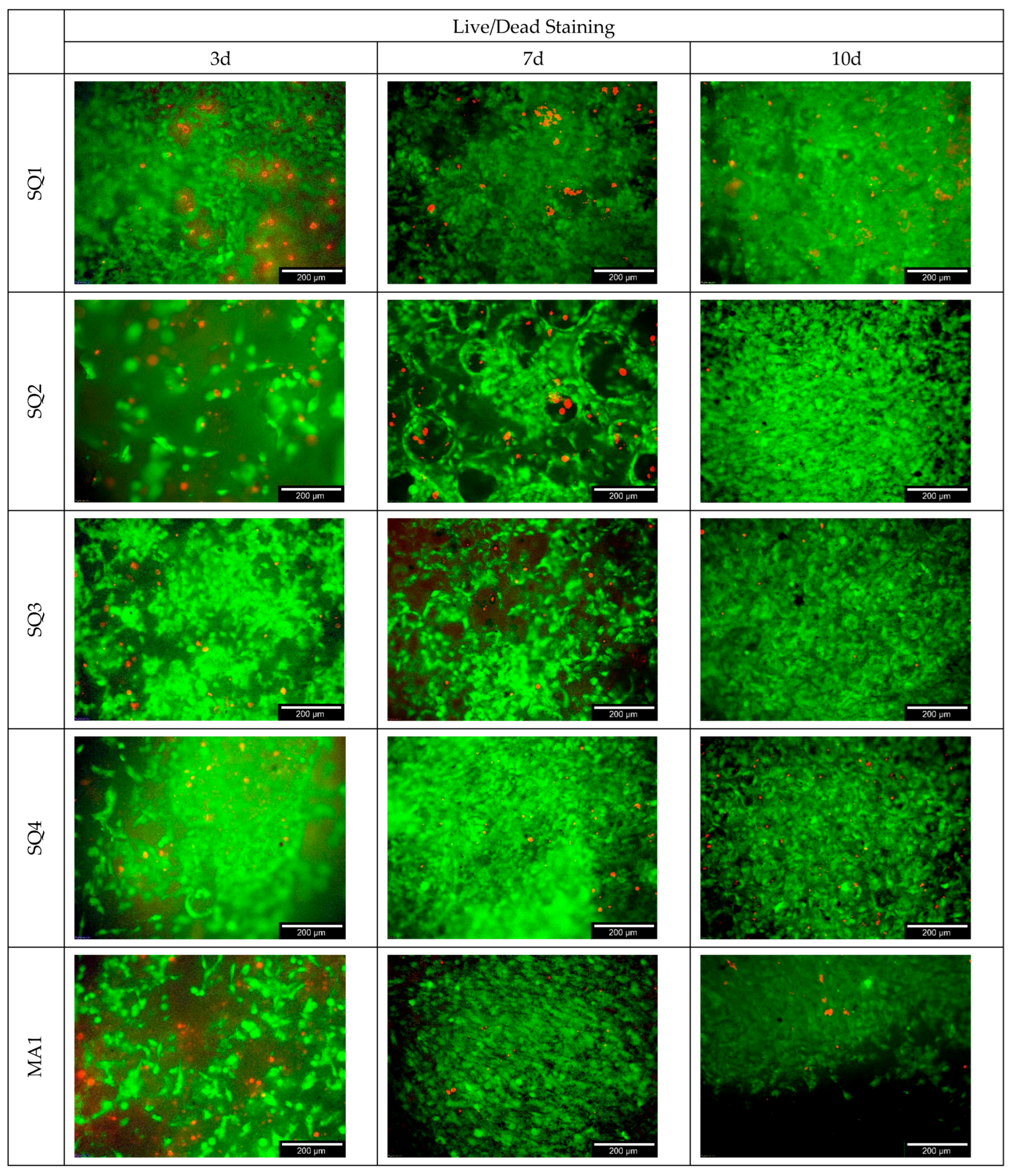
3.4.1. Live/Dead Assay
3.4.2. Cell Proliferation
3.4.3. Cytotoxicity
4. Discussion
4.1. Foaming Behaviour
4.2. Drying, Debinding and Sintering
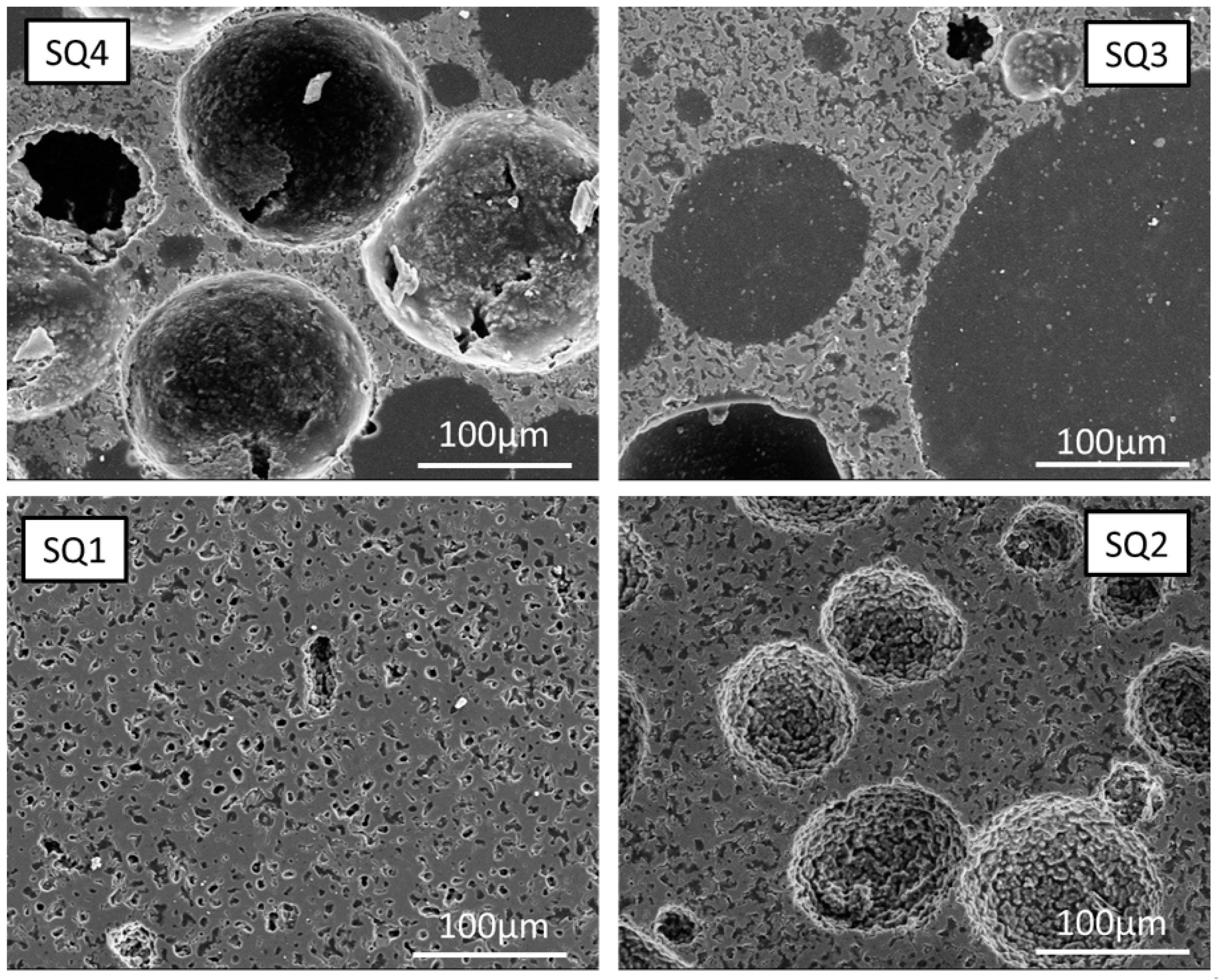
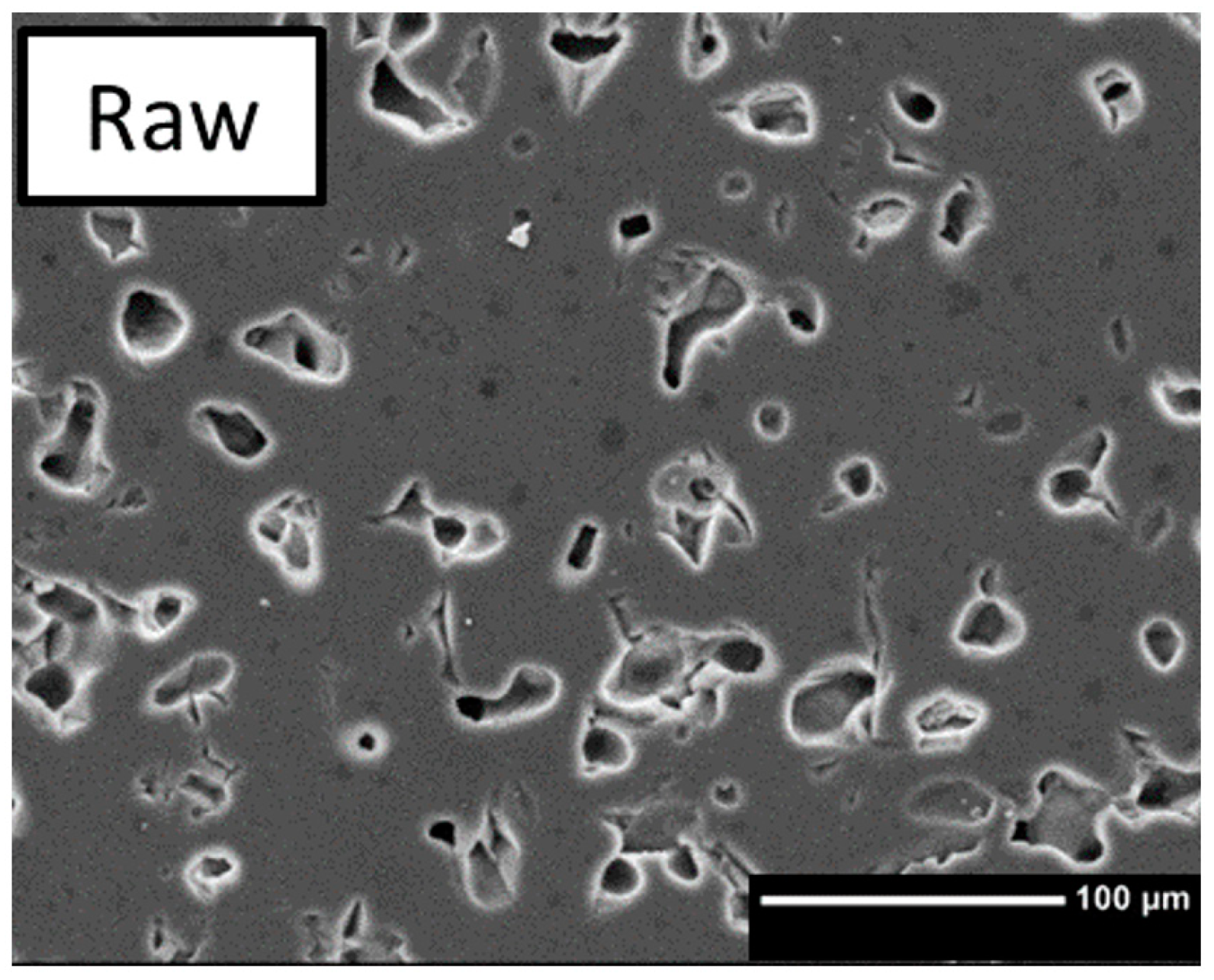
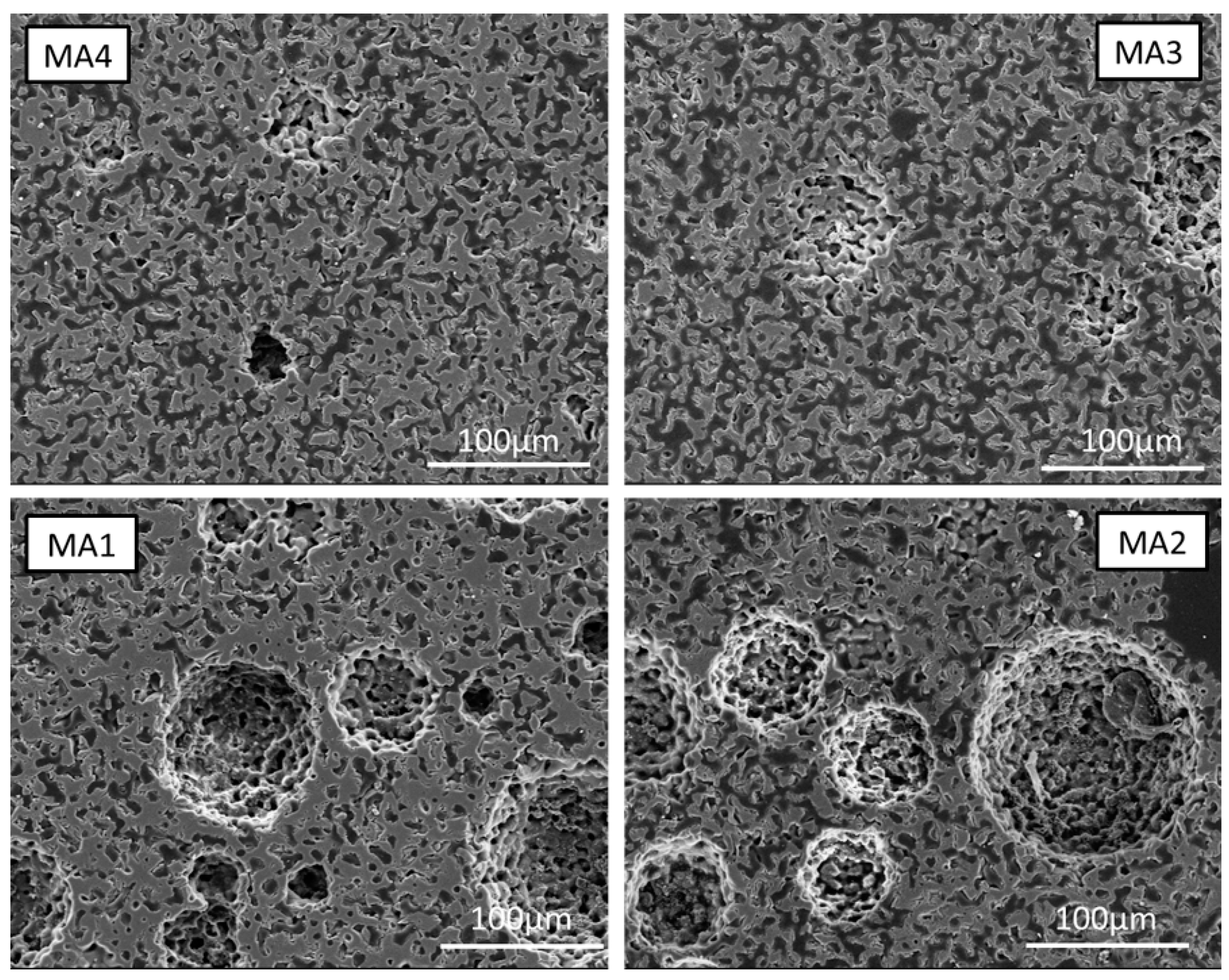
4.3. Pore Analysis
4.3.1. Macroporosity
4.3.2. Microporosity
4.4. Biocompatibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liebscher, M.A. Computer-Aided Tissue Engineering, 1. Auflage; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4939-5718-7. [Google Scholar]
- Frost, H.M. Defining osteopenias and osteoporoses: Another view (with insights from a new paradigm). Bone 1997, 20, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Vesvoranan, O.; Anup, A.; Hixon, K.R. Current Concepts and Methods in Tissue Interface Scaffold Fabrication. Biomimetics 2022, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.; Han, S.H.; Cho, M.; Choi, Y.-J.; Jin, Y.-Z.; Park, H.; Lee, J.H.; Yun, H. Effect of porosity and phase composition in 3D printed calcium phosphate scaffolds on bone tissue regeneration in vivo. Mater. Des. 2022, 219, 110819. [Google Scholar] [CrossRef]
- Pecqueux, F.; Tancret, F.; Payraudeau, N.; Bouler, J.M. Influence of microporosity and macroporosity on the mechanical properties of biphasic calcium phosphate bioceramics: Modelling and experiment. J. Eur. Ceram. Soc. 2010, 30, 819–829. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Seidenstuecker, M.; Ruehe, J.; Suedkamp, N.P.; Serr, A.; Wittmer, A.; Bohner, M.; Bernstein, A.; Mayr, H.O. Composite material consisting of microporous β-TCP ceramic and alginate for delayed release of antibiotics. Acta Biomater. 2017, 51, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Gudehus, H.T. Untersuchung des Einwachsverhaltens von Zirkoniumdioxid-Implantaten in die Kieferknochenstruktur—Eine Experimentelle Studie am Miniaturschwein. Inaugural Dissertation, Institute of Veterinary Pathology, Ludwig Maximilian University of Munich, Munich, Germany, 2006. Available online: https://d-nb.info/981950930/34 (accessed on 16 December 2022).
- Sánchez-Salcedo, S.; Nieto, A.; Vallet-Regí, M. Hydroxyapatite/β-tricalcium phosphate/agarose macroporous scaffolds for bone tissue engineering. Chem. Eng. J. 2008, 137, 62–71. [Google Scholar] [CrossRef]
- Dittmann, J. Verwendung von Kapillarsuspensionen als Precursor für die Herstellung Hochporöser Sinterwerkstoffe. Inaugural Dissertation, Institute of Mechanical Process Engineering and Mechanics, Karlsruhe Institute of Technology, Karlsruhe, Germany, 2015. [Google Scholar]
- Ohji, T.; Fukushima, M. Macro-porous ceramics: Processing and properties. Int. Mater. Rev. 2012, 57, 115–131. [Google Scholar] [CrossRef]
- Anita Lett, J.; Sundareswari, M.; Ravichandran, K. Porous hydroxyapatite scaffolds for orthopedic and dental applications—The role of binders. Mater. Today Proc. 2016, 3, 1672–1677. [Google Scholar] [CrossRef]
- Khallok, H.; Ojala, S.; Ezzahmouly, M.; Elouahli, A.; Gourri, E.H.; Jamil, M.; Hatim, Z. Porous foams based hydroxyapatite prepared by direct foaming method using egg white as a pore promoter. J. Aust. Ceram. Soc. 2019, 55, 611–619. [Google Scholar] [CrossRef]
- Dapporto, M.; Sprio, S.; Fabbi, C.; Figallo, E.; Tampieri, A. A novel route for the synthesis of macroporous bioceramics for bone regeneration. J. Eur. Ceram. Soc. 2016, 36, 2383–2388. [Google Scholar] [CrossRef]
- Kalemtas, A.; Topates, G.; Özcoban, H.; Mandal, H.; Kara, F.; Janssen, R. Mechanical characterization of highly porous β-Si3N4 ceramics fabricated via partial sintering & starch addition. J. Eur. Ceram. Soc. 2013, 33, 1507–1515. [Google Scholar] [CrossRef]
- Dejob, L.; Toury, B.; Tadier, S.; Grémillard, L.; Gaillard, C.; Salles, V. Electrospinning of In Situ Synthesized Silica-Based and Calcium Phosphate Bioceramics for Applications in Bone Tissue Engineering: A Review; Université Claude Bernard Lyon 1: Lyon, France, 2021. [Google Scholar]
- Gaudillere, C.; Serra, J.M. Freeze-casting: Fabrication of highly porous and hierarchical ceramic supports for energy applications. Boletín Soc. Española Cerámica Vidr. 2016, 55, 45–54. [Google Scholar] [CrossRef]
- Lee, H.; Jang, T.-S.; Song, J.; Kim, H.-E.; Jung, H.-D. The Production of Porous Hydroxyapatite Scaffolds with Graded Porosity by Sequential Freeze-Casting. Materials 2017, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Maurath, J.; Dittmann, J.; Schultz, N.; Willenbacher, N. Fabrication of highly porous glass filters using capillary suspension processing. Sep. Purif. Technol. 2015, 149, 470–478. [Google Scholar] [CrossRef]
- Madhavan, N.; Mukherjee, M.; Basavaraj, M.G. Porous Ceramics via Processable Pickering Emulsion Stabilized by Oppositely Charged Colloids. Langmuir 2020, 36, 11645–11654. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hu, Y.; Wang, C.; Binks, B.P. Fabrication of Hierarchical Macroporous Biocompatible Scaffolds by Combining Pickering High Internal Phase Emulsion Templates with Three-Dimensional Printing. ACS Appl. Mater. Interfaces 2017, 9, 22950–22958. [Google Scholar] [CrossRef] [PubMed]
- Gonzenbach, U.T.; Studart, A.R.; Steinlin, D.; Tervoort, E.; Gauckler, L.J. Processing of Particle-Stabilized Wet Foams into Porous Ceramics. J. Am. Ceram. Soc. 2007, 90, 3407–3414. [Google Scholar] [CrossRef]
- Ahn, M.-K.; Moon, Y.-W.; Maeng, W.-Y.; Koh, Y.-H.; Kim, H.-E. Design and Production of Continuously Gradient Macro/Microporous Calcium Phosphate (CaP) Scaffolds Using Ceramic/Camphene-Based 3D Extrusion. Materials 2017, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.-W.; Franco, R.A.; Sarkar, S.K.; Lee, B.-T. Fabrication of porous hydroxyapatite scaffolds as artificial bone preform and its biocompatibility evaluation. ASAIO J. 2014, 60, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Esslinger, S. Additive Fertigung Bioaktiver Keramiken zur Herstellung von Knochenersatzstrukturen; Shaker Verlag: Herzogenrath, Germany, 2020. [Google Scholar]
- Koepp, H.E.; Schorlemmer, S.; Kessler, S.; Brenner, R.E.; Claes, L.; Günther, K.-P.; Ignatius, A.A. Biocompatibility and osseointegration of beta-TCP: Histomorphological and biomechanical studies in a weight-bearing sheep model. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 70, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Q.; Wootton, D.; Chiou, R.; Li, D.; Lu, B.; Lelkes, P.; Zhou, J. Biocompatibility and biodegradation studies of PCL/β-TCP bone tissue scaffold fabricated by structural porogen method. J. Mater. Sci. Mater. Med. 2012, 23, 2217–2226. [Google Scholar] [CrossRef]
- Safronova, T.V.; Selezneva, I.I.; Tikhonova, S.A.; Kiselev, A.S.; Davydova, G.A.; Shatalova, T.B.; Larionov, D.S.; Rau, J.V. Biocompatibility of biphasic α,β-tricalcium phosphate ceramics in vitro. Bioact. Mater. 2020, 5, 423–427. [Google Scholar] [CrossRef]
- Garcia, R.A.; Tennent, D.J.; Chang, D.; Wenke, J.C.; Sanchez, C.J. An In Vitro Comparison of PMMA and Calcium Sulfate as Carriers for the Local Delivery of Gallium (III) Nitrate to Staphylococcal Infected Surgical Sites. BioMed Res. Int. 2016, 2016, 7078989. [Google Scholar] [CrossRef] [PubMed]
- Lan Levengood, S.K.; Polak, S.J.; Poellmann, M.J.; Hoelzle, D.J.; Maki, A.J.; Clark, S.G.; Wheeler, M.B.; Wagoner Johnson, A.J. The effect of BMP-2 on micro- and macroscale osteointegration of biphasic calcium phosphate scaffolds with multiscale porosity. Acta Biomater. 2010, 6, 3283–3291. [Google Scholar] [CrossRef]
- Parhizkar, M.; Sofokleous, P.; Stride, E.; Edirisinghe, M. Novel preparation of controlled porosity particle/fibre loaded scaffolds using a hybrid micro-fluidic and electrohydrodynamic technique. Biofabrication 2014, 6, 45010. [Google Scholar] [CrossRef] [PubMed]
- Biggemann, J.; Hoffmann, P.; Hristov, I.; Simon, S.; Müller, P.; Fey, T. Injection Molding of 3-3 Hydroxyapatite Composites. Materials 2020, 13, 1907. [Google Scholar] [CrossRef]
- Fujii, S.; Ryan, A.J.; Armes, S.P. Long-range structural order, moiré patterns, and iridescence in latex-stabilized foams. J. Am. Chem. Soc. 2006, 128, 7882–7886. [Google Scholar] [CrossRef]
- Horozov, T. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Kaptay, G. On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 387–401. [Google Scholar] [CrossRef]
- Haußmann, M.; Reznik, B.; Bockhorn, H.; Denev, J.A. Thermal degradation of polymethylsilsesquioxane and microstructure of the derived glasses. J. Anal. Appl. Pyrolysis 2011, 91, 224–231. [Google Scholar] [CrossRef]
- Alargova, R.G.; Warhadpande, D.S.; Paunov, V.N.; Velev, O.D. Foam superstabilization by polymer microrods. Langmuir 2004, 20, 10371–10374. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Hong, F.C.-N. Improved adhesion of PMSQ hard coatings on polymer substrates. J. Coat. Technol. Res. 2011, 8, 779–783. [Google Scholar] [CrossRef]
- Bohner, M.; Le Santoni, B.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhou, Y.; Ma, Y.; Xiao, L.; Ji, W.; Zhang, Y.; Wang, X. Current Application of Beta-Tricalcium Phosphate in Bone Repair and Its Mechanism to Regulate Osteogenesis. Front. Mater. 2021, 8, 8915. [Google Scholar] [CrossRef]
- Mayr, H.O.; Hube, R.; Bernstein, A.; Seibt, A.B.; Hein, W.; von Eisenhart-Rothe, R. Beta-tricalcium phosphate plugs for press-fit fixation in ACL reconstruction—A mechanical analysis in bovine bone. Knee 2007, 14, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Rajababu, P.; Rohini, S.; Butchibabu, K.; Naveen, A.; Reddy, P.K.; Vidyasagar, S.; Satyanarayana, D.; Pavan Kumar, S. Multi-centre, randomized clinical trial on the efficacy and safety of recombinant human platelet-derived growth factor with β-tricalcium phosphate in human intra-osseous periodontal defects. J. Clin. Periodontol. 2011, 38, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kishore, D.T.; Bandiwadekar, T.; Padma, R.; Debunath, S.; Profulla; Reddy, A. Evaluation of relative efficacy of β-tricalcium phosphate with and without type I resorbable collagen membrane in periodontal infrabony defects: A clinical and radiographic study. J. Contemp. Dent. Pract. 2013, 14, 193–201. [Google Scholar] [CrossRef]
- Eitenmüller, J.; Peters, G.; Golsong, W.; Weltin, R.; Gellissen, G.; Reichmann, W. Die Freisetzungsverzögerung verschiedener Antibiotica aus resorbierbarem Tricalciumphosphat-Keramikgranulat durch die Verwendung löslicher Uberzüge zur lokalen Behandlung der Osteomyelitis. Eine tierexperimentelle Untersuchung. Langenbecks Arch. Chir. 1983, 360, 193–206. [Google Scholar] [CrossRef]
- Silverman, L.D.; Lukashova, L.; Herman, O.T.; Lane, J.M.; Boskey, A.L. Release of gentamicin from a tricalcium phosphate bone implant. J. Orthop. Res. 2007, 25, 23–29. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Kerr, L.; Bernstein, A.; Mayr, H.O.; Suedkamp, N.P.; Gadow, R.; Krieg, P.; Hernandez Latorre, S.; Thomann, R.; Syrowatka, F.; et al. 3D Powder Printed Bioglass and β-Tricalcium Phosphate Bone Scaffolds. Materials 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Seidenstuecker, M.; Lange, S.; Esslinger, S.; Latorre, S.H.; Krastev, R.; Gadow, R.; Mayr, H.O.; Bernstein, A. Inversely 3D-Printed β-TCP Scaffolds for Bone Replacement. Materials 2019, 12, 3417. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Schilling, P.; Ritschl, L.; Lange, S.; Schmal, H.; Bernstein, A.; Esslinger, S. Inverse 3D Printing with Variations of the Strand Width of the Resulting Scaffolds for Bone Replacement. Materials 2021, 14, 1964. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.-I.; Lin, K.; Zreiqat, H. Fabrication of bioinspired structured glass–ceramics with enhanced fracture toughness. J. Mater. Sci. 2017, 52, 9202–9210. [Google Scholar] [CrossRef]
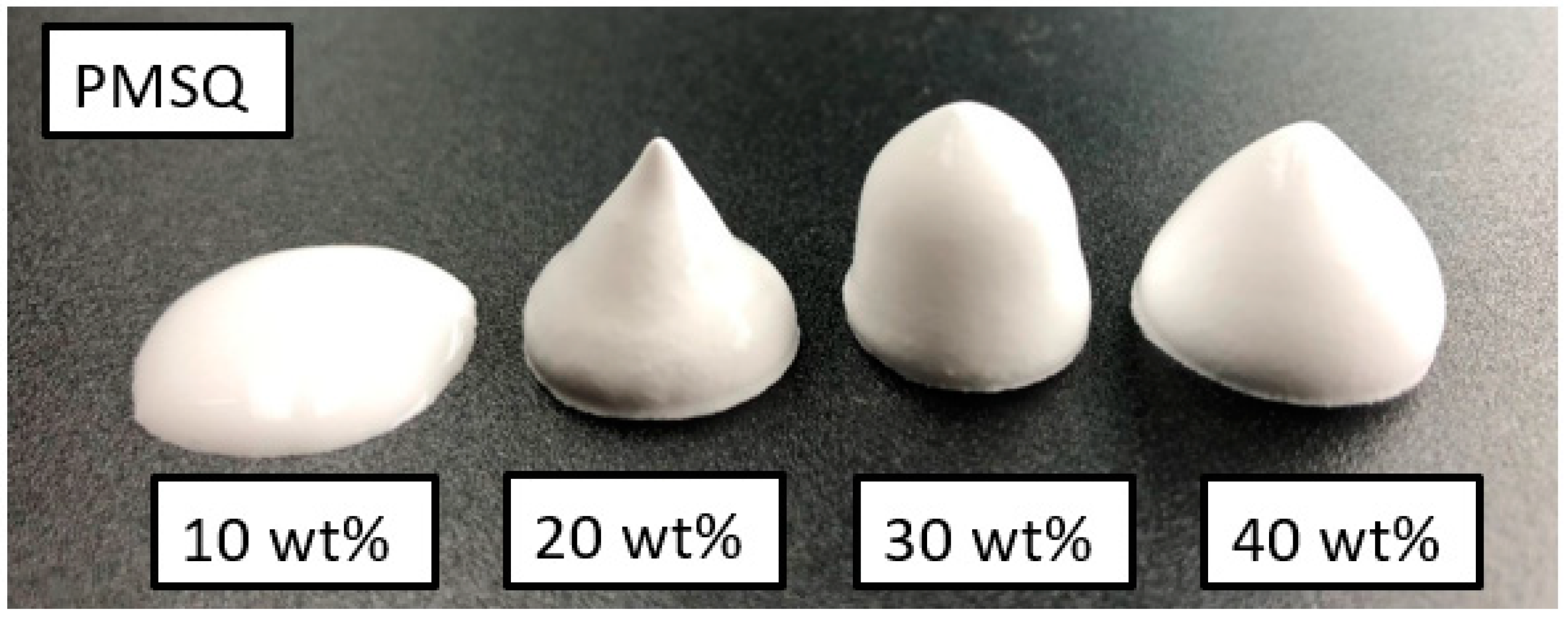

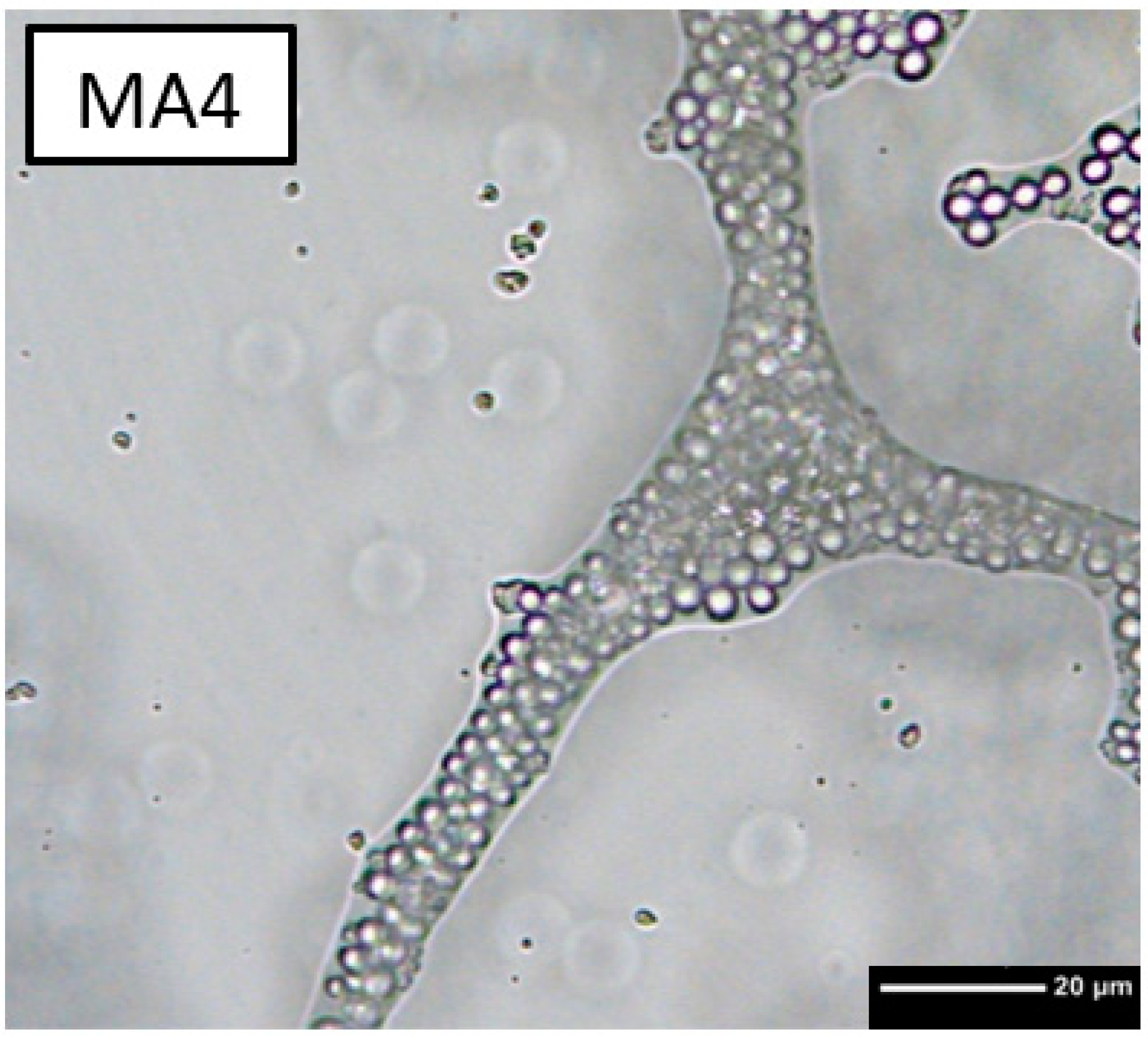
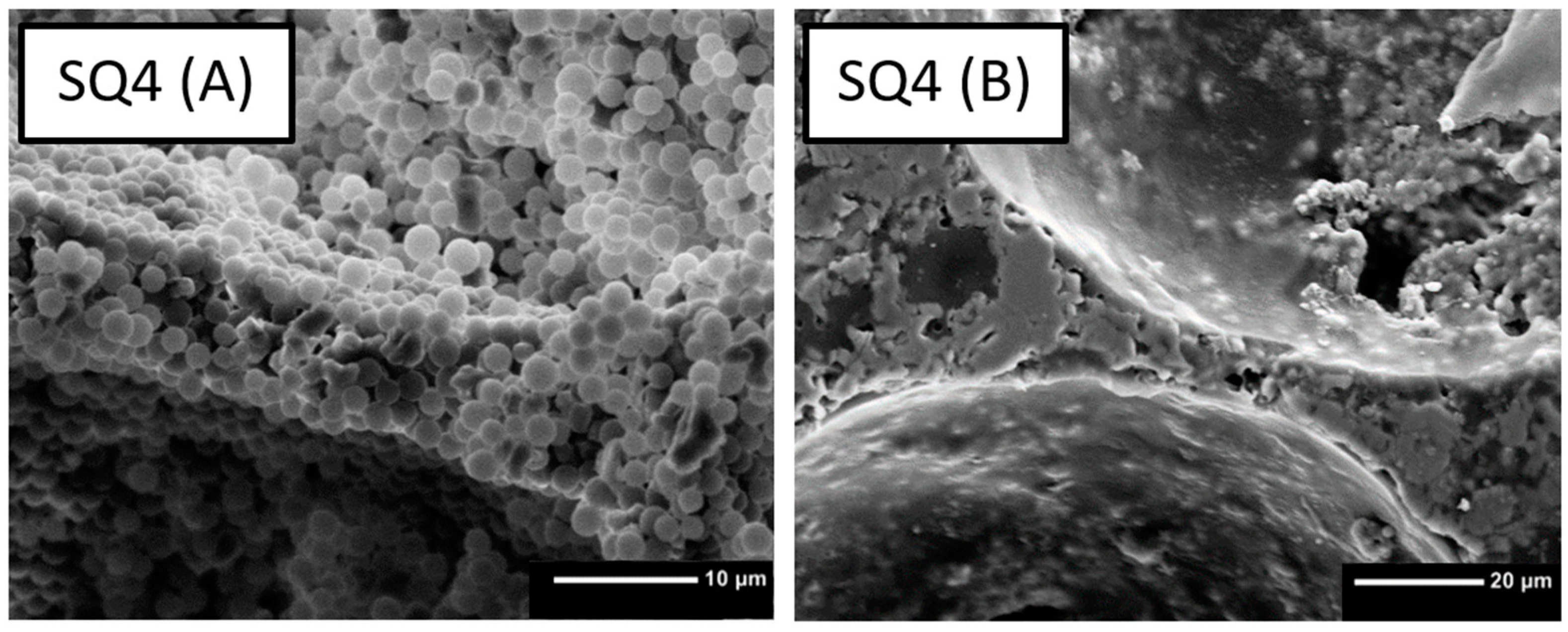






| No. | Sample | PMSQ [wt%] | PMMA [wt%] | ß-TCP [wt%] |
|---|---|---|---|---|
| 1 | SQ1 | 10 | 0 | 90 |
| 2 | SQ2 | 20 | 0 | 80 |
| 3 | SQ3 | 30 | 0 | 70 |
| 4 | SQ4 | 40 | 0 | 60 |
| 5 | MA1 | 0 | 10 | 90 |
| 6 | MA2 | 0 | 20 | 80 |
| 7 | MA3 | 0 | 30 | 70 |
| 8 | MA4 | 0 | 40 | 60 |
| 9 | Raw | 0 | 0 | 100 |
| SQ1 | SQ2 | SQ3 | SQ4 | MA1 | MA2 | MA3 | MA4 | |
|---|---|---|---|---|---|---|---|---|
| Average pore radius [µm] | 1.14 | 2.17 | 6.68 | 7.88 | 2.25 | 2.59 | 3.01 | 2.76 |
| Total porosity [%] | 23.2 | 55.0 | 68.5 | 75.2 | 46.5 | 55.6 | 50.1 | 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dufner, L.; Oßwald, B.; Eberspaecher, J.; Riedel, B.; Kling, C.; Kern, F.; Seidenstuecker, M. Adjustment of Micro- and Macroporosity of ß-TCP Scaffolds Using Solid-Stabilized Foams as Bone Replacement. Bioengineering 2023, 10, 256. https://doi.org/10.3390/bioengineering10020256
Dufner L, Oßwald B, Eberspaecher J, Riedel B, Kling C, Kern F, Seidenstuecker M. Adjustment of Micro- and Macroporosity of ß-TCP Scaffolds Using Solid-Stabilized Foams as Bone Replacement. Bioengineering. 2023; 10(2):256. https://doi.org/10.3390/bioengineering10020256
Chicago/Turabian StyleDufner, Lukas, Bettina Oßwald, Jan Eberspaecher, Bianca Riedel, Chiara Kling, Frank Kern, and Michael Seidenstuecker. 2023. "Adjustment of Micro- and Macroporosity of ß-TCP Scaffolds Using Solid-Stabilized Foams as Bone Replacement" Bioengineering 10, no. 2: 256. https://doi.org/10.3390/bioengineering10020256
APA StyleDufner, L., Oßwald, B., Eberspaecher, J., Riedel, B., Kling, C., Kern, F., & Seidenstuecker, M. (2023). Adjustment of Micro- and Macroporosity of ß-TCP Scaffolds Using Solid-Stabilized Foams as Bone Replacement. Bioengineering, 10(2), 256. https://doi.org/10.3390/bioengineering10020256