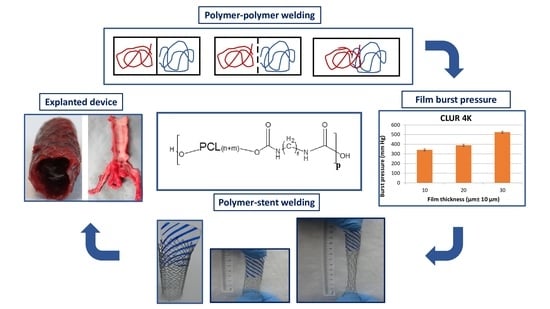
Engineering In Situ Weldable Vascular Devices
Abstract
:1. Introduction
2. Materials and Methods
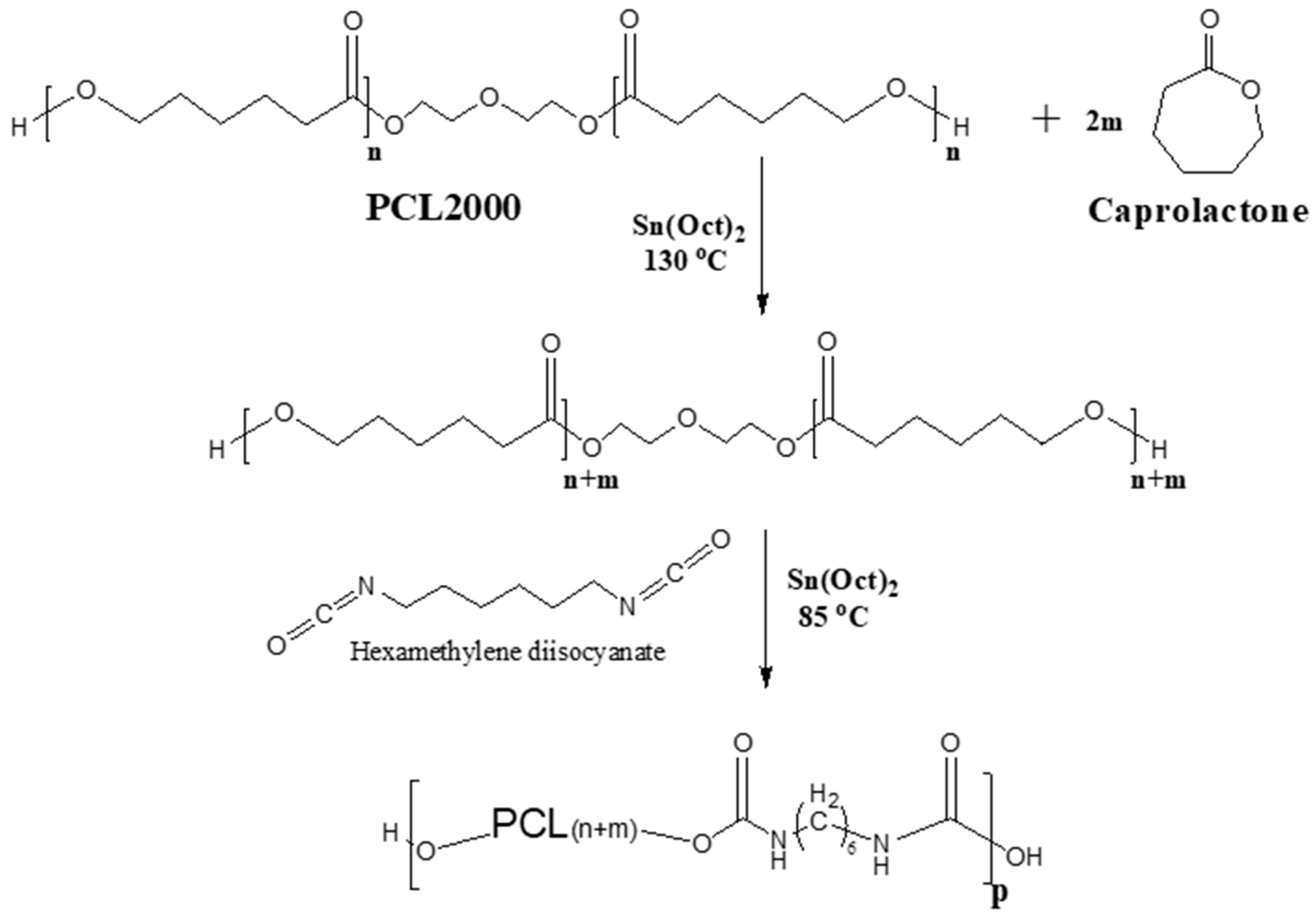
2.1. Synthesis and Characterization of the Polyether Urethane Elastomers
2.2. Synthesis and Characterization of the Polyester Urethane Elastomers
2.3. Preparation and Analysis of Polymer Sleeves
2.4. Cytotoxicity Testing
2.5. Preparation of the Bi-Component Endovascular Device
2.6. In Vivo Studies
3. Results and Discussion
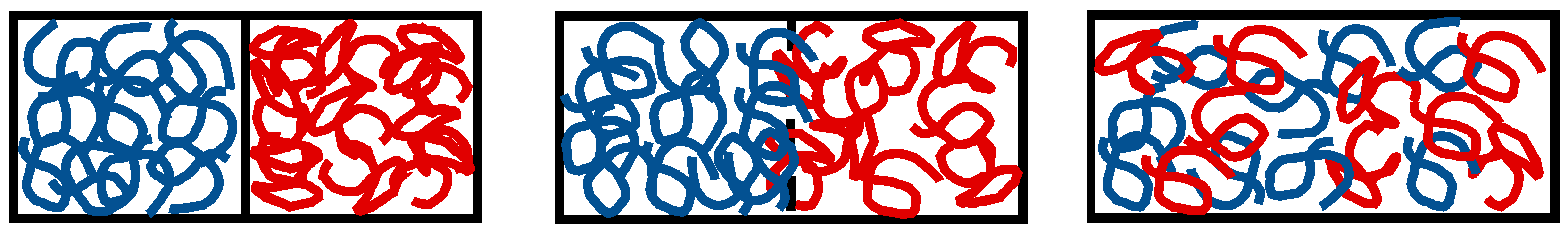
3.1. The In Situ Welding Working Concept
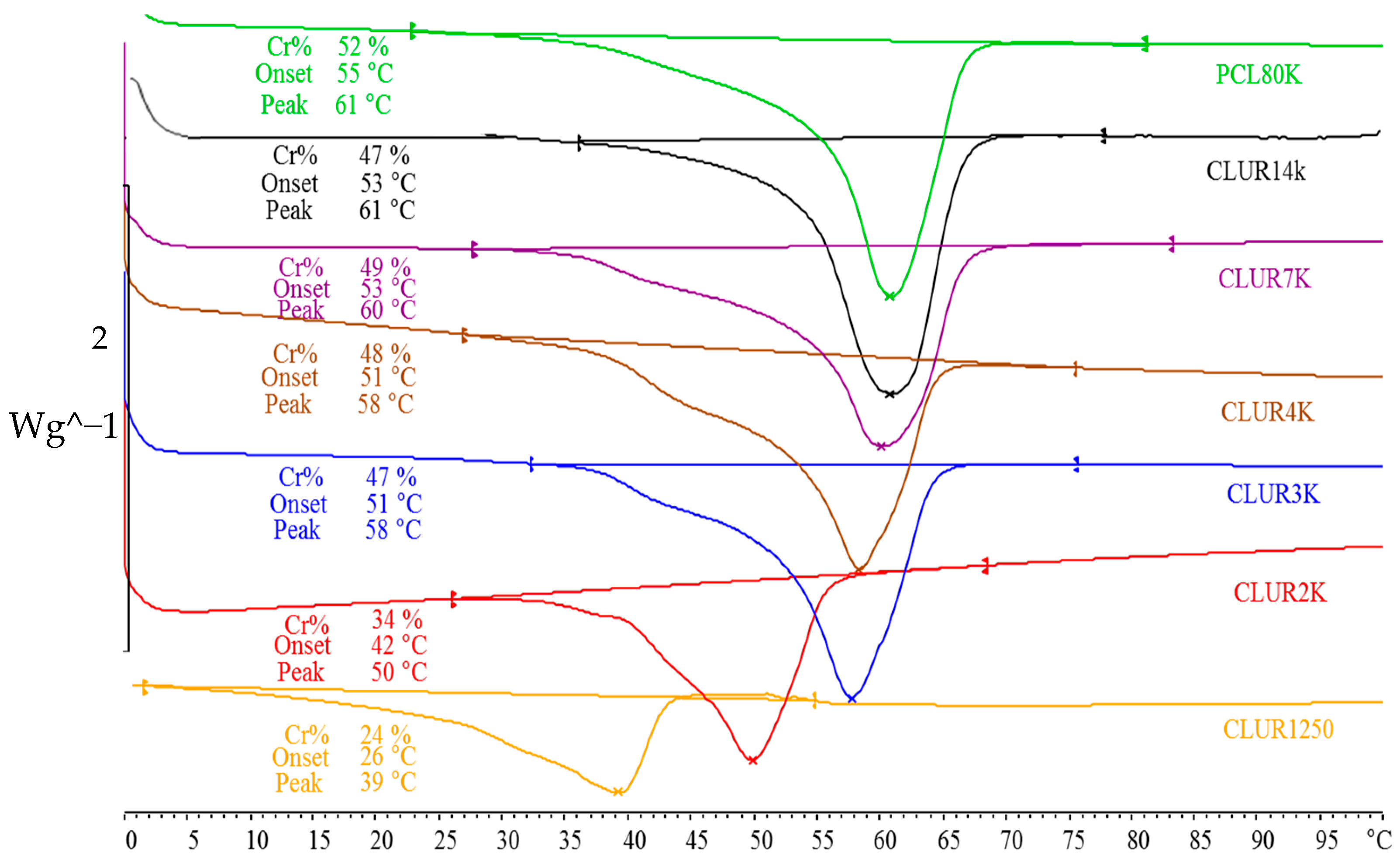
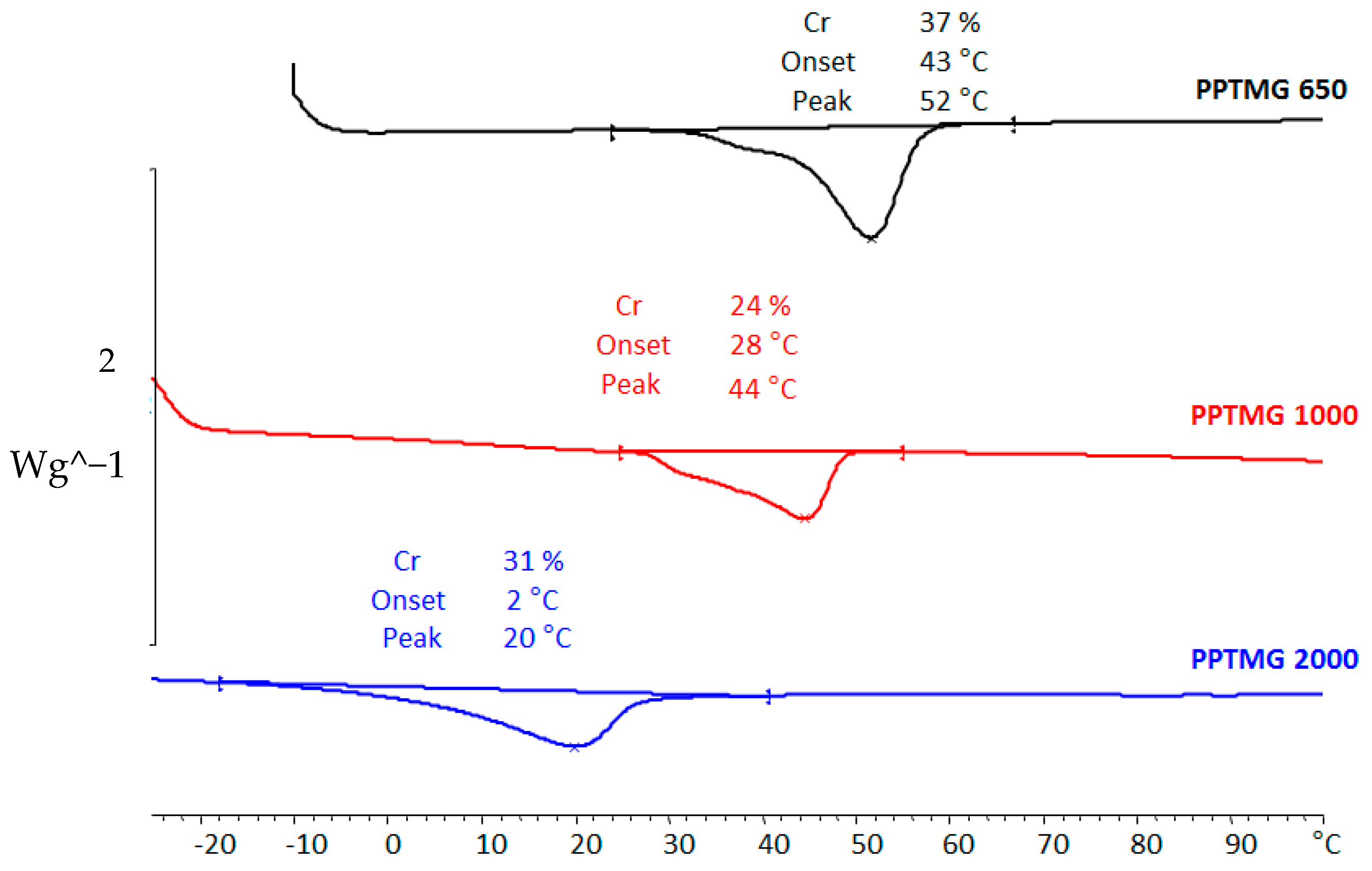
3.2. Synthesis, Characterization, and Performance of Weldable Polymers
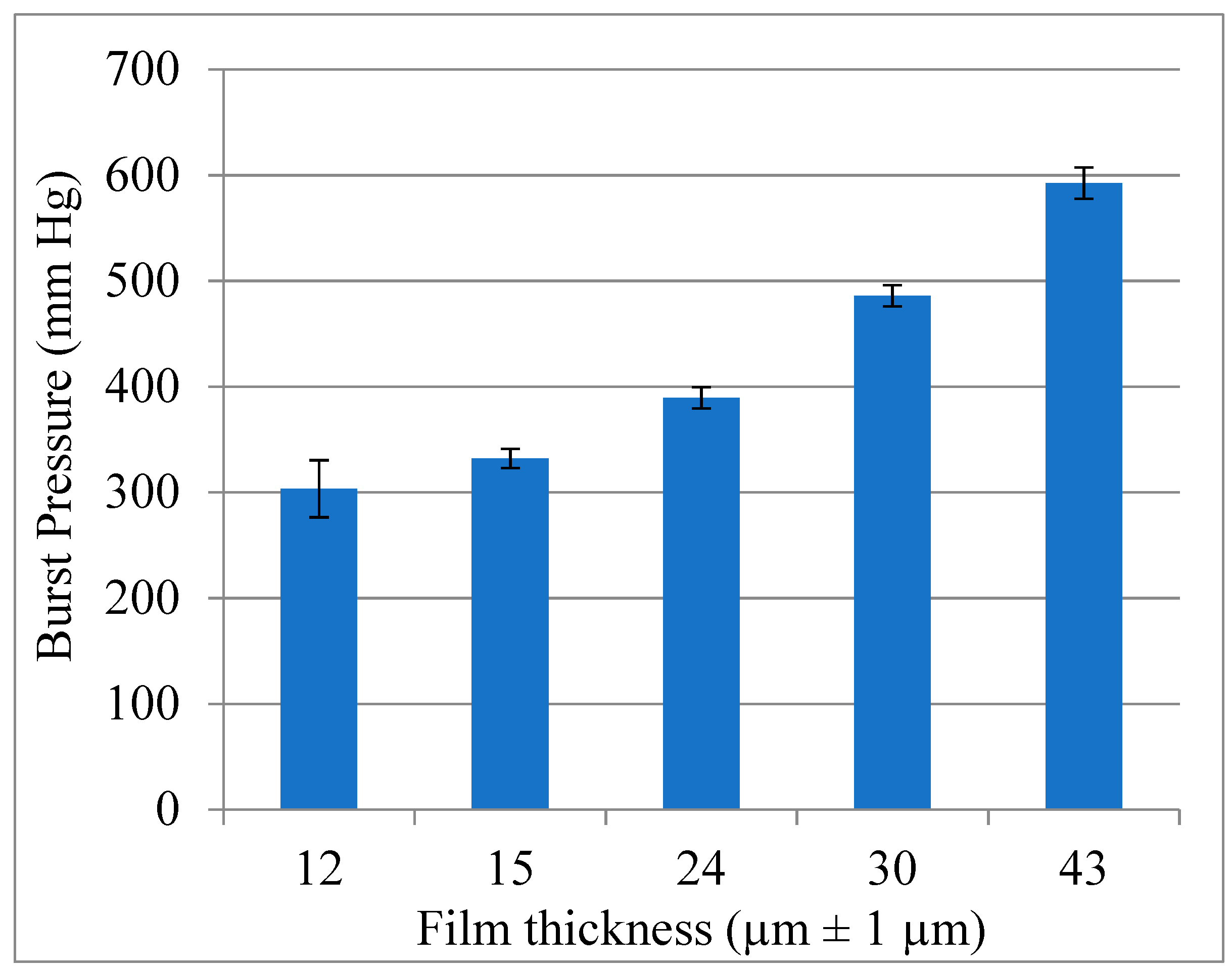
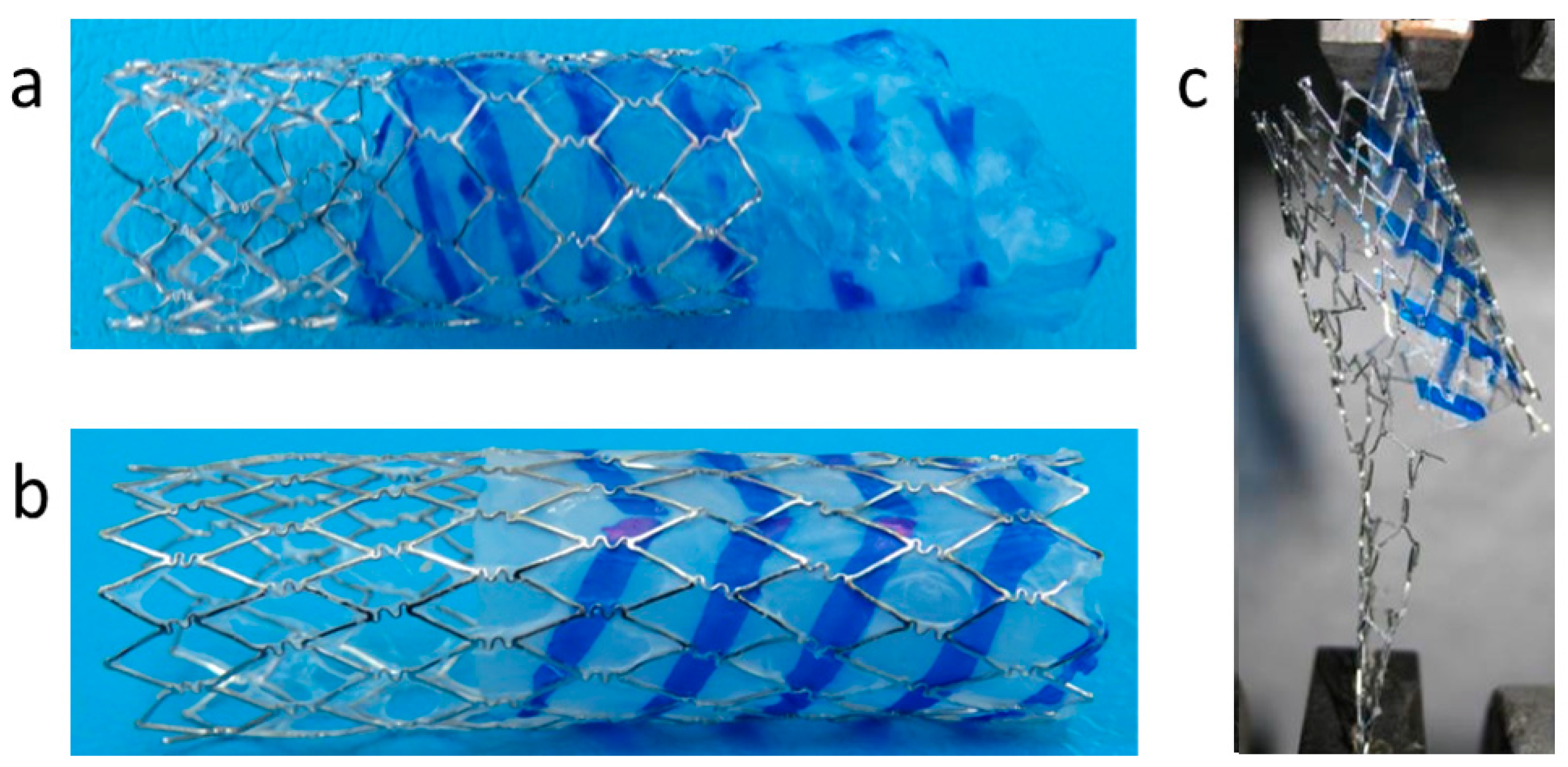
3.3. Pre-Implantation Performance of the Bi-Component Device
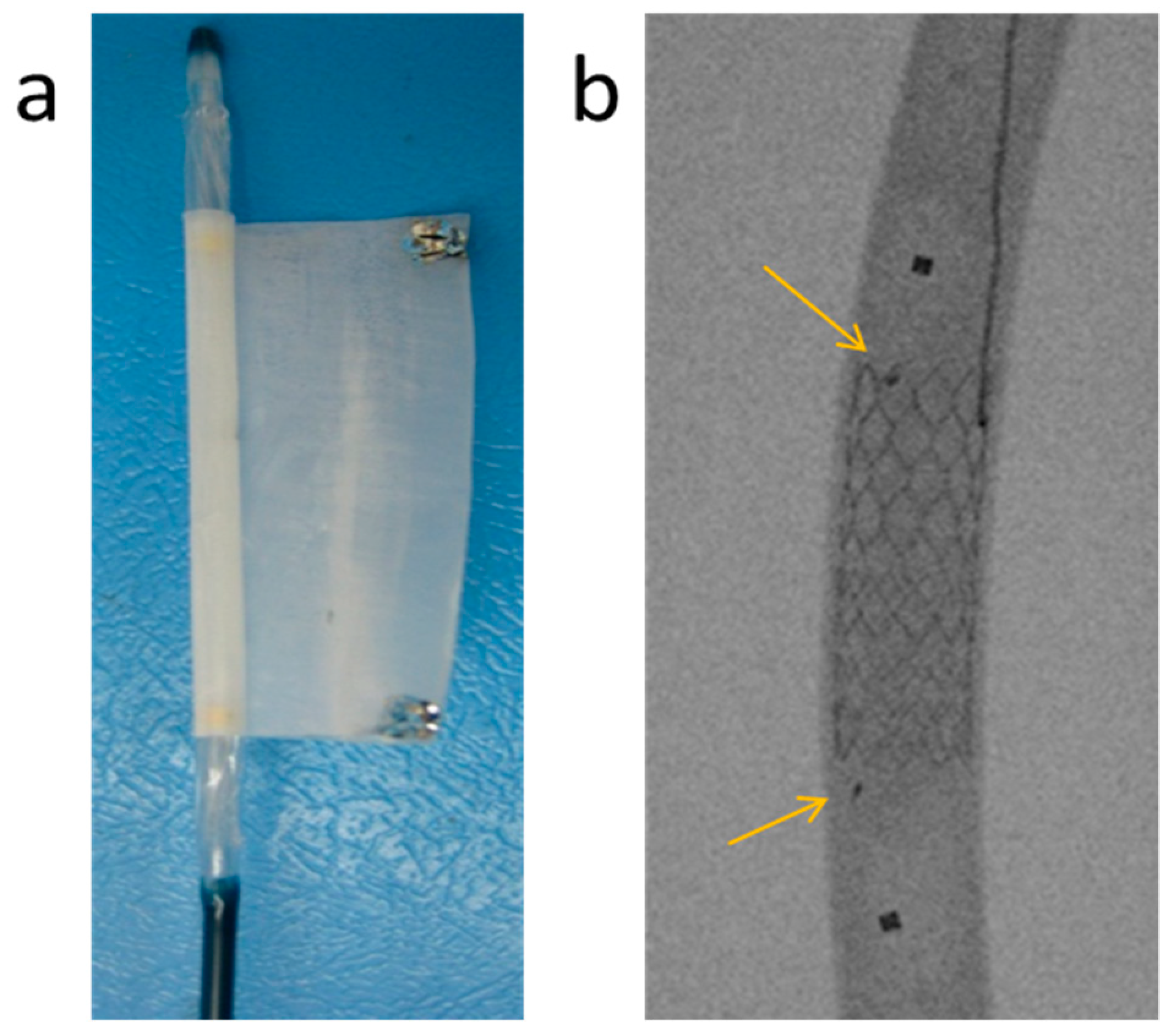
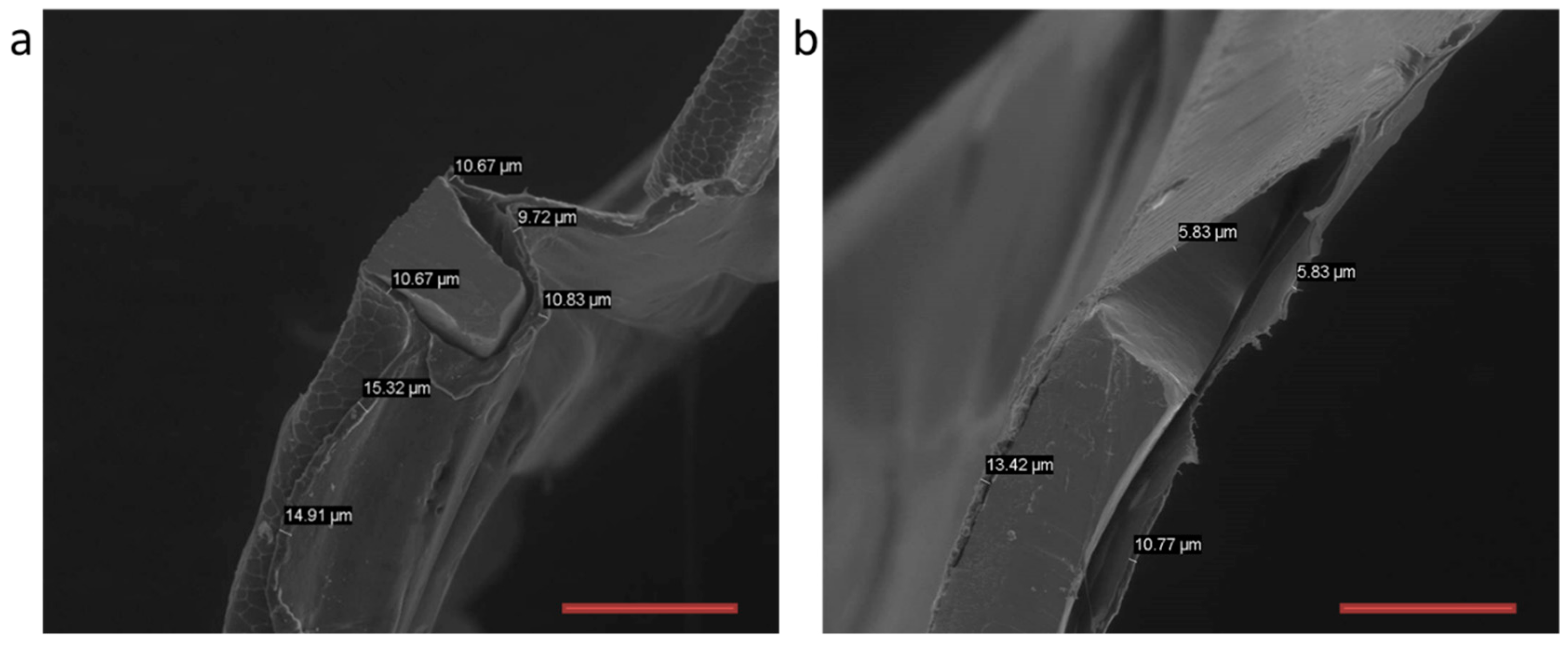
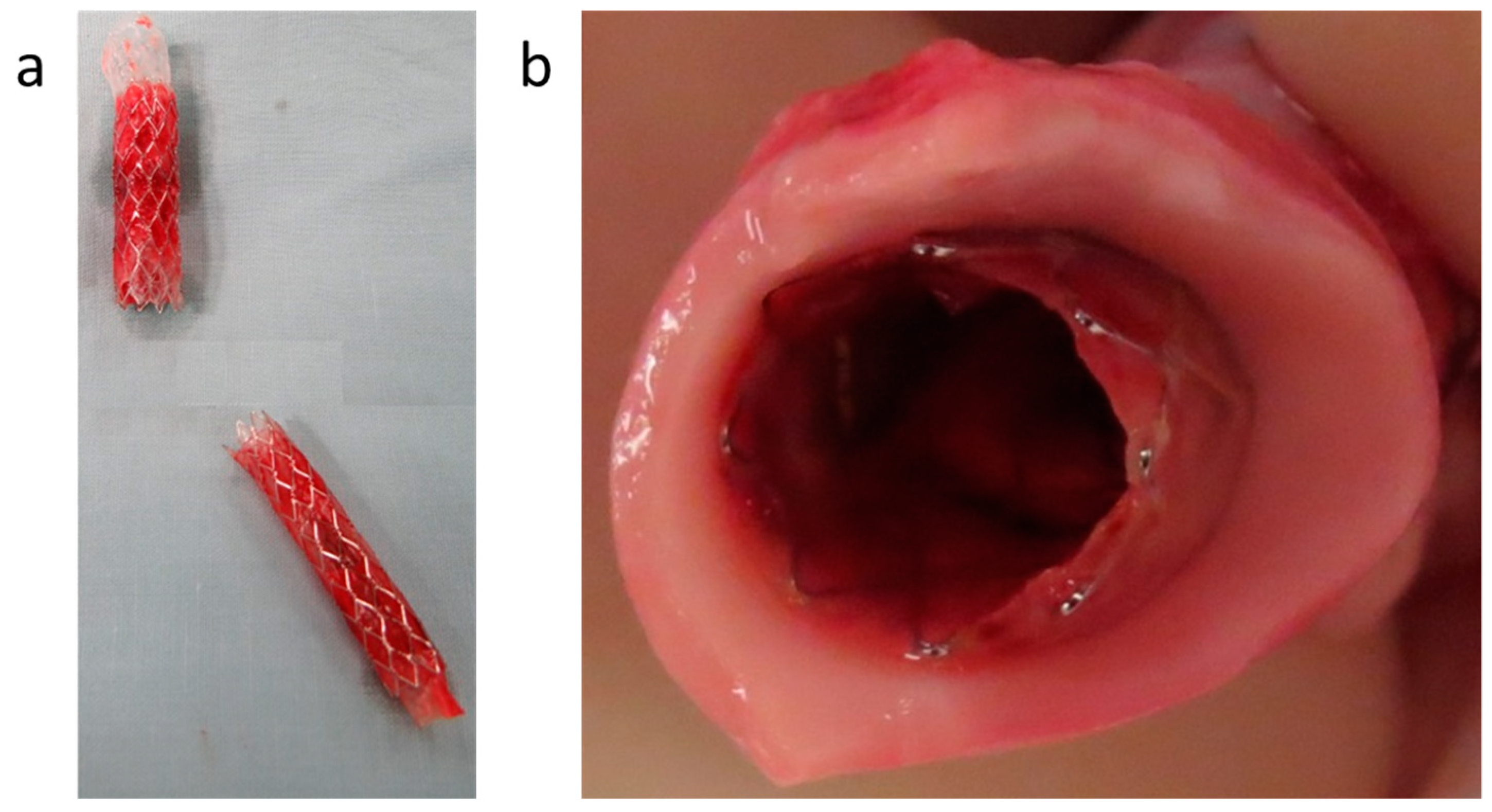
3.4. In Vivo Evaluation of Bi-Component Device
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felix, B.; Kalatar, S.B.; Moatz, B.; Hofstetter, C.; Karsy, M.; Parr, R.; Gibby, W. Augmented Reality Spine Surgery Navigation: Increasing Pedicle Screw Insertion Accuracy for Both Open and Minimally Invasive Spine Surgeries. Spine 2022, 47, 865–872. [Google Scholar] [CrossRef]
- Neufeld, S.K.; Dean, D.; Hussaini, S. Outcomes and Surgical Strategies of Minimally Invasive Chevron/Akin Procedures. Foot Ankle Int. 2021, 42, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lyu, L.; Xu, Y.; Liang, H.; Zhang, X.; Ding, H.; Wu, Z. Intelligent Soft Surgical Robots for Next-Generation Minimally Invasive Surgery. Adv. Intell. Syst. 2021, 3, 2100011. [Google Scholar] [CrossRef]
- Hunter, J.G.; Sackier, J.M. Minimally Invasive Surgery; McGraw-Hill Book Co.: New York, NY, USA, 1993. [Google Scholar]
- Rosen, M.J.; Heniford, B.T. Endoluminal gastric surgery: The modern era of minimally invasive surgery. Surg. Clin. 2005, 85, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Zouridakis, G.; Moore Jr, J.E.; Maitland, D.J. Biomedical Technology and Devices; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Seriki, S.A.; Otoikhila, O.C. Age, smoking, hypertension, and aortic aneurysm: Interactions and risks. J. Cardiol. Cardiovasc. Med. 2022, 7, 001–005. [Google Scholar] [CrossRef]
- Sunderland, K.; Jiang, J.; Zhao, F. Disturbed flow’s impact on cellular changes indicative of vascular aneurysm initiation, expansion, and rupture: A pathological and methodological review. J. Cell Physiol. 2022, 237, 278–300. [Google Scholar] [CrossRef]
- Molenberg, R.; Aalbers, M.W.; Appelman, A.P.A.; Uyttenboogaart, M.; van Dijk, J.M.C. Intracranial aneurysm wall enhancement as an indicator of instability: A systematic review and meta-analysis. Eur. J. Neurol. 2021, 28, 3837–3848. [Google Scholar] [CrossRef]
- Lee, A.M.; Chaikof, E.L. Is the abdominal aortic aneurysm rupture rate decreasing? Adv. Surg. 2013, 47, 271–286. [Google Scholar] [CrossRef]
- Parodi, J.C. Endovascular repair of abdominal aortic aneurysms and other arterial lesions. J. Vasc. Surg. 1995, 21, 549–557. [Google Scholar] [CrossRef]
- Jacobs, C.R.; Scali, S.T.; Khan, T.; Cadavid, F.; Staton, K.M.; Feezor, R.J.; Back, M.R.; Upchurch, G.R., Jr.; Huber, T.S. Endovascular aneurysm repair conversion is an increasingly common indication for open abdominal aortic aneurysm repair. J. Vasc. Surg. 2022, 75, 144–152.e1. [Google Scholar] [CrossRef]
- Zoethout, A.C.; Hochstenbach, I.; van der Laan, M.J.; de Vries, J.P.M.; Reijnen, M.; Zeebregts, C.J. Systematic Review on the Mid-Term Outcomes of Elective Endovascular Aneurysm Sealing in Comparison to Endovascular Aneurysm Repair. J. Endovasc. 2022, 29, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Mesnard, T.; Pruvot, L.; Oliver Patterson, B.; Preville, A.; Azzaoui, R.; Sobocinski, J. Early Institutional Experience with One-Piece Bifurcated-Fenestrated Stentgraft in the Treatment of Abdominal Aortic Aneurysms. J. Endovasc. 2022. [Google Scholar] [CrossRef]
- De Mestral, C.; Croxford, R.; Eisenberg, N.; Roche-Nagle, G. The impact of compliance with imaging follow-up on mortality after endovascular abdominal aortic aneurysm repair: A population based cohort study. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 315–323. [Google Scholar] [CrossRef]
- Kristmundsson, T.; Sonesson, B.; Dias, N.; Malina, M.; Resch, T. Anatomic suitability for endovascular repair of abdominal aortic aneurysms and possible benefits of low profile delivery systems. Vascular 2014, 22, 112–115. [Google Scholar] [CrossRef]
- Schanzer, A.; Greenberg, R.K.; Hevelone, N.; Robinson, W.P.; Eslami, M.H.; Goldberg, R.J.; Messina, L. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011, 123, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Droc, I.; Calinescu, F.B.; Droc, G.; Blaj, C.; Dammrau, R. Aortic stenting. Minim. Invasive Ther. Allied Technol. 2015, 24, 296–304. [Google Scholar]
- Kasprzak, P.M.; Pfister, K.; Kuczmik, W.; Schierling, W.; Sachsamanis, G.; Oikonomou, K. Novel Technique for the Treatment of Type Ia Endoleak After Endovascular Abdominal Aortic Aneurysm Repair. J. Endovasc. 2021, 28, 519–523. [Google Scholar] [CrossRef]
- Juraszek, A.; Czerny, M.; Rylski, B. Thoracic endovascular aortic repair: Current evidence and challenges. Kardiologia Polska 2022, 80, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, R.; Luzzani, L.; Carugati, C.; Cossu, L.; Sarcina, A. Endovascular Repair of a Ruptured Abdominal Aortic Aneurysm in a Patient With Unfavorable Anatomy. Vasc. Endovasc. Surg. 2010, 44, 48–49. [Google Scholar] [CrossRef]
- Iezzi, R.; Cotroneo, A. Endovascular repair of abdominal aortic aneurysms: CTA evaluation of contraindications. Abdom. Imaging 2006, 31, 722–731. [Google Scholar] [CrossRef]
- Choke, E.; Munneke, G.; Morgan, R.; Belli, A.-M.; Loftus, I.; McFarland, R.; Loosemore, T.; Thompson, M.M. Outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile neck anatomy. Cardiovasc. Interv. Radiol. 2006, 29, 975–980. [Google Scholar] [CrossRef]
- Hoshina, K.; Ishimaru, S.; Sasabuchi, Y.; Yasunaga, H.; Komori, K. Outcomes of endovascular repair for abdominal aortic aneurysms: A nationwide survey in Japan. Ann. Surg. 2019, 269, 564. [Google Scholar] [CrossRef]
- Murray, D.; Szeberin, Z.; Benevento, D.; Abdallah, F.; Palasciano, G.; Lescan, M.; Uberoi, R.; Setacci, C. A comparison of clinical outcomes of abdominal aortic aneurysm patients with favorable and hostile neck angulation treated by endovascular repair with the Treovance stent graft. J. Vasc. Surg. 2019, 71, 1881–1889. [Google Scholar] [CrossRef]
- Carbon, R.T.; Baar, S.; Kriegelstein, S.; Huemmer, H.P.; Baar, K.; Simon, S.-I. Evaluating the in vitro adhesive strength of biomaterials. Biosimulator for selective leak closure. Biomaterials 2003, 24, 1469–1475. [Google Scholar] [CrossRef]
- de Gennes, P.G. Reptation of a Polymer Chain in the Presence of Fixed Obstacles. J. Chem. Phys. 1971, 55, 572–579. [Google Scholar] [CrossRef]
- Wool, R.; Yuan, B.L.; McGarel, O. Welding of polymer interfaces. Polym. Eng. Sci. 1989, 29, 1340–1367. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Báez, J.E.; Kenny, J.M.; Marcos-Fernández, A. Effect of the molecular weight on the crystallinity of PCL-b-PLLA di-block copolymers. Polymer 2012, 53, 4561–4568. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Migliaresi, C.; De Lollis, A.; Fambri, L.; Cohn, D. The Effect of Thermal History on the Crystallinity of Different Molecular Weight PLLA Biodegradable Polymers. Clin. Mater. 1991, 8, 111–118. [Google Scholar] [CrossRef]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly(ε-caprolactone): A potential polymer for biodegradable food packaging applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- dos Santos Filho, E.A.; Siqueira, D.D.; Araújo, E.M.; Luna, C.B.B.; de Medeiros, E.P. The Impact of the Macaíba Components Addition on the Biodegradation Acceleration of Poly (Ɛ-Caprolactone) (PCL). J. Polym. Environ. 2021, 30, 443–460. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- van den Born, B.H.; Lip, G.Y.H.; Brguljan-Hitij, J.; Cremer, A.; Segura, J.; Morales, E.; Mahfoud, F.; Amraoui, F.; Persu, A.; Kahan, T.; et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur. Heart J. Cardiovasc. Pharm. 2019, 5, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Mancia, G.; Kreutz, R.; Bundy, J.D.; Williams, B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines. Eur. Heart J. 2022, 43, 3302–3311. [Google Scholar] [CrossRef]
- Chobanian, A.; Bakris, G.; Black, H.; Cushman, W.; Green, L.; Izzo, J., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertens 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Kuramitsu, S.; Sonoda, S.; Ando, K.; Otake, H.; Natsuaki, M.; Anai, R.; Honda, Y.; Kadota, K.; Kobayashi, Y.; Kimura, T. Drug-eluting stent thrombosis: Current and future perspectives. Cardiovasc. Interv. Ther. 2021, 36, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, N.; Zirak, N.; Champmartin, S.; Shirinbayan, M.; Bakir, F. An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents. Polymer 2022, 14, 2751. [Google Scholar] [CrossRef]
- Liffman, K.; Šutalo, I.D.; Bui, A.; Lawrence-Brown, M.M.; Semmens, J.B. Experimental Measurement and Mathematical Modeling of Pulsatile Forces on a Symmetric, Bifurcated Endoluminal Stent Graft Model. Vascular 2009, 17, 201–209. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohn, D.; Widlan, F.; Zarek, M.; Peselev, Z.; Bloom, A.I. Engineering In Situ Weldable Vascular Devices. Bioengineering 2023, 10, 221. https://doi.org/10.3390/bioengineering10020221
Cohn D, Widlan F, Zarek M, Peselev Z, Bloom AI. Engineering In Situ Weldable Vascular Devices. Bioengineering. 2023; 10(2):221. https://doi.org/10.3390/bioengineering10020221
Chicago/Turabian StyleCohn, Daniel, Fany Widlan, Matt Zarek, Ziv Peselev, and Allan Isaac Bloom. 2023. "Engineering In Situ Weldable Vascular Devices" Bioengineering 10, no. 2: 221. https://doi.org/10.3390/bioengineering10020221
APA StyleCohn, D., Widlan, F., Zarek, M., Peselev, Z., & Bloom, A. I. (2023). Engineering In Situ Weldable Vascular Devices. Bioengineering, 10(2), 221. https://doi.org/10.3390/bioengineering10020221