Frozen Stored Teeth: Autogenous Dentin as an Alternative Augmentation Material in Dentistry
Abstract
1. Introduction
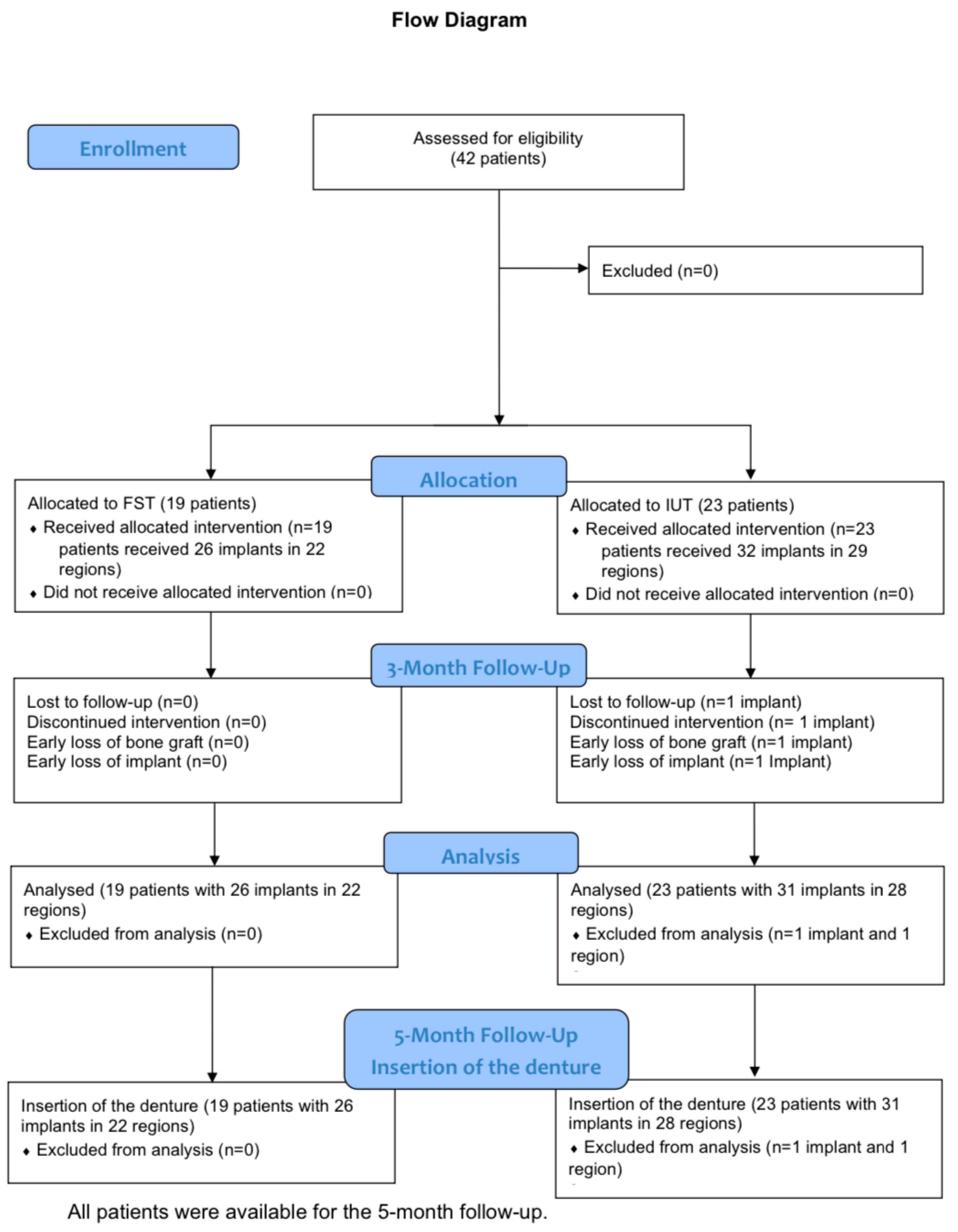
2. Materials and Methods
- Study group 1: Freeze-stored teeth (FST): 19 patients (10 female, 9 male) and 22 regions with 26 implants.
- Control group 2: Immediately used teeth (IUT): 23 patients (12 female, 11 male) with 29 regions and 32 implants.
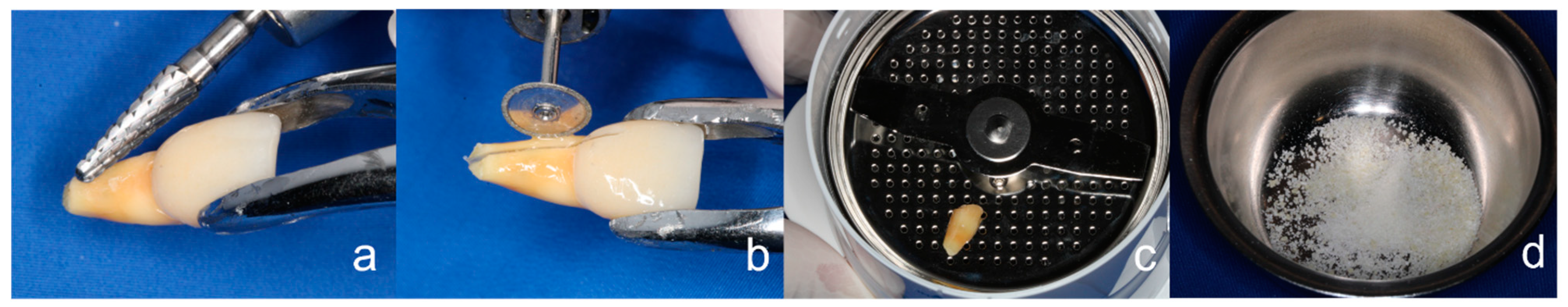
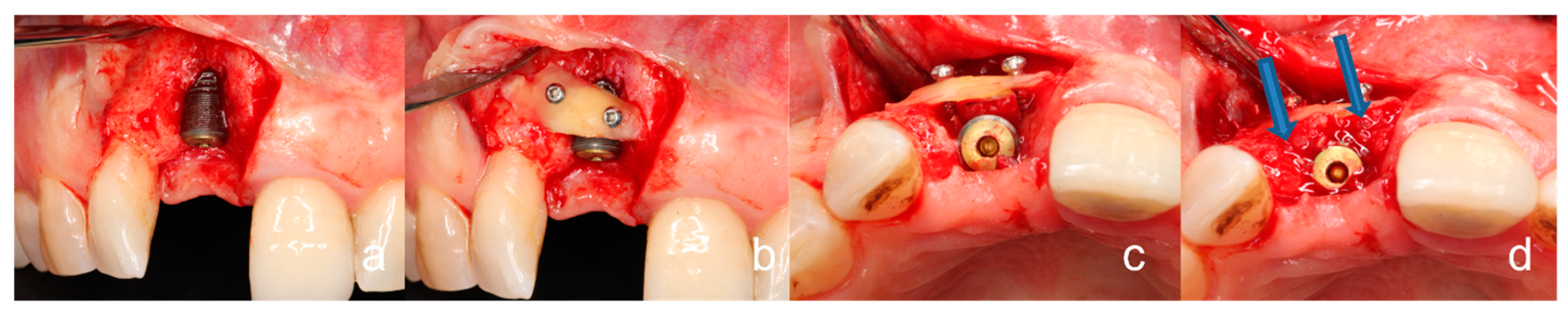
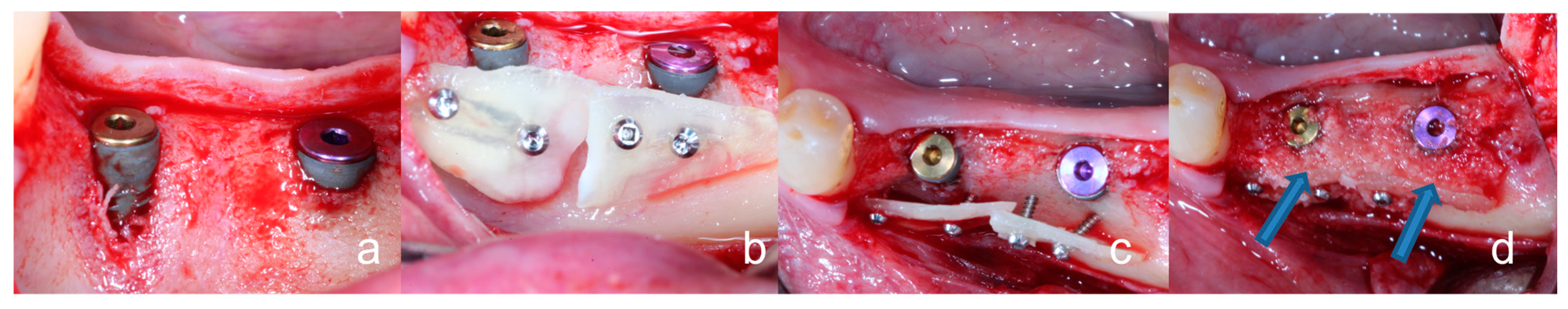
2.1. General Surgical Procedure of the Tooth Shell Technique (TST)
2.2. Radiographic Evaluation
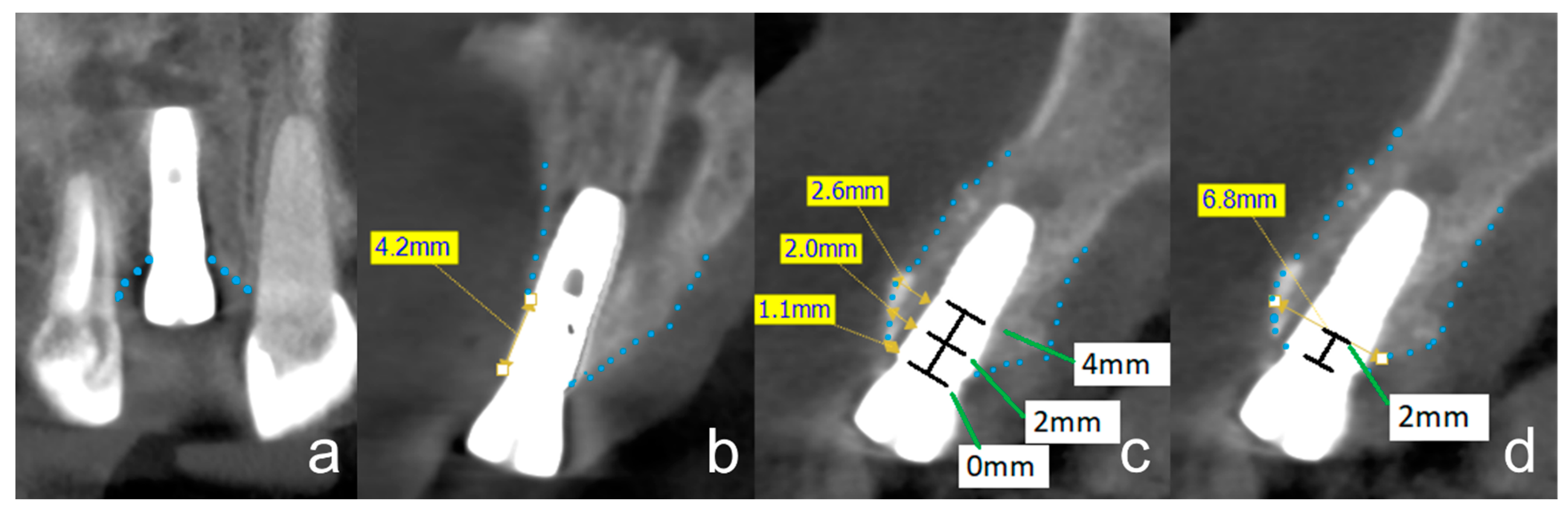
2.3. Osseointegration
- -
- A decrease in peri-implant structures of less than one millimeter at four measurement points.
- -
- ISQ values above sixty.
- -
- Radiologically covered implant in CBCT.
- -
- The vestibular lamella loses less than one millimeter in the CBCT.
2.4. Statistical Analyses
3. Results
3.1. Non-Severe Clinical Complications
3.2. Radiographic Evaluation
3.3. Peri-Implant Tissue Probing, Implant Stability, Osseointegration, and Prosthetic Restoration
- -
- All other implants had a probing depth that did not exceed 0.5 mm.
- -
- ISQ values between 62 and 87 were registered for all implants and mean values of 74 (IUT group) to 76 (FST group) were determined so that there can be no talk of significant differences.
- -
- All implants could be defined as fully osseointegrated as there was no increased probing depth, all ISQ values were above 60 and all implant surfaces were covered with bone and hard tissue.
- -
- All implants could be restored with fixed prostheses after implant exposure without any complications.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papageorgiou, S.N.; Papageorgiou, P.N.; Deschner, J.; Götz, W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: Systematic review and network meta-analysis of parallel and cluster randomized controlled trials. J. Dent. 2016, 48, 1–8. [Google Scholar] [CrossRef]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implant. 2014, 7 (Suppl. S2), S203–S217. [Google Scholar]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implant. 2018, 44, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Baldini, N.; De Sanctis, M.; Ferrari, M. Deproteinized bovine bone in periodontal and implant surgery. Dent. Mater. 2011, 27, 61–70. [Google Scholar] [CrossRef]
- Barone, A.; Aldini, N.N.; Fini, M.; Giardino, R.; Guirado, J.L.C.; Covani, U. Xenograft versus Extraction Alone for Ridge Preservation after Tooth Removal: A Clinical and Histomorphometric Study. J. Periodontol. 2008, 79, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Gennai, S.; Cei, S.; Ducci, F.; Discepoli, N.; Carmignani, A.; Tonetti, M. Does enamel matrix derivative application provide additional clinical benefits in residual periodontal pockets associated with suprabony defects? A systematic review and meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2014, 41, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Chisci, G.; Fredianelli, L. Therapeutic Efficacy of Bromelain in Alveolar Ridge Preservation. Antibiotics 2022, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—Is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant. Dent. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Korsch, M. Tooth shell technique: A proof of concept with the use of autogenous dentin block grafts. Aust. Dent. J. 2020, 66, 159–168. [Google Scholar] [CrossRef]
- Schwarz, F.; Golubovic, V.; Becker, K.; Mihatovic, I. Extracted tooth roots used for lateral alveolar ridge augmentation: A proof-of-concept study. J. Clin. Periodontol. 2016, 43, 345–353. [Google Scholar] [CrossRef]
- Schwarz, F.; Golubovic, V.; Mihatovic, I.; Becker, J. Periodontally diseased tooth roots used for lateral alveolar ridge augmentation. A proof-of-concept study. J. Clin. Periodontol. 2016, 43, 797–803. [Google Scholar] [CrossRef]
- Linde, A. Dentin matrix proteins: Composition and possible functions in calcification. Anat. Rec. 1989, 224, 154–166. [Google Scholar] [CrossRef]
- Cole, A.; Eastoe, J. Biochemistry and Oral Biology; Wright: Bristol, UK, 1977. [Google Scholar]
- Quelch, K.J.; Melick, R.; Bingham, P.J.; Mercuri, S.M. Chemical composition of human bone. Arch. Oral Biol. 1983, 28, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.A.T.; Mitsugi, M.; Kabir, M.A.; Um, I.W.; Minamida, Y.; Kim, K.W.; Kim, Y.K.; Sun, Y.; Qin, C. Autograft of Dentin Materials for Bone Regeneration. Adv. Biomater. Sci. Biomed. Appl. 2013, 27, 391–403. [Google Scholar]
- Kim, Y.K.; Lee, J.H.; Um, I.W.; Kim, K.W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-derived bone graft material. J. Korean Assoc. Oral. Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef]
- Al-Asfour, A.; Andersson, L.; Kamal, M.; Joseph, B. New bone formation around xenogenic dentin grafts to rabbit tibia marrow. Dent. Traumatol. 2013, 29, 455–460. [Google Scholar] [CrossRef]
- Schroeder, H.E. Orale Strukturbiologie; Georg-Thieme-Verlag: Stuttgart, Germany, 2000; Volume 5. [Google Scholar]
- Khoury, F.; Hanser, T. Three-Dimensional Vertical Alveolar Ridge Augmentation in the Posterior Maxilla: A 10-year Clinical Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 471–480. [Google Scholar] [CrossRef]
- Korsch, M.; Peichl, M. Retrospective Study: Lateral Ridge Augmentation Using Autogenous Dentin: Tooth-Shell Technique vs. Bone-Shell Technique. Int. J. Environ. Res. Public Heal 2021, 18, 3174. [Google Scholar] [CrossRef]
- Hürzeler, M.B.; Zuhr, O.; Schupbach, P.; Rebele, S.F.; Emmanouilidis, N.; Fickl, S. The socket-shield technique: A proof-of-principle report. J. Clin. Periodontol. 2010, 37, 855–862. [Google Scholar] [CrossRef]
- Mellonig, J.T. Freeze-dried bone allografts in periodontal reconstructive surgery. Dent. Clin. North Am. 1991, 35, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Korsch, M.; Peichl, M. Retrospective Study on Tooth Shell Technique Using Endodontically Treated Teeth in Lateral Ridge Augmentation. Appl. Sci. 2021, 11, 5882. [Google Scholar] [CrossRef]
- Minetti, E.; Pisani, F. Bone Regeneration in Implantology: Tooth as a Graft; Edra: Milano, Italy, 2021. [Google Scholar]
- Bäumer, D.; Zuhr, O.; Rebele, S.; Hürzeler, M. Socket Shield Technique for immediate implant placement—Clinical, radiographic and volumetric data after 5 years. Clin. Oral Implant. Res. 2017, 28, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, C.; Schwass, D.R. The socket-shield technique: A critical literature review. Int. J. Implant Dent. 2020, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Kastel, I.; de Quincey, G.; Neugebauer, J.; Sader, R.; Gehrke, P. Does the manual insertion torque of smartpegs affect the outcome of implant stability quotients (ISQ) during resonance frequency analysis (RFA)? Int. J. Implant. Dent. 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.B.F.; Moura, C.C.G.; Claudino, M.; Carvalho, V.F.; Rocha, F.S.; Zanetta-Barbosa, D. Influence of Implant Surfaces on Osseointegration: A Histomorphometric and Implant Stability Study in Rabbits. Braz. Dent. J. 2015, 26, 451–457. [Google Scholar] [CrossRef]
- Petzold, G.; Aguilera, J.M. Ice Morphology: Fundamentals and Technological Applications in Foods. Food Biophys. 2009, 4, 378–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Ertbjerg, P. On the origin of thaw loss: Relationship between freezing rate and protein denaturation. Food Chem. 2019, 299, 125104. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, J.-Y.; Park, K.-M.; Chang, P.-S. Effects of freezing rate on structural changes in l-lactate dehydrogenase during the freezing process. Sci. Rep. 2021, 11, 13643. [Google Scholar] [CrossRef]
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte cryopreservation: Where are we now? Hum. Reprod Update 2016, 22, 440–449. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef]
- Daniel, R.M.; Danson, M.J. A new understanding of how temperature affects the catalytic activity of enzymes. Trends Biochem. Sci. 2010, 35, 584–591. [Google Scholar] [CrossRef]
- Cao, E.; Chen, Y.; Cui, Z.; Foster, P.R. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol. Bioeng. 2003, 82, 684–690. [Google Scholar] [CrossRef]
- Tan, M.; Mei, J.; Xie, J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021, 11, 68. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Nevins, M.; Schupbach, P. New Bone Formation Using an Extracted Tooth as a Biomaterial: A Case Report with Histologic Evidence. Int. J. Periodontics Restor. Dent. 2019, 39, 157–163. [Google Scholar] [CrossRef]
- Becker, K.; Drescher, D.; Hönscheid, R.; Golubovic, V.; Mihatovic, I.; Schwarz, F. Biomechanical, micro-computed tomographic and immunohistochemical analysis of early osseous integration at titanium implants placed following lateral ridge augmentation using extracted tooth roots. Clin. Oral Implant. Res. 2017, 28, 334–340. [Google Scholar] [CrossRef]
- Qin, X.; Raj, R.M.; Liao, X.; Shi, W.; Ma, B.; Gong, S.; Chen, W.; Zhou, B. Using rigidly fixed autogenous tooth graft to repair bone defect: An animal model. Dent. Traumatol. 2014, 30, 380–384. [Google Scholar] [CrossRef] [PubMed]
| Study Groups | Significance | |||
|---|---|---|---|---|
| Starting Characteristic | Total | FST | IUT | p-Value |
| Age (years) | ||||
| - mean (SD) | 61.4 (10.6) | 60.5 (8.2) | 62.2 (12.4) | n.s. |
| - range | 28–80 | 38–74 | 28–80 | |
| Sex (male) | ||||
| n (%) | 20 of 42 (48) | 9 of 19 (47) | 11 of 23 (48) | n.s. |
| Study Groups | Fisher’s Exact Test (2-Sided) | |||
|---|---|---|---|---|
| Clinical Complication | Total | FST | IUT | p-Value |
| Total severe complications | ||||
| n (%) on PL | 1 of 42 (2) | 0 of 19 (0) | 1 of 23 (4) | 0.548 |
| n (%) on RL | 1 of 51 (2) | 0 of 22 (0) | 1 of 29 (3) | 0.569 |
| n (%) on IL | 1 of 58 (2) | 0 of 26 (0) | 1 of 32 (3) | 0.552 |
| Wound dehiscence | ||||
| n (%) on PL | 2 of 42 (5) | 0 of 19 (0) | 2 of 23 (9) | 0.294 |
| n (%) on RL | 2 of 51 (4) | 0 of 22 (0) | 2 of 29 (7) | 0.318 |
| n (%) on IL | 2 of 58 (3) | 0 of 26 (0) | 2 of 32 (6) | 0.300 |
| Inflammation (pus) | ||||
| n (%) on PL | 1 of 42 (2) | 0 of 19 (0) | 1 of 23 (4) | 0.548. |
| n (%) on RL | 1 of 51 (2) | 0 of 22 (0) | 1 of 29 (3) | 0.569 |
| n (%) on IL | 1 of 58 (2) | 0 of 26 (0) | 1 of 32 (3) | 0.552 |
| Total complications at all | ||||
| n (%) on PL | 3 of 42 (7) | 0 of 19 (0) | 3 of 23 (13) | 0.239 |
| n (%) on RL | 4 of 51 (6) | 0 of 22 (0) | 4 of 29 (14) | 0.120 |
| n (%) on IL | 4 of 58 (5) | 0 of 26 (0) | 4 of 32 (13) | 0.085 |
| Study Groups | Two-Sample t-Test | |||
|---|---|---|---|---|
| Time of Measurement | Mean | FST | IUT | (p-Value) |
| T1 | ||||
| Mean ucco-oral alveolar ridge width (mm) | ||||
| PL, n = 42 (SD) | 8.8 (1.6) | 8.6 (2.1) | 8.9 (1.1) | 0.520 |
| RL, n = 51 (SD) | 8.7 (1.6) | 8.5 (2.1) | 8.9 (1.1) | 0.373 |
| IL, n = 58 (SD) | 8.7 (1.6) | 8.4 (2.1) | 8.9 (1.1) | 0.212 |
| Mean buccal lamella width L0 (mm) | ||||
| PL, n = 42 (SD) | 2.7 (1.0) | 2.8 (1.2) | 2.6 (0.8) | 0.586 |
| RL, n = 51 (SD) | 2.6 (1.0) | 2.7 (1.1) | 2.6 (0.9) | 0.762 |
| IL, n = 58 (SD) | 2.6 (1.0) | 2.7 (1.1) | 2.6 (0.9) | 0.733 |
| Mean buccal lamella width L2 (mm) | ||||
| PL, n = 42 (SD) | 3.2 (1.0) | 3.3 (1.3) | 3.1 (0.7) | 0.475 |
| RL, n = 51 (SD) | 3.1 (1.0) | 3.2 (1.2) | 3.0 (0.7) | 0.542 |
| IL, n = 58 (SD) | 3.1 (1.0) | 3.2 (1.2) | 3.1 (0.8) | 0.581 |
| Mean buccal lamella width L4 (mm) | ||||
| PL, n = 42 (SD) | 3.4 (1.3) | 3.7 (1.6) | 3.2 (1.0) | 0.283 |
| RL, n = 51 (SD) | 3.4 (1.3) | 3.6 (1.6) | 3.2 (1.0) | 0.303 |
| IL, n = 58 (SD) | 3.4 (1.3) | 3.5 (1.5) | 3.2 (1.1) | 0.359 |
| T2 | ||||
| Mean bucco-oral alveolar ridge width (mm) | ||||
| PL, n = 42 (SD) | 8.3 (1.6) | 8.1 (2.0) | 8.5 (1.1) | 0.446 |
| RL, n = 50 (SD) | 8.3 (1.5) | 8.0 (2.0) | 8.5 (1.0) | 0.244 |
| IL, n = 57 (SD) | 8.3 (1.6) | 7.9 (1.9) | 8.5 (1.1) | 0.127 |
| Mean buccal lamella width L0 (mm) | ||||
| PL, n = 42 (SD) | 2.2 (1.0) | 2.2 (1.1) | 2.2 (0.9) | 0.967 |
| RL, n = 50 (SD) | 2.2 (1.0) | 2.1 (1.1) | 2.2 (0.9) | 0.888 |
| IL, n = 57 (SD) | 2.2 (1.0) | 2.2 (1.1) | 2.2 (0.9) | 1.000 |
| Mean buccal lamella width L2 (mm) | ||||
| PL, n = 42 (SD) | 2.9 (1.0) | 3.0 (1.3) | 2.8 (0.8) | 0.573 |
| RL, n = 50 (SD) | 2.8 (1.0) | 2.9 (1.2) | 2.7 (0.8) | 0.576 |
| IL, n = 57 (SD) | 2.8 (1.0) | 2.9 (1.2) | 2.8 (0.8) | 0.647 |
| Mean buccal lamella width L4 (mm) | ||||
| PL, n = 42 (SD) | 3.1 (1.4) | 3.3 (1.7) | 3.0 (1.0) | 0.545 |
| RL, n = 50 (SD) | 3.1 (1.3) | 3.2 (1.7) | 3.0 (0.9) | 0.634 |
| IL, n = 57 (SD) | 3.1 (1.3) | 3.2 (1.6) | 3.1 (1.0) | 0.766 |
| Study Groups | Two-Sample t-Test | |||
|---|---|---|---|---|
| Mean Resorption in mm | Mean | FST | IUT | (p-Value) |
| bucco-oral alveolar ridge | ||||
| PL, n = 42 (SD) | 0.44 (0.71) | 0.46 (0.69) | 0.42 (0.74) | 0.874 |
| RL, n = 50 (SD) | 0.46 (0.76) | 0.50 (0.82) | 0.42 (0.73) | 0.688 |
| IL, n = 57 (SD) | 0.44 (0.74) | 0.48 (0.79) | 0.41 (0.70) | 0.709 |
| L0 | ||||
| PL, n = 42 (SD) | 0.45 (0.68) | 0.53 (0.76) | 0.37 (0.61) | 0.460 |
| RL, n = 50 (SD) | 0.47 (0.68) | 0.54 (0.79) | 0.41 (0.60) | 0.510 |
| IL, n = 57 (SD) | 0.45 (0.68) | 0.50 (0.76) | 0.40 (0.61) | 0.611 |
| L2 | ||||
| PL, n = 42 (SD) | 0.31 (0.55) | 0.33 (0.50) | 0.30 (0.61) | 0.871 |
| RL, n = 50 (SD) | 0.31 (0.55) | 0.30 (0.51) | 0.31 (0.59) | 0.955 |
| IL, n = 57 (SD) | 0.30 (0.54) | 0.31 (0.49) | 0.30 (0.58) | 0.958 |
| L4 | ||||
| PL, n = 42 (SD) | 0.35 (0.56) | 0.42 (0.45) | 0.28 (0.64) | 0.450 |
| RL, n = 50 (SD) | 0.32 (0.57) | 0.39 (0.44) | 0.26 (0.65) | 0.451 |
| IL, n = 57 (SD) | 0.30 (0.55) | 0.38 (0.42) | 0.24 (0.63) | 0.333 |
| Study Groups | Two-Sample t-Test | |||
|---|---|---|---|---|
| Ratio from T1 to T2 | Mean | FST | IUT | (p-Value) |
| bucco-oral alveolar ridge | ||||
| PL, n = 42 (SD) | 0.95 (0.08) | 0.95 (0.08) | 0.96 (0.08) | 0.845 |
| RL, n = 50 (SD) | 0.95 (0.08) | 0.95 (0.09) | 0.96 (0.08) | 0.632 |
| IL, n = 57 (SD) | 0.95 (0.08) | 0.95 (0.09) | 0.96 (0.08) | 0.688 |
| L0 | ||||
| PL, n = 42 (SD) | 0.84 (0.27) | 0.82 (0.32) | 0.86 (0.23) | 0.659 |
| RL, n = 50 (SD) | 0.83 (0.27) | 0.82 (0.32) | 0.84 (0.23) | 0.825 |
| IL, n = 57 (SD) | 0.84 (0.28) | 0.84 (0.34) | 0.84 (0.23) | 0.990 |
| L2 | ||||
| PL, n = 42 (SD) | 0.91 (0.18) | 0.90 (0.16) | 0.91 (0.20) | 0.814 |
| RL, n = 50 (SD) | 0.91 (0.19) | 0.91 (0.17) | 0.90 (0.20) | 0.906 |
| IL, n = 57 (SD) | 0.91 (0.18) | 0.91 (0.16) | 0.91 (0.19) | 0.996 |
| L4 | ||||
| PL, n = 42 (SD) | 0.90 (0.19) | 0.86 (0.18) | 0.93 (0.20) | 0.244 |
| RL, n = 50 (SD) | 0.91 (0.19) | 0.87 (0.17) | 0.94 (0.21) | 0.245 |
| IL, n = 57 (SD) | 0.91 (0.19) | 0.87 (0.16) | 0.94 (0.20) | 0.160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korsch, M.; Alt, K.W.; Mock, F.R. Frozen Stored Teeth: Autogenous Dentin as an Alternative Augmentation Material in Dentistry. Bioengineering 2023, 10, 456. https://doi.org/10.3390/bioengineering10040456
Korsch M, Alt KW, Mock FR. Frozen Stored Teeth: Autogenous Dentin as an Alternative Augmentation Material in Dentistry. Bioengineering. 2023; 10(4):456. https://doi.org/10.3390/bioengineering10040456
Chicago/Turabian StyleKorsch, Michael, Kurt Werner Alt, and Frederick Reza Mock. 2023. "Frozen Stored Teeth: Autogenous Dentin as an Alternative Augmentation Material in Dentistry" Bioengineering 10, no. 4: 456. https://doi.org/10.3390/bioengineering10040456
APA StyleKorsch, M., Alt, K. W., & Mock, F. R. (2023). Frozen Stored Teeth: Autogenous Dentin as an Alternative Augmentation Material in Dentistry. Bioengineering, 10(4), 456. https://doi.org/10.3390/bioengineering10040456





