Home Spirometry in Children with Cystic Fibrosis
Abstract
:1. Introduction
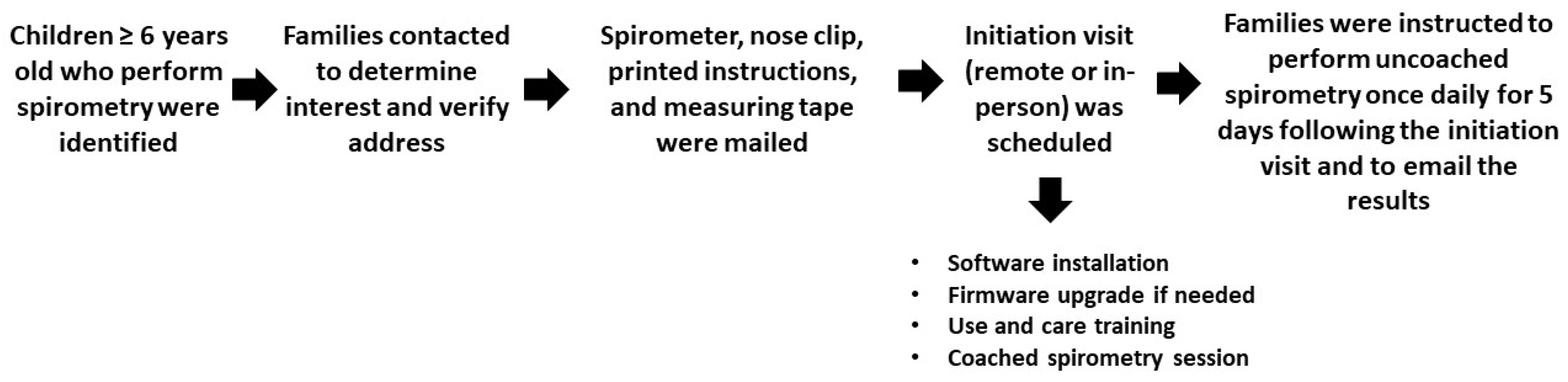
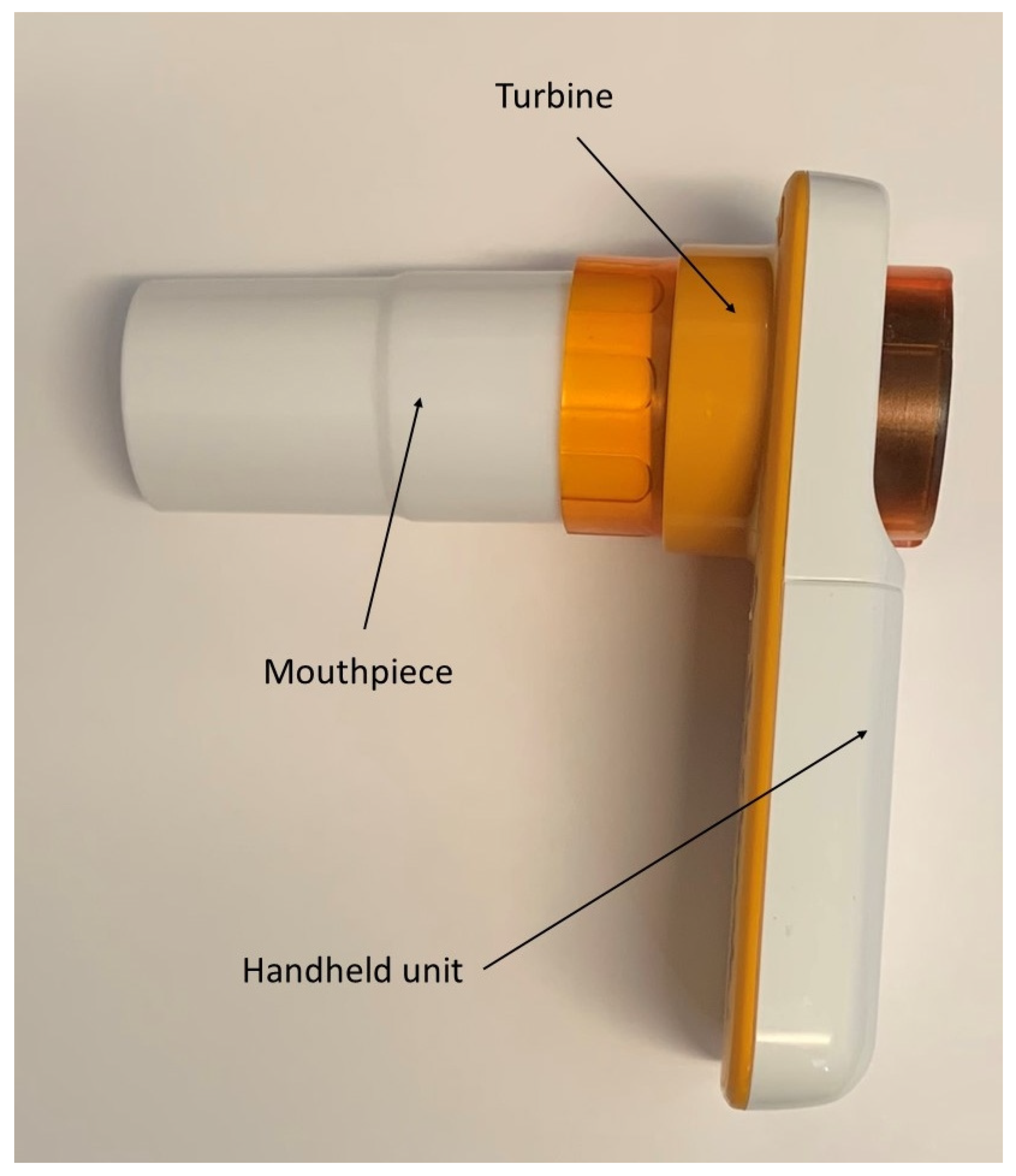
2. Methods
3. Results
3.1. Coached Spirometry
3.2. Uncoached Spirometry
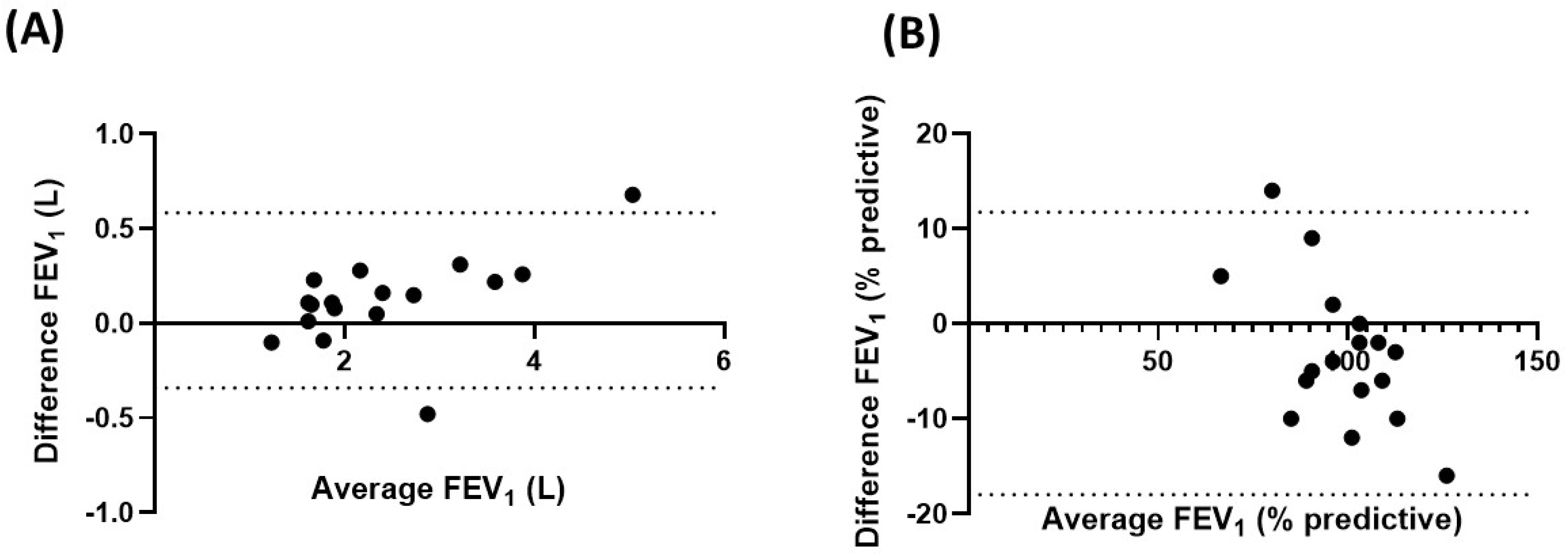
3.3. Paired Comparisons
3.4. Adherence
4. Discussion
Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Duff, A.J.; Bell, S.C.; Heijerman, H.G.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef]
- Sanders, D.B.; Bittner, R.C.L.; Rosenfeld, M.; Hoffman, L.R.; Redding, G.J.; Goss, C.H. Failure to Recover to Baseline Pulmonary Function after Cystic Fibrosis Pulmonary Exacerbation. Am. J. Respir. Crit. Care Med. 2010, 182, 627–632. [Google Scholar] [CrossRef]
- Prentice, B.J.; Jaffe, A.; Hameed, S.; Verge, C.F.; Waters, S.; Widger, J. Cystic fibrosis-related diabetes and lung disease: An update. Eur. Respir. Rev. 2021, 30, 200293. [Google Scholar] [CrossRef] [PubMed]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Wijbenga, N.; Hoek, R.A.S.; Mathot, B.J.; Seghers, L.; Van Weezel, J.J.; Ouden, J.D.; Wijsenbeek, M.S.; Aerts, J.G.J.V.; Hellemons, M.E.; Moor, C.C. Evaluation of a Home Monitoring Application for Follow Up after Lung Transplantation—A Pilot Study. J. Pers. Med. 2020, 10, 240. [Google Scholar] [CrossRef]
- Logie, K.; Welsh, L.; Ranganathan, S. Telehealth spirometry for children with cystic fibrosis. Arch. Dis. Child. 2019, 105, 1203–1205. [Google Scholar] [CrossRef]
- Bastian-Lee, Y.; Chavasse, R.; Richter, H.; Seddon, P. Assessment of a low-cost home monitoring spirometer for children. Pediatr. Pulmonol. 2002, 33, 388–394. [Google Scholar] [CrossRef]
- Shakkottai, A.; Nasr, S.Z. The Use of Home Spirometry in Pediatric Cystic Fibrosis Patients: Results of a Feasibility Study. Glob. Pediatr. Health 2017, 4, 2333794–17690315. [Google Scholar] [CrossRef]
- Shakkottai, A.; Kaciroti, N.; Kasmikha, L.; Nasr, S.Z. Impact of home spirometry on medication adherence among adolescents with cystic fibrosis. Pediatr. Pulmonol. 2018, 53, 431–436. [Google Scholar] [CrossRef]
- Rad, E.J.; Mirza, A.A.; Chhatwani, L.; Purington, N.; Mohabir, P.K. Cystic fibrosis telemedicine in the era of COVID-19. JAMIA Open 2022, 5, ooac005. [Google Scholar] [CrossRef]
- Ong, T.; Van Citters, A.D.; Dowd, C.; Fullmer, J.; List, R.; Pai, S.-A.; Ren, C.L.; Scalia, P.; Solomon, G.M.; Sawicki, G.S. Remote monitoring in telehealth care delivery across the U.S. cystic fibrosis care network. J. Cyst. Fibros. 2021, 20, 57–63. [Google Scholar] [CrossRef]
- Moor, C.C.; Gür-Demirel, Y.; Wijsenbeek, M.S. Feasibility of a Comprehensive Home Monitoring Program for Sarcoidosis. J. Pers. Med. 2019, 9, 23. [Google Scholar] [CrossRef]
- VanDevanter, E.J.; Heltshe, S.L.; Skalland, M.; Lechtzin, N.; Nichols, D.; Goss, C.H. The effect of oral and intravenous antimicrobials on pulmonary exacerbation recovery in cystic fibrosis. J. Cyst. Fibros. 2021, 20, 932–936. [Google Scholar] [CrossRef]
- Compton, M.; List, R.; Starheim, E.; Somerville, L.; Williamson, L.; Murray, R.; Jennings, D.; Bruschwein, H.; Albon, D. Home spirometry utilisation in telemedicine clinic for cystic fibrosis care during COVID-19 pandemic: A quality improvement process. BMJ Open Qual. 2021, 10, e001529. [Google Scholar] [CrossRef]
- Bell, J.M.; Sivam, S.; Dentice, R.L.; Dwyer, T.J.; Jo, H.E.; Lau, E.M.; Munoz, P.A.; Nolan, S.A.; Taylor, N.A.; Visser, S.K.; et al. Quality of home spirometry performance amongst adults with cystic fibrosis. J. Cyst. Fibros. 2021, 21, 84–87. [Google Scholar] [CrossRef]
- Wasilewska, E.; Sobierajska-Rek, A.; Małgorzewicz, S.; Soliński, M.; Jassem, E. Benefits of Telemonitoring of Pulmonary Func-tion-3-Month Follow-Up of Home Electronic Spirometry in Patients with Duchenne Muscular Dystrophy. J. Clin. Med. 2022, 11, 856. [Google Scholar] [CrossRef]
- Nakshbandi, G.; Moor, C.C.; Nossent, E.J.; Geelhoed, J.J.M.; Baart, S.J.; Boerrigter, B.G.; Aerts, J.G.J.V.; Nijman, S.F.M.; Santema, H.Y.; Hellemons, M.E.; et al. Home monitoring of lung function, symptoms and quality of life after admission with COVID-19 in-fection: The HOMECOMIN’ study. Respirology 2022, 27, 501–509. [Google Scholar] [CrossRef]
- Turner, J.; He, Q.; Baker, K.; Chung, L.; Lazarevic-Fogelquist, A.; Bethune, D.; Hubbard, J.; Guerriero, M.; Sheshadri, A.; Syrjala, K.L.; et al. Home Spirometry Telemonitoring for Early Detection of Bronchiolitis Obliterans Syndrome in Patients with Chronic Graft-versus-Host Disease. Transplant. Cell. Ther. 2021, 27, 616.e1–616.e6. [Google Scholar] [CrossRef]
- Van Opstal, J.; Zhao, A.T.; Kaplan, S.J.; Sung, A.D.; Schoemans, H. eHealth-Generated Patient Data in an Outpatient Setting after Hematopoietic Stem Cell Transplantation: A Scoping Review. Transplant. Cell. Ther. 2022, 28, 463–471. [Google Scholar] [CrossRef]
- Russell, A.-M.; Adamali, H.; Molyneaux, P.L.; Lukey, P.T.; Marshall, R.P.; Renzoni, E.A.; Wells, A.U.; Maher, T.M. Daily Home Spirometry: An Effective Tool for Detecting Progression in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 989–997. [Google Scholar] [CrossRef]
- Wijsenbeek, M.S.; Moor, C.C.; Johannson, K.A.; Jackson, P.D.; Khor, Y.H.; Kondoh, Y.; Rajan, S.K.; Tabaj, G.C.; Varela, B.E.; van der Wal, P.; et al. Home monitoring in interstitial lung diseases. Lancet Respir. Med. 2022, 11, 97–110. [Google Scholar] [CrossRef]
- Kerwin, E.; Pascoe, S.; Bailes, Z.; Nathan, R.; Bernstein, D.; Dahl, R.; von Maltzahn, R.; Robbins, K.; Fowler, A.; Lee, L. A phase IIb, ran-domised, parallel-group study: The efficacy, safety and tolerability of once-daily umeclidinium in patients with asthma re-ceiving inhaled corticosteroids. Respir. Res. 2020, 21, 148. [Google Scholar] [CrossRef]
- Bindler, R.; Haverkamp, H.C.; O’Flanagan, H.; Whicker, J.; Rappold, A.G.; Walden, V.; Postma, J. Feasibility and acceptability of home monitoring with portable spirometry in young adults with asthma. J. Asthma 2023, 1–6. [Google Scholar] [CrossRef]
- Avdimiretz, N.; Wilson, D.; Grasemann, H. Comparison of a handheld turbine spirometer to conventional spirometry in children with cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 1394–1399. [Google Scholar] [CrossRef]
- Gerzon, F.L.; Jöbsis, Q.; Bannier, M.A.; Winkens, B.; Dompeling, E. Discrepancy between Lung Function Measurements at Home and in the Hospital in Children with Asthma and CF. J. Clin. Med. 2020, 9, 1617. [Google Scholar] [CrossRef]
- Kruizinga, M.D.; Essers, E.; Stuurman, F.E.; Zhuparris, A.; van Eik, N.; Janssens, H.M.; Groothuis, I.; Sprij, A.J.; Nuijsink, M.; Cohen, A.F.; et al. Technical validity and usability of a novel smartphone-connected spirometry device for pediatric patients with asthma and cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 2463–2470. [Google Scholar] [CrossRef]
- Davis, J.; Ryan, M.; Marchetti, P.; Dahlberg, S.E.; Greenberg, J.; Bacon, C.; Kaur, R.; Scalia, S.; Sawicki, G.S. Real-world feasibility of short-term, unsupervised home spirometry in CF. Pediatr. Pulmonol. 2022, 57, 3129–3135. [Google Scholar] [CrossRef]
- Doumit, M.; Ledwos, R.; Plush, L.; Chuang, S.; Gray, M.; Jaffe, A.; McBride, J. Telehealth application of an ultrasonic home spirometer. Arch. Dis. Child. 2022, 107, 752–754. [Google Scholar] [CrossRef]
- Fettes, E.; Riley, M.; Brotherston, S.; Doughty, C.; Griffiths, B.; Laverty, A.; Aurora, P. “You’re on mute!” Does pediatric CF home spirometry require physiologist supervision? Pediatr. Pulmonol. 2022, 57, 278–284. [Google Scholar] [CrossRef]
- Berlinski, A. Implementation of pediatric home spirometry: Potential height bias. J. Cyst. Fibros. 2020, 20, 719–720. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Bolcato, M.; Beverina, I.; Rodriguez, D.; Aprile, A.; Trabucco Aurilio, M. Organizational Strategies for the Management of Intra-venous Iron Therapy in Non-Hospitalized Settings: A Safe Opportunity to Implement Patient Blood Management in Italy. Healthcare 2021, 9, 1222. [Google Scholar] [CrossRef]
- Foster, J.M.; Usherwood, T.; Smith, L.; Sawyer, S.M.; Xuan, W.; Rand, C.S.; Reddel, H.K. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J. Allergy Clin. Immunol. 2014, 134, 1260–1268.e3. [Google Scholar] [CrossRef]
- De Simoni, A.; Fleming, L.; Holliday, L.; Horne, R.; Priebe, S.; Bush, A.; Sheikh, A.; Griffiths, C. Electronic reminders and rewards to improve adherence to inhaled asthma treatment in adolescents: A non-randomised feasibility study in tertiary care. BMJ Open 2021, 11, e053268. [Google Scholar] [CrossRef]
- D’Errico, S.; Zanon, M.; Radaelli, D.; Padovano, M.; Santurro, A.; Scopetti, M.; Frati, P.; Fineschi, V. Medication Errors in Pediatrics: Proposals to Improve the Quality and Safety of Care Through Clinical Risk Management. Front. Med. 2022, 8, 814100. [Google Scholar] [CrossRef]


| Subjects | n | Age (Years) | Sex (Male/Female) | Ethnicity (Caucasian) | Weight (kg) | Height (cm) |
|---|---|---|---|---|---|---|
| ALL | 52 | 12.7 ± 4 | 30/22 | 48 | 45.2 ± 18.3 | 145 ± 18 |
| <3 uncoached tests | 18 | 12.6 ± 4.5 | 9/9 | 17 | 43.6 ± 23.3 | 142 ± 20 |
| ≥3 uncoached tests | 34 | 13 ± 3.7 | 21/13 | 31 | 46 ± 15.3 | 146 ± 17 |
| p value | 0.88 | 0.56 | 0.99 | 0.69 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlinski, A.; Leisenring, P.; Willis, L.; King, S. Home Spirometry in Children with Cystic Fibrosis. Bioengineering 2023, 10, 242. https://doi.org/10.3390/bioengineering10020242
Berlinski A, Leisenring P, Willis L, King S. Home Spirometry in Children with Cystic Fibrosis. Bioengineering. 2023; 10(2):242. https://doi.org/10.3390/bioengineering10020242
Chicago/Turabian StyleBerlinski, Ariel, Pamela Leisenring, Lauren Willis, and Sandra King. 2023. "Home Spirometry in Children with Cystic Fibrosis" Bioengineering 10, no. 2: 242. https://doi.org/10.3390/bioengineering10020242
APA StyleBerlinski, A., Leisenring, P., Willis, L., & King, S. (2023). Home Spirometry in Children with Cystic Fibrosis. Bioengineering, 10(2), 242. https://doi.org/10.3390/bioengineering10020242







