Design and Modeling of Ultra-Compact Wideband Implantable Antenna for Wireless ISM Band
Abstract
:1. Introduction
2. Background
2.1. Size Reduction Techniques
2.2. Properties of Human Tissues
3. Antenna Design and Simulations
3.1. Proposed Antenna Configuration
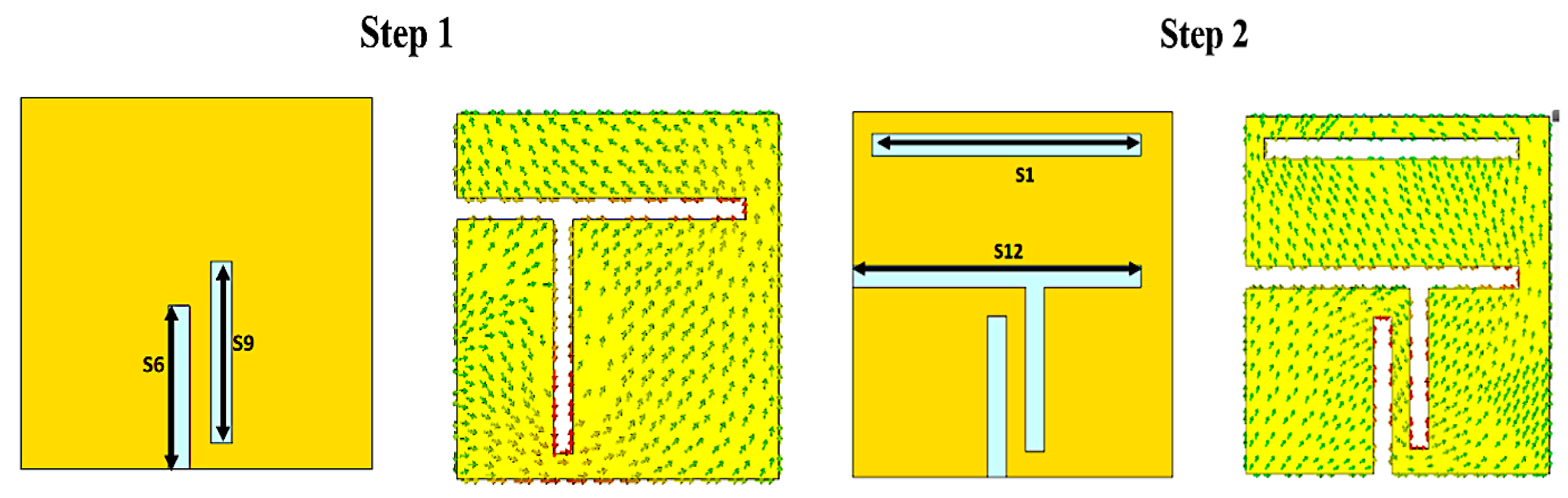
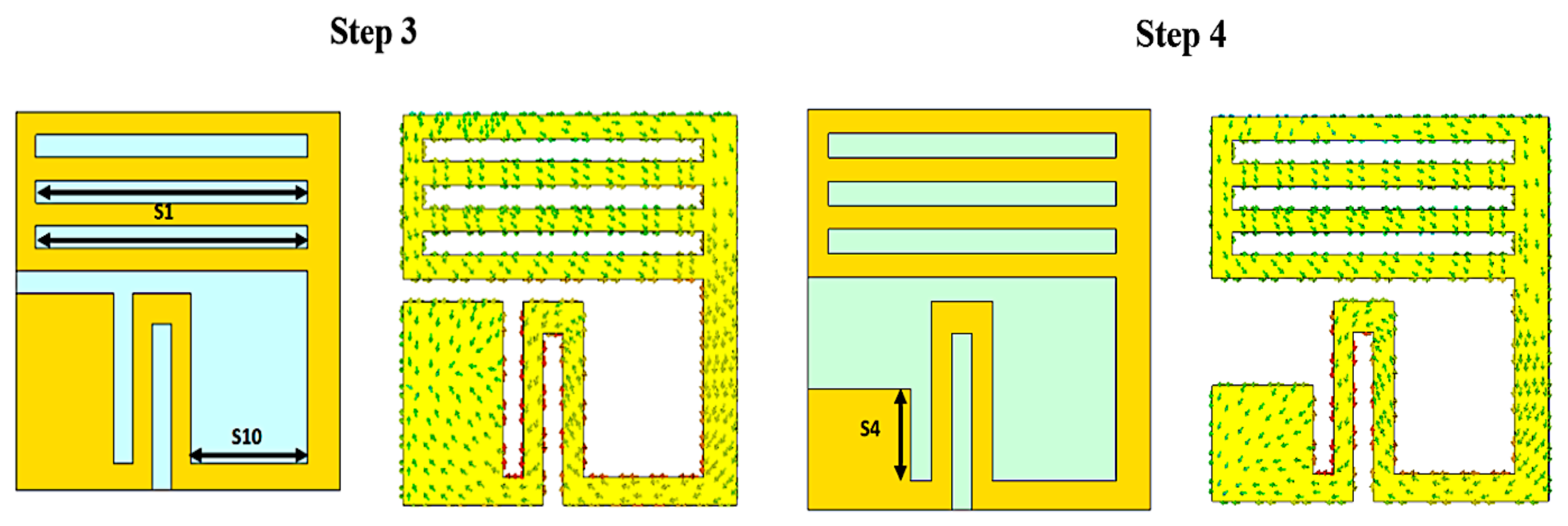
3.2. Size Reduction Steps
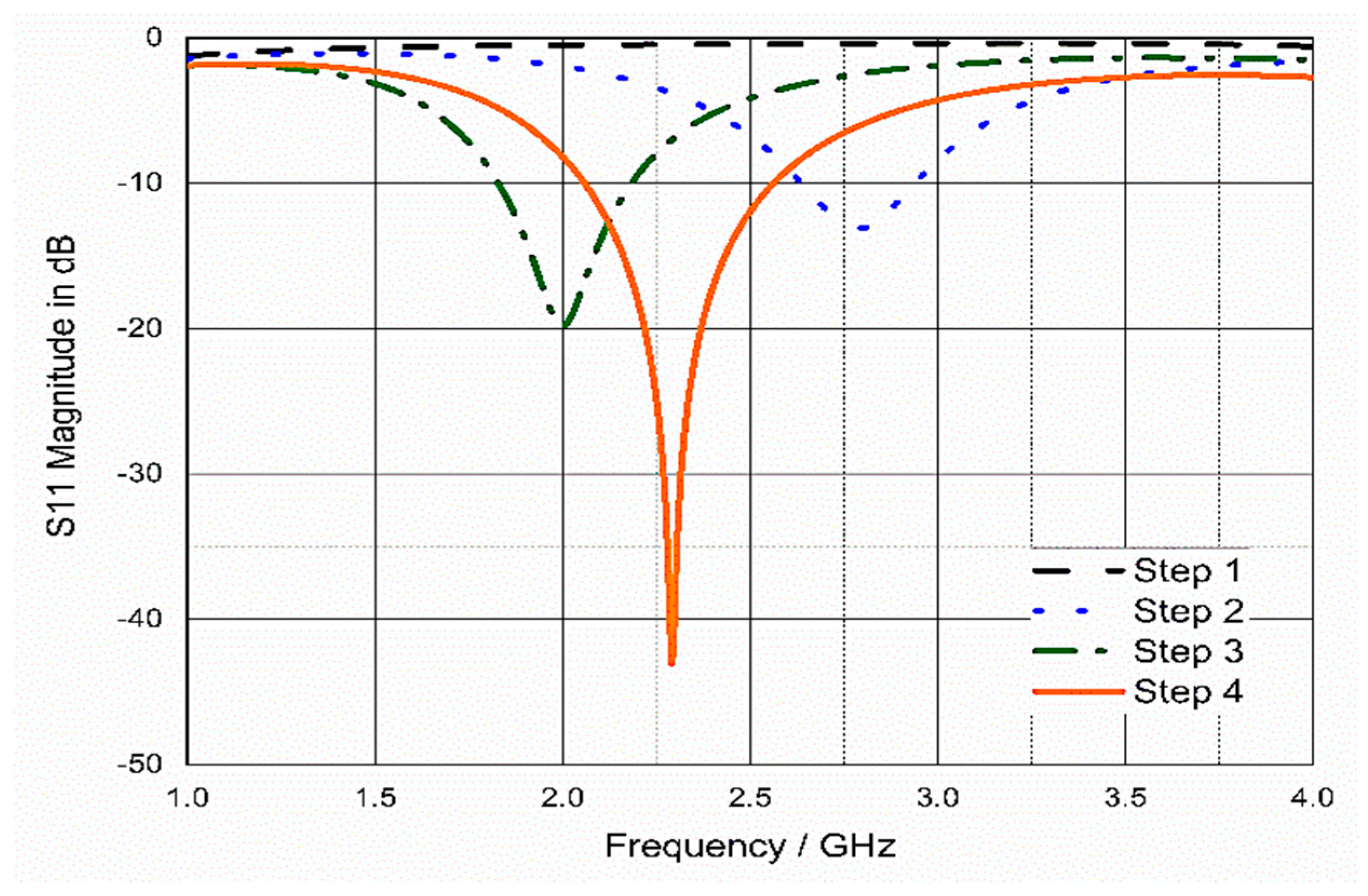
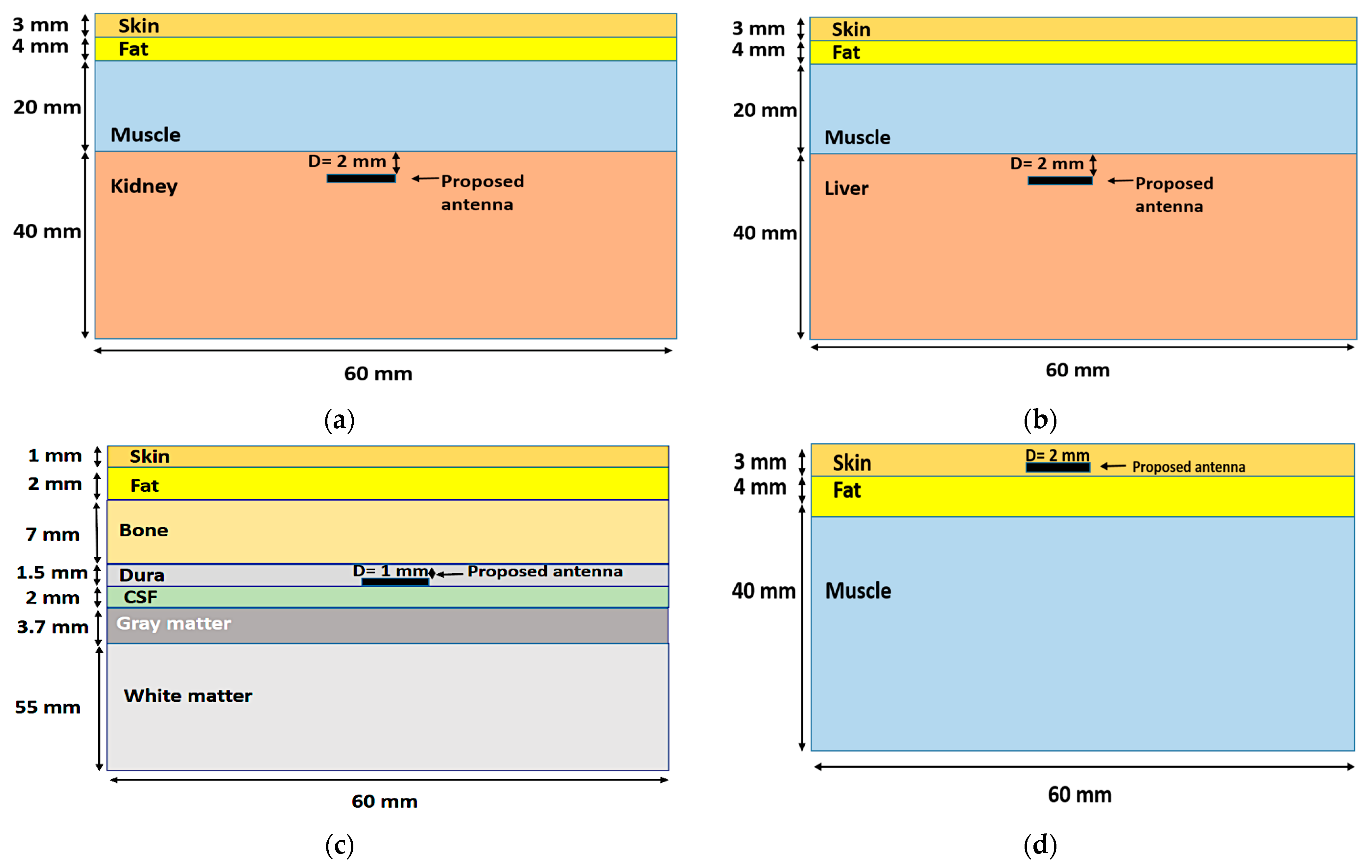
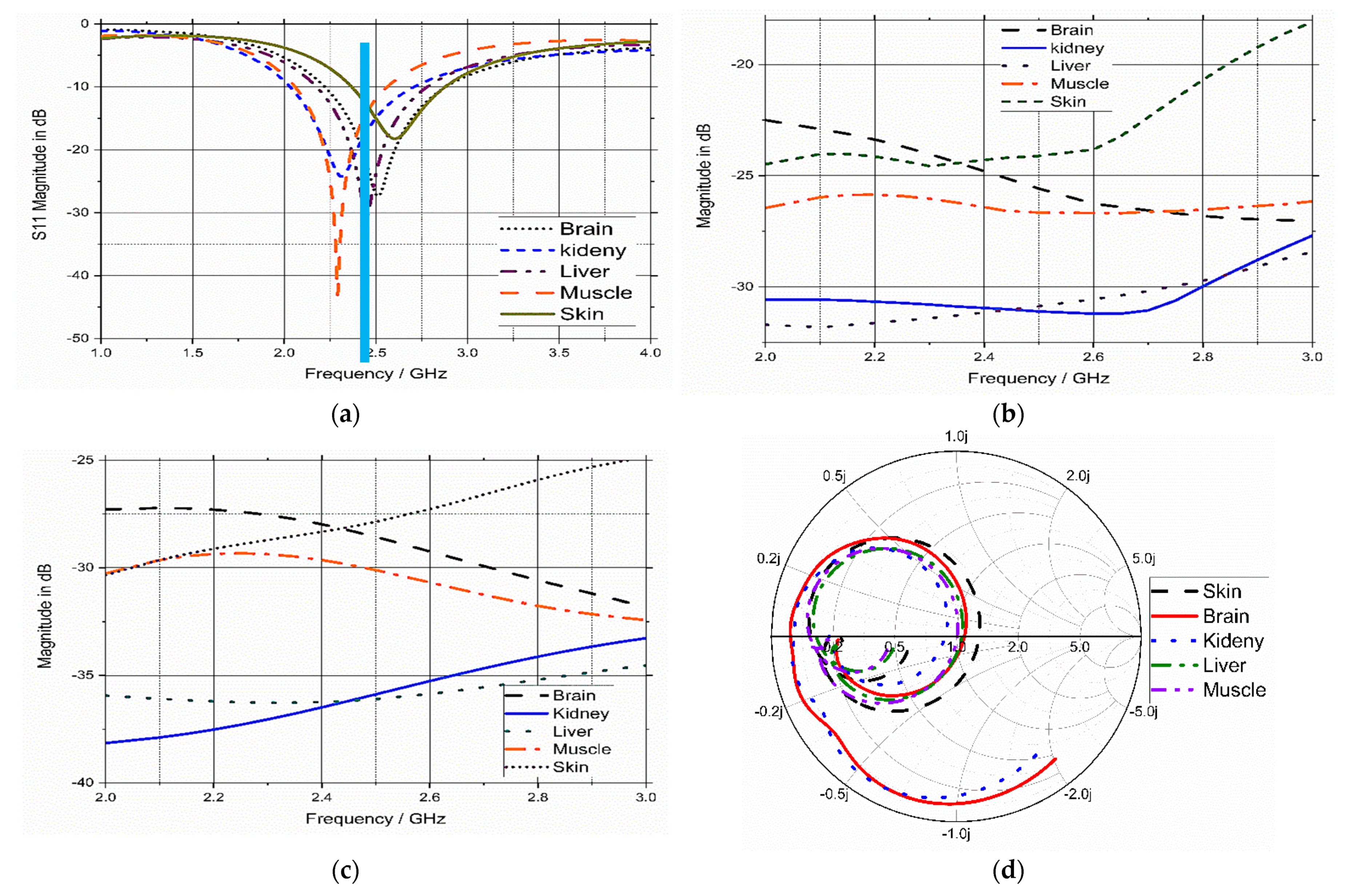
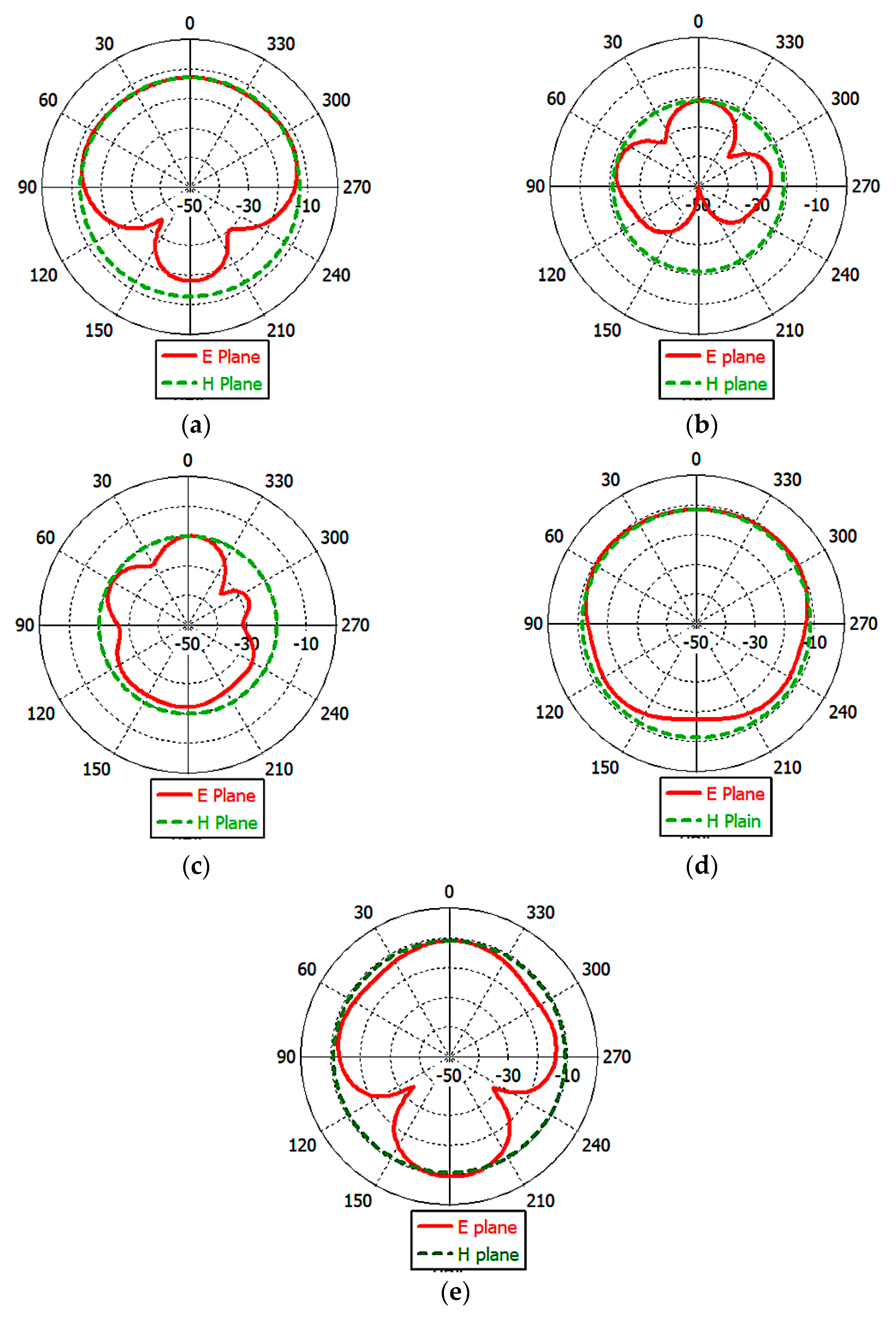
3.3. Analysis and Discussion
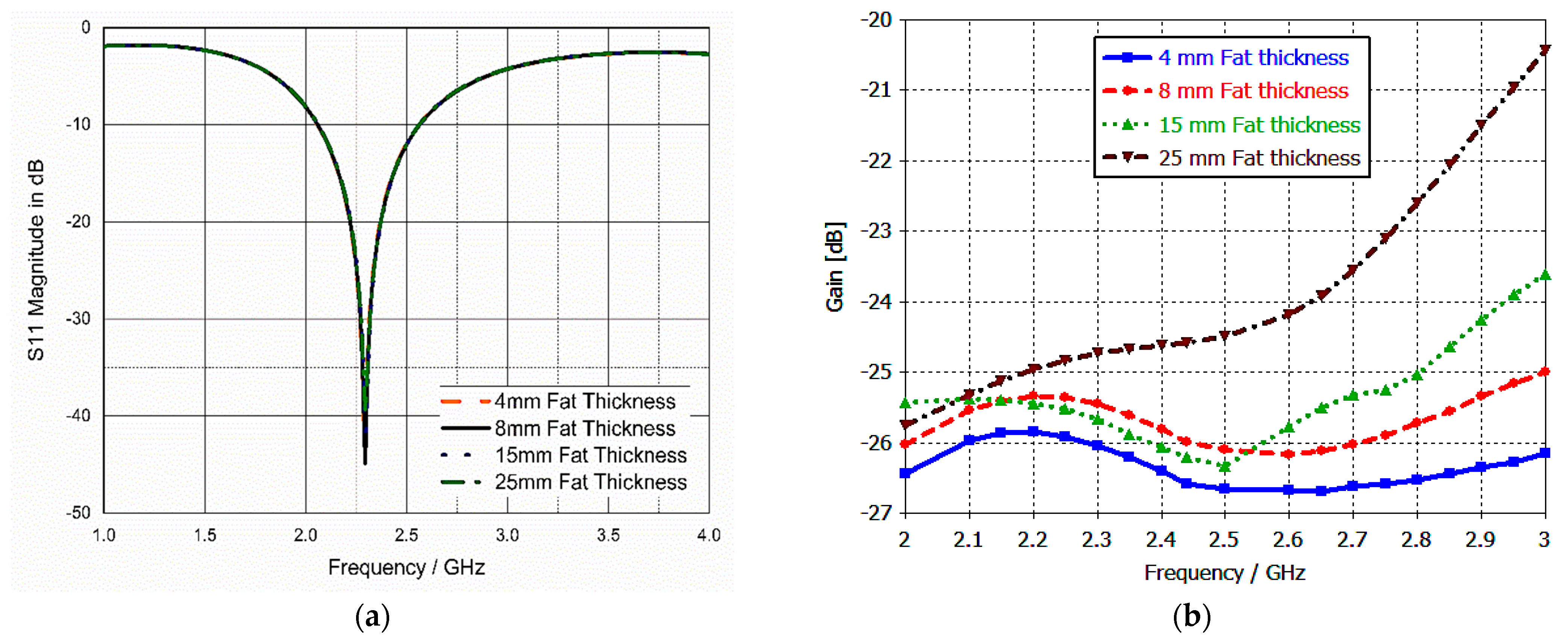
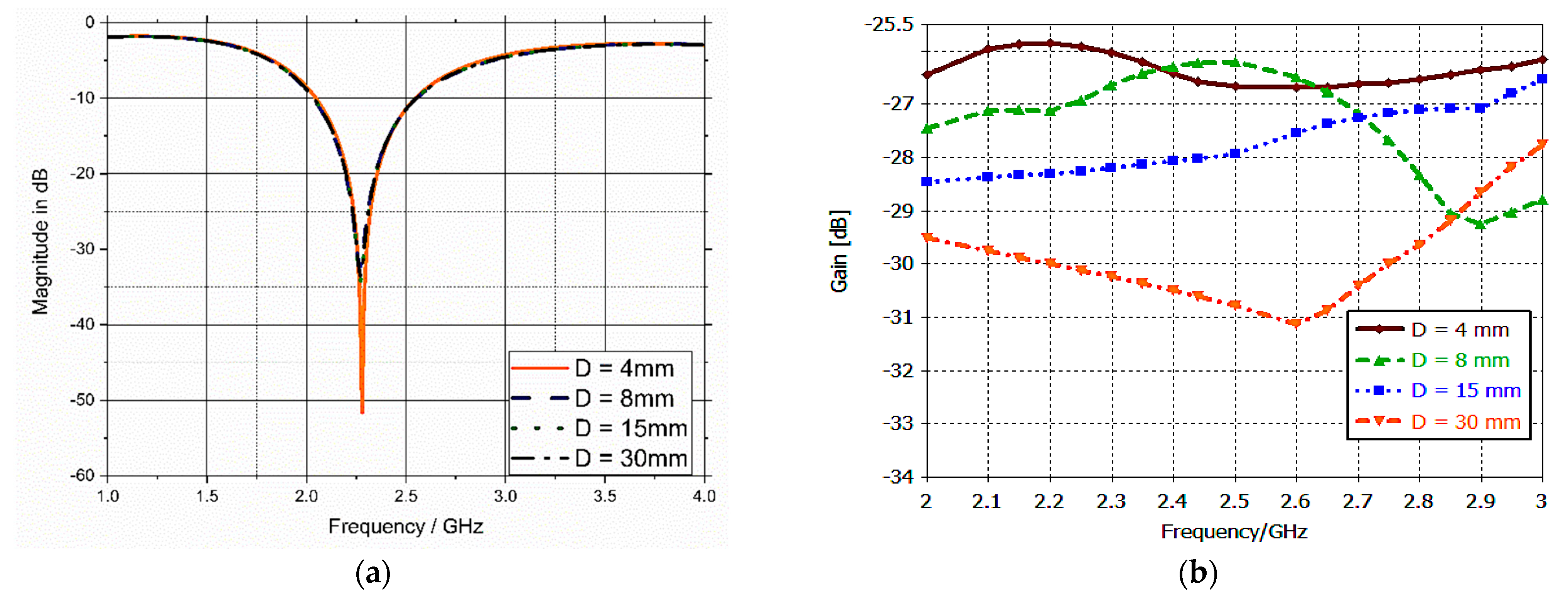
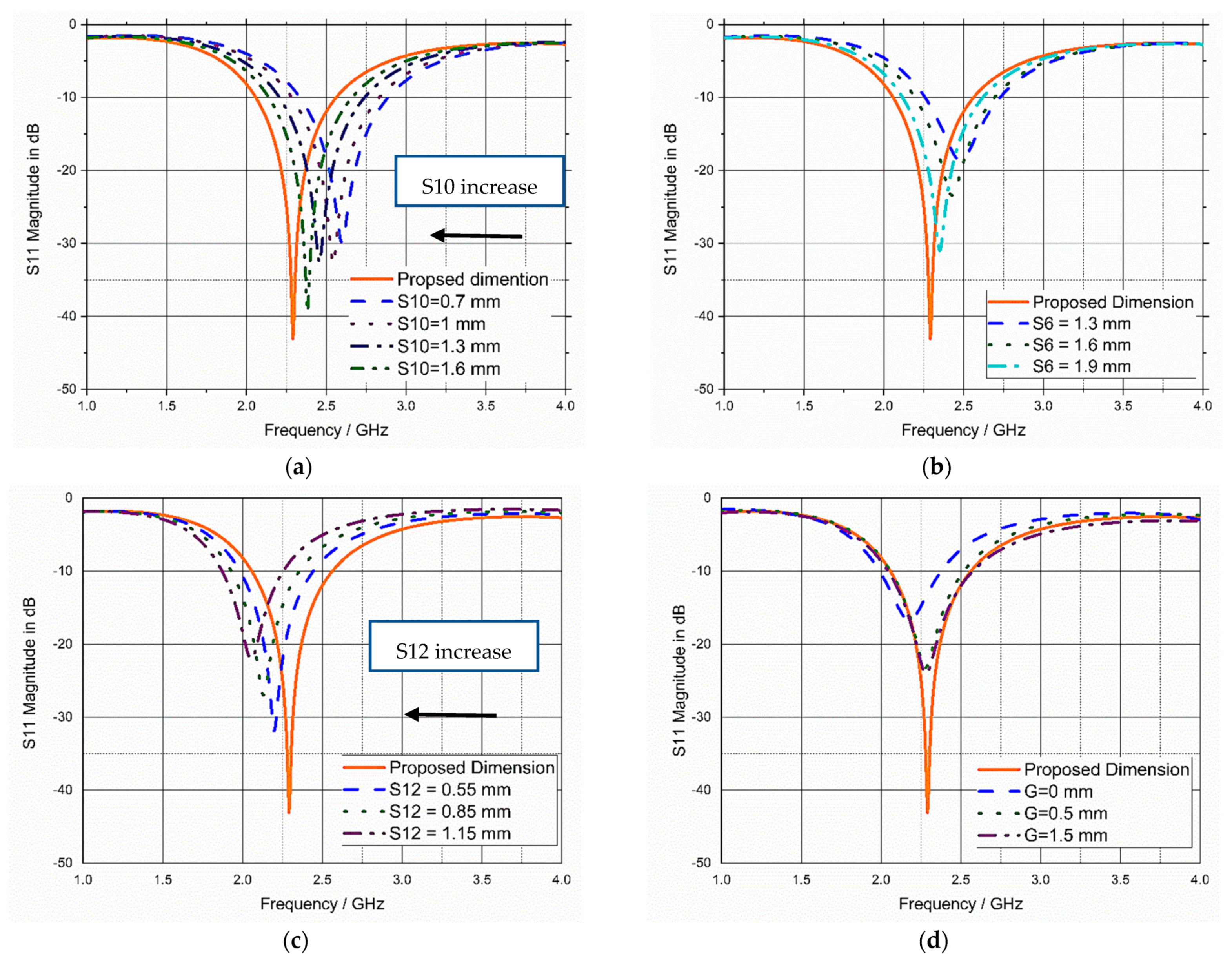
3.4. Sensitivity Analysis and Parametric Study
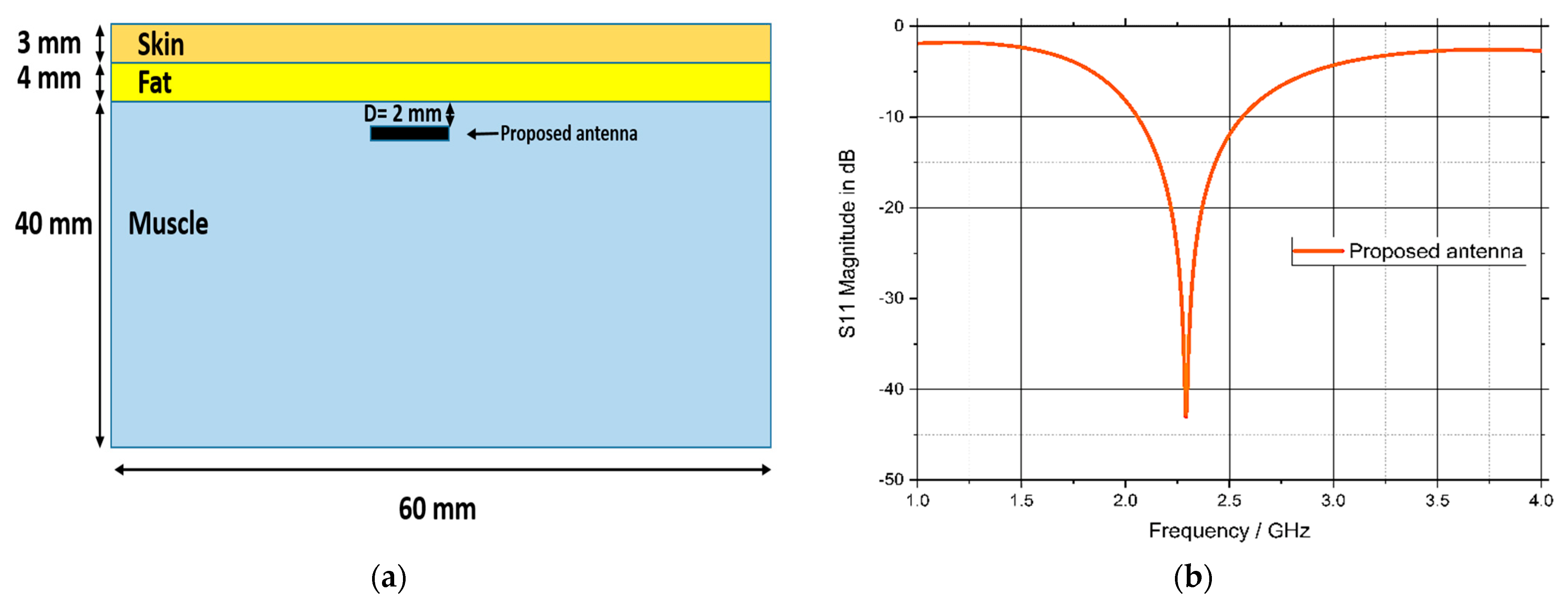
3.5. Model Integrity Analysis
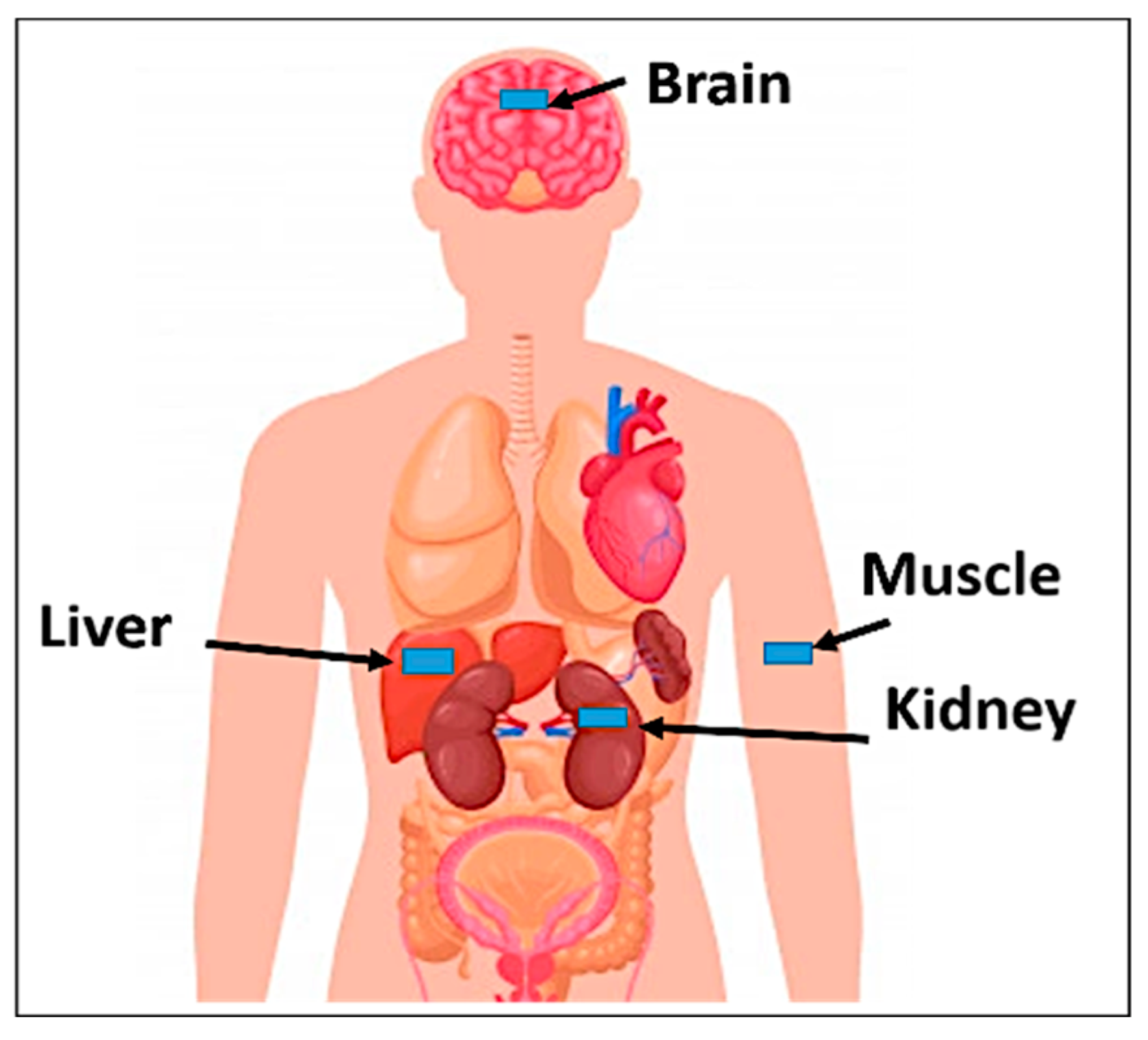
4. Specific Absorption Rate Calculations
5. Link Budget
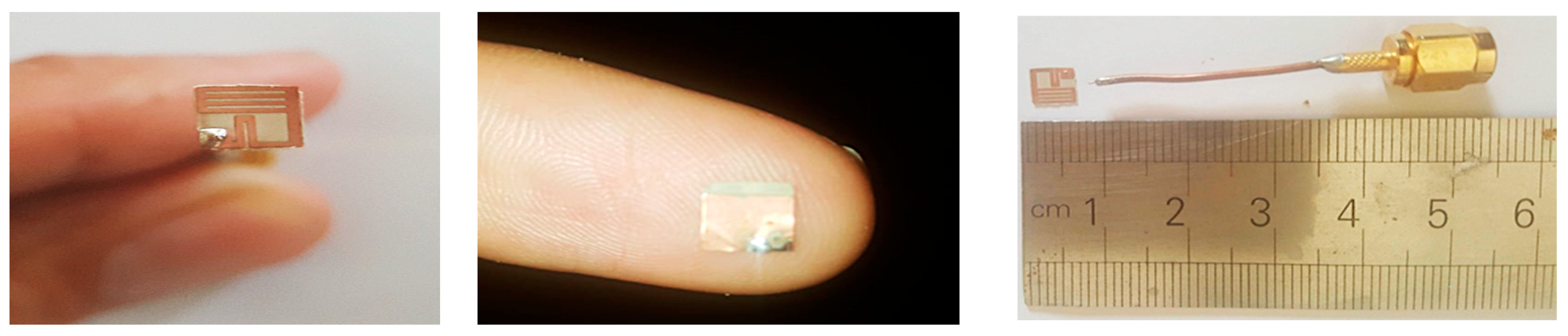
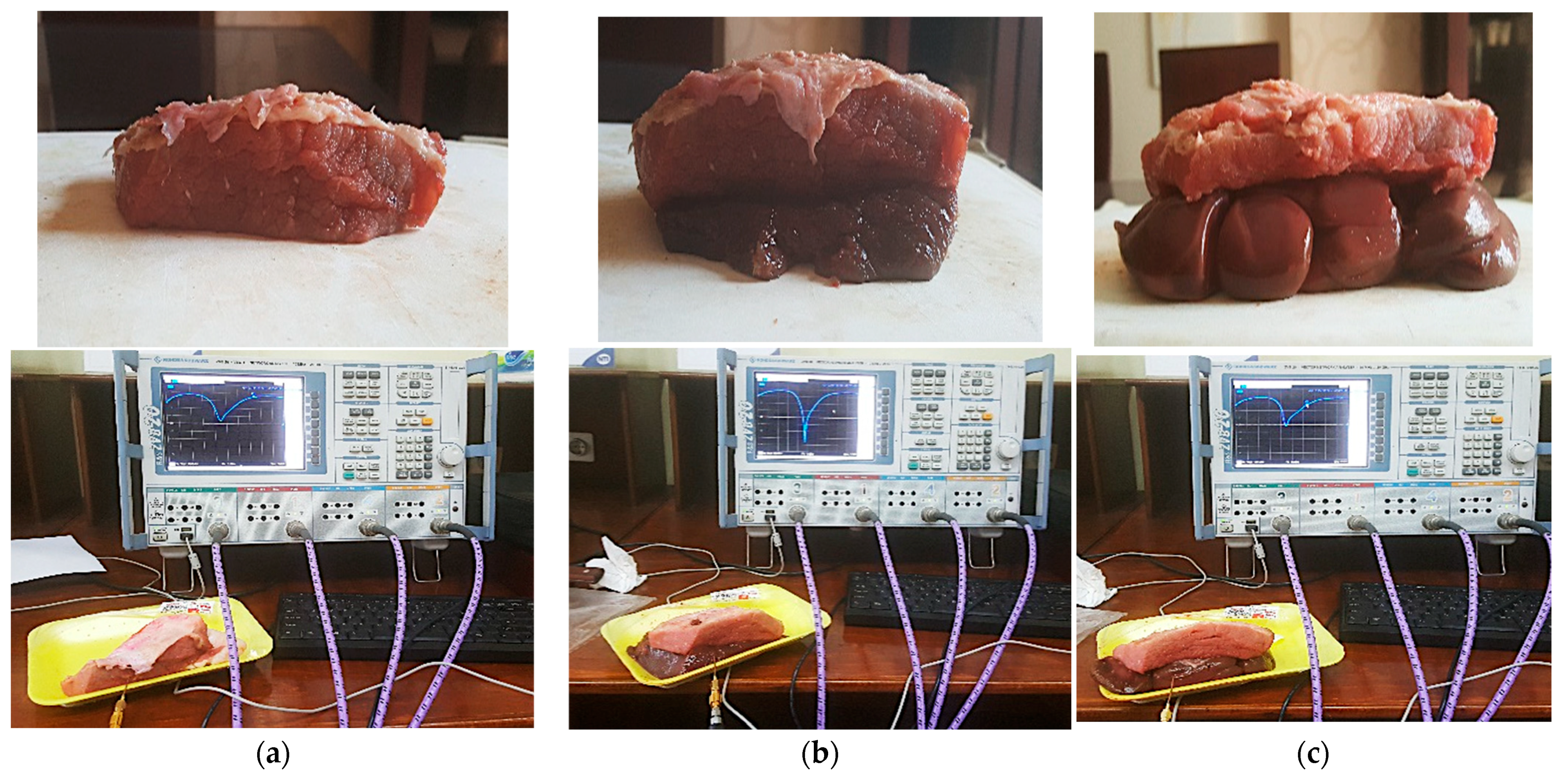
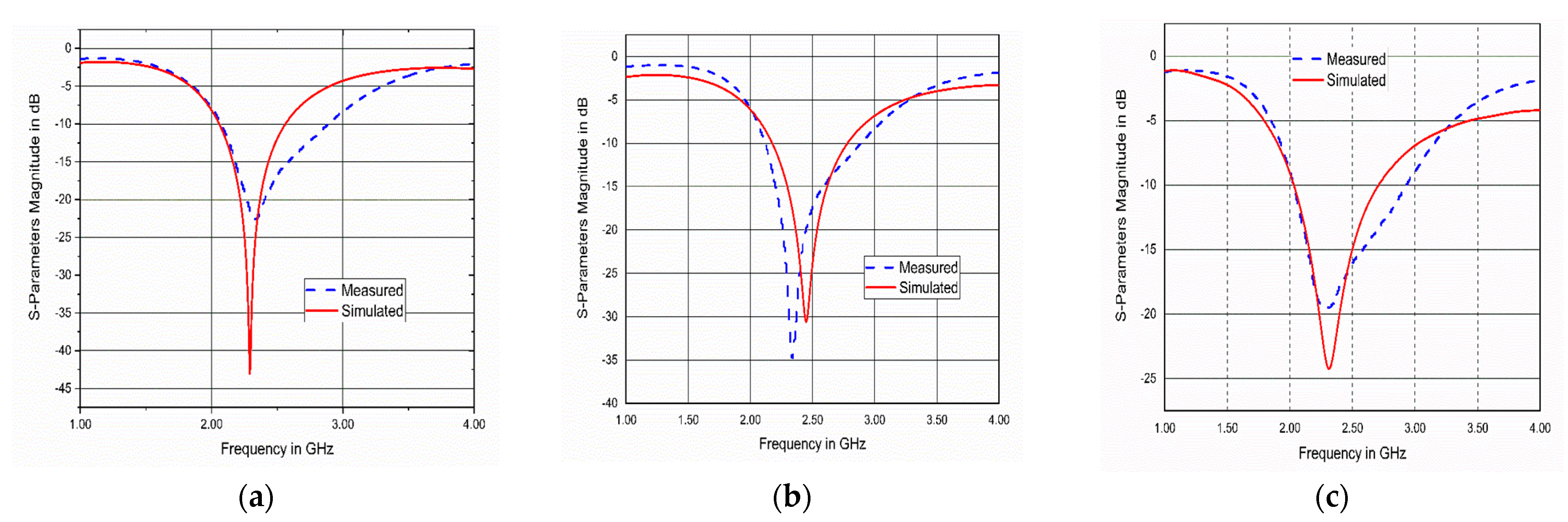
6. Fabrication and Measurements
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Status Report On Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Debén, E.; Gallego, T.; Perez, M.; Gomez, M.; Serra, J.; Ramirez, E.; Ibañez, A.; Martinez, C.; Alañon, E.; Morell, A. DI-024 Cetuximab in the treatment of advanced metastatic colorectal cancer. Eur. J. Hosp. Pharm. 2014, 21, A79.2–A80. [Google Scholar] [CrossRef]
- Werner, D.H.; Jiang, Z. Electromagnetics of Body Area Networks: Antennas, Propagation, and RF Systems, 1st ed.; Wiley-IEEE Press: State Collage, PA, USA, 2016. [Google Scholar]
- Latré, B.; Braem, B.; Moerman, I.; Blondia, C.; Demeester, P. A survey on wireless body area networks. Wirel. Netw. 2010, 17, 1–18. [Google Scholar] [CrossRef]
- Das, R.; Yoo, H. A triple-band deep-tissue implantable antenna incorporating biotelemetry and unidirectional wireless power transfer system. In Proceedings of the IEEE Antennas and Propagation Society International Symposium, San Diego, CA, USA, 9–14 July 2017; pp. 2489–2490. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Yoo, H. Scalp-Implantable Antenna Systems for Intracranial Pressure Monitoring. IEEE Trans. Antennas Propag. 2018, 66, 2170–2173. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Yang, X.; Liu, X.; Guo, H. An ingestible capsule system for in-body core temperature monitoring. Microw. Opt. Technol. Lett. 2017, 59, 2670–2675. [Google Scholar] [CrossRef]
- Heller, A. Implanted Electrochemical Glucose Sensors for the Management of Diabetes. Annu. Rev. Biomed. Eng. 1999, 1, 153–175. [Google Scholar] [CrossRef]
- Patel, R.; Upadhyaya, T. An electrically small antenna for nearfield biomedical applications. Microw. Opt. Technol. Lett. 2018, 60, 556–561. [Google Scholar] [CrossRef]
- Patel, R.; Desai, A.; Upadhyaya, T.K. An electrically small antenna using defected ground structure for RFID, GPS and IEEE 802.11 A/B/G/S applications. Prog. Electromagn. Res. Lett. 2018, 75, 75–81. [Google Scholar] [CrossRef]
- Gyselinckx, B.; Vullers, R.; Van Hoof, C.; Ryckaert, J.; Yazicioglu, R.F. Human++: Emerging technology for body area networks. Wirel. Technol. Circ. Syst. Devices 2017, 249, 221–240. [Google Scholar] [CrossRef]
- C95.1-2005; IEEE Standard for Safety Levels With Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz. IEEE-SA Standards Board: Piscataway, NJ, USA, 2006.
- Zhang, S.; Qin, Y.; Kuang, J.; Mak, P.U.; Pun, S.H.; Vai, M.I.; Liu, Y. Development and Prospect of Implantable Intra-Body Communication Technology. J. Comput. 2014, 9, 463–474. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, L. Dual-band implantable antenna with circular polarisation property for ingestible capsule application. Electron. Lett. 2017, 53, 1090–1092. [Google Scholar] [CrossRef]
- Nikolayev, D.; Zhadobov, M.; Le Coq, L.; Karban, P.; Sauleau, R. Robust Ultraminiature Capsule Antenna for Ingestible and Implantable Applications. IEEE Trans. Antennas Propag. 2017, 65, 6107–6119. [Google Scholar] [CrossRef]
- Patel, R.H.; Upadhyaya, T.; Desai, A. Electrically small inverted L planar patch antenna for wireless application. Microw. Opt. Technol. Lett. 2018, 60, 2351–2357. [Google Scholar] [CrossRef]
- Gemio, J.; Parron, J.; Soler, J. Human body effects on implantable antennas for ism bands applications: Models comparison and propagation losses study. Prog. Electromagn. Res. 2010, 110, 437–452. [Google Scholar] [CrossRef]
- Basir, A.; Bouazizi, A.; Zada, M.; Iqbal, A.; Ullah, S.; Naeem, U. A dual-band implantable antenna with wide-band characteristics at MICS and ISM bands. Microw. Opt. Technol. Lett. 2018, 60, 2944–2949. [Google Scholar] [CrossRef]
- Kiourti, A.; Psathas, K.A.; Nikita, K.S. Implantable and ingestible medical devices with wireless telemetry functionalities: A review of current status and challenges. Bioelectromagnetics 2013, 35, 1–15. [Google Scholar] [CrossRef]
- Zaki, A.Z.A.; Hamad, E.K.I.; Abouelnaga, T.G.; Elsadek, H.A. Design of ultra-compact ISM band implantable patch antenna for bio-medical applications. Int. J. Microw. Wirel. Technol. 2022, 14, 1279–1288. [Google Scholar] [CrossRef]
- Kiourti, A.; Nikita, K.S. Numerical assessment of the performance of a scalp-implantable antenna: Effects of head anatomy and dielectric parameters. Bioelectromagnetics 2012, 34, 167–179. [Google Scholar] [CrossRef]
- Magill, M.K.; Conway, G.A.; Scanlon, W.G. Tissue-Independent Implantable Antenna for In-Body Communications at 2.36–2.5 GHz. IEEE Trans. Antennas Propag. 2017, 65, 4406–4417. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Maity, S.; Chaudhuri, S.R.B.; Mitra, M. Metamaterial-inspired wideband biocompatible antenna for implantable applications. IET Microw. Antennas Propag. 2018, 12, 1799–1805. [Google Scholar] [CrossRef]
- Vidal, N.; Curto, S.; Lopez-Villegas, J.M.; Sieiro, J.; Ramos, F.M. Detuning study of implantable antennas inside the human body. Prog. Electromagn. Res. 2012, 124, 265–283. [Google Scholar] [CrossRef]
- Chen, Y. Reduction of detuning effects using robust parameter design for implantable antennas. Electron. Lett. 2015, 51, 1971–1973. [Google Scholar] [CrossRef]
- Basir, A.; Yoo, H. A Stable Impedance-Matched Ultrawideband Antenna System Mitigating Detuning Effects for Multiple Biotelemetric Applications. IEEE Trans. Antennas Propag. 2019, 67, 3416–3421. [Google Scholar] [CrossRef]
- Balanis, C.A. Antenna Theory: Analysis and Design, 3rd ed.; Wiely: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Lee, C.-M.; Yo, T.-C.; Huang, F.-J.; Luo, C.-H. Bandwidth enhancement of planar inverted-F antenna for implantable biotelemetry. Microw. Opt. Technol. Lett. 2009, 51, 749–752. [Google Scholar] [CrossRef]
- Liu, W.-C.; Chen, S.-H.; Wu, C.-M. Bandwidth enhancement and size reduction of an implantable PIFA antenna for biotelemetry devices. Microw. Opt. Technol. Lett. 2009, 51, 755–757. [Google Scholar] [CrossRef]
- Luo, L.; Hu, B.; Wu, J.; Yan, T.; Xu, L. Compact dual-band antenna with slotted ground for implantable applications. Microw. Opt. Technol. Lett. 2019, 61, 1314–1319. [Google Scholar] [CrossRef]
- Faisal, F.; Yoo, H. A Miniaturized Novel-Shape Dual-Band Antenna for Implantable Applications. IEEE Trans. Antennas Propag. 2018, 67, 774–783. [Google Scholar] [CrossRef]
- Yousaf, M.; Ben Mabrouk, I.; Zada, M.; Akram, A.; Amin, Y.; Nedil, M.; Yoo, H. An Ultra-Miniaturized Antenna With Ultra-Wide Bandwidth Characteristics for Medical Implant Systems. IEEE Access 2021, 9, 40086–40097. [Google Scholar] [CrossRef]
- Basir, A.; Zada, M.; Yoo, H. Compact and Flexible Wideband Antenna for Intraoral Tongue-Drive System for People With Disabilities. IEEE Trans. Antennas Propag. 2019, 68, 2405–2409. [Google Scholar] [CrossRef]
- Zaki, A.Z.A.; Abouelnaga, T.G.; Hamad, E.K.I.; Elsadek, H.A. Design of dual-band implanted patch antenna system for bio-medical applications. J. Electr. Eng. 2021, 72, 240–248. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Takahashi, M.; Saito, K.; Ito, K. Performance of Implantable Folded Dipole Antenna for In-Body Wireless Communication. IEEE Trans. Antennas Propag. 2013, 61, 1363–1370. [Google Scholar] [CrossRef]
- Kiourti, A.; Christopoulou, M.; Nikita, K.S. Performance of a novel miniature antenna implanted in the human head for wireless biotelemetry. In Proceedings of the 2011 IEEE International Symposium on Antennas and Propagation (APSURSI), Spokane, WA, USA, 3–8 July 2011; pp. 392–395. [Google Scholar] [CrossRef]
- Yang, G. Body Sensor Networks, 2nd ed.; Springer: London, UK, 2006. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Polk, C. CRC Handbook of Biological Effects of Electromagnetic Fields; Taylor & Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Kiourti, A.; Nikita, K.S. A Review of Implantable Patch Antennas for Biomedical Telemetry: Challenges and Solutions [Wireless Corner]. IEEE Antennas Propag. Mag. 2012, 54, 210–228. [Google Scholar] [CrossRef]
- Samanta, G.; Mitra, D. Miniaturised and radiation efficient implantable antenna using reactive impedance surface for biotelemetry. IET Microw. Antennas Propag. 2019, 14, 177–184. [Google Scholar] [CrossRef]
- Huang, W.; Kishk, A.A. Embedded Spiral Microstrip Implantable Antenna. Int. J. Antennas Propag. 2011, 2011, 919821. [Google Scholar] [CrossRef]
- Mahalakshmi, N.; Thenmozhi, A. Design of hexagon shape bow-tie patch antenna for implantable bio-medical applications. Alex. Eng. J. 2017, 56, 235–239. [Google Scholar] [CrossRef]
- Alamri, S.; AlAmoudi, A.; Langley, R. Gain enhancement of implanted antenna using lens and parasitic ring. Electron. Lett. 2016, 52, 800–801. [Google Scholar] [CrossRef]
- Das, S.; Mitra, D. A Compact Wideband Flexible Implantable Slot Antenna Design With Enhanced Gain. IEEE Trans. Antennas Propag. 2018, 66, 4309–4314. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Zhu, L.; Xiao, S. An Implantable Wideband Microstrip Patch Antenna Based on High-Loss Property of Human Tissue. IEEE Access 2020, 8, 93048–93057. [Google Scholar] [CrossRef]
- Tunstall, R.; Tubbs, R.S. Gray’s Anatomy—The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Hout, S.; Chung, J.-Y. Design and Characterization of a Miniaturized Implantable Antenna in a Seven-Layer Brain Phantom. IEEE Access 2019, 7, 162062–162069. [Google Scholar] [CrossRef]
- Zada, M.; Yoo, H. A Miniaturized Triple-Band Implantable Antenna System for Bio-Telemetry Applications. IEEE Trans. Antennas Propag. 2018, 66, 7378–7382. [Google Scholar] [CrossRef]
- Xia, W.; Saito, K.; Takahashi, M.; Ito, K. Performances of an Implanted Cavity Slot Antenna Embedded in the Human Arm. IEEE Trans. Antennas Propag. 2009, 57, 894–899. [Google Scholar] [CrossRef]
- Laird, E.R.; Ferguson, K. Dielectric properties of some animal tissues at meter and centimeter wave lengths. Can. J. Res. 1949, 27a, 218–230. [Google Scholar] [CrossRef]
- Farag, K.; Lyng, J.; Morgan, D.; Cronin, D. Dielectric and thermophysical properties of different beef meat blends over a temperature range of −18 to +10 °C. Meat Sci. 2008, 79, 740–747. [Google Scholar] [CrossRef]
- Brunton, N.; Lyng, J.; Zhang, L.; Jacquier, J. The use of dielectric properties and other physical analyses for assessing protein denaturation in beef biceps femoris muscle during cooking from 5 to 85 °C. Meat Sci. 2006, 72, 236–244. [Google Scholar] [CrossRef]
- Brandy, M.M.; Symons, S.A.; Stuchly, S.S. Dielectric Behavior of Selected Animal Tissues in Vitro at Frequencies from 2 to 4 GHz. IEEE Trans. Biomed. Eng. 1981, BME-28, 305–307. [Google Scholar] [CrossRef]
- Tran, V.; Stuchly, S. Dielectric Properties of Beet, Beer Liver, Chicken and Salmon at Frequencies from 100 to 2500 MHz. J. Microw. Power Electromagn. Energy 1987, 22, 29–33. [Google Scholar] [CrossRef]
- Cui, W.; Liu, R.; Wang, L.; Wang, M.; Zheng, H.; Li, E. Design of Wideband Implantable Antenna for Wireless Capsule Endoscope System. IEEE Antennas Wirel. Propag. Lett. 2019, 18, 2706–2710. [Google Scholar] [CrossRef]
- Ketavath, K.N.; Gopi, D.; Rani, S.S. In-Vitro Test of Miniaturized CPW-Fed Implantable Conformal Patch Antenna at ISM Band for Biomedical Applications. IEEE Access 2019, 7, 43547–43554. [Google Scholar] [CrossRef]
- Shah, I.A.; Zada, M.; Yoo, H. Design and Analysis of a Compact-Sized Multiband Spiral-Shaped Implantable Antenna for Scalp Implantable and Leadless Pacemaker Systems. IEEE Trans. Antennas Propag. 2019, 67, 4230–4234. [Google Scholar] [CrossRef]
- Kangeyan, R.; Karthikeyan, M. Implantable dual band semi-circular slotted patch with DGS antenna for biotelemetry applications. Microw. Opt. Technol. Lett. 2023, 65, 225–230. [Google Scholar] [CrossRef]
- Hayat, S.; Shah, S.A.A.; Yoo, H. Miniaturized Dual-Band Circularly Polarized Implantable Antenna for Capsule Endoscopic System. IEEE Trans. Antennas Propag. 2020, 69, 1885–1895. [Google Scholar] [CrossRef]




| Parameters | Values (mm) | Parameters | Values (mm) |
|---|---|---|---|
| W | 5 | S6 | 2.2 |
| L | 5 | S7 | 0.3 |
| S1 | 4.2 | S8 | 0.3 |
| S2 | 0.3 | S9 | 2.25 |
| S3 | 1.5 | S10 | 1.8 |
| S4 | 1.15 | S11 | 2.55 |
| S5 | 0.3 | G | 1 |
| Human Tissue | Max. SAR (W/Kg) | Max. Allowed Input Power (mW) | ||
|---|---|---|---|---|
| 1 g | 10 g | 1 g | 10 g | |
| Muscle | 712.2 | 78.86 | 2.8 | 20.28 |
| Kidney | 771.4 | 82.98 | 2.59 | 19.28 |
| Liver | 758.2 | 83.4 | 2.64 | 19.18 |
| Brain | 788.7 | 85.24 | 2.535 | 18.77 |
| Skin | 715.7 | 77.6 | 2.79 | 20.6 |
| Muscle | 712.2 | 78.86 | 2.8 | 20.28 |
| Transmission | Receiver | ||
|---|---|---|---|
| Frequency (GHz) | 2.45 | Rx antennae gain Gr (dBi) | 2.15 |
| Tx power (dBm) | −40 | PLF (dB) | 1 |
| Tx antenna gain, Gt (dBi) | Scenario dependent | Temperature T0 (K) | 293 |
| Boltzmann constant (K) | 1.38 × 10−23 | ||
| Signal quality | |||
| Bit rate Br (Kb/s) | 100/1000 | ||
| Bit error rate | 1 × 10−5 | ||
| Eb/N0 (ideal PSK) (dB) | 9.6 | ||
| Coding gain Gc (dB) | 0 | ||
| Ref. | Freq. (GHz) | Volume mm3 | BW (MHz) | Gain (dBi) | SAR W/kg | Implantation Scenario | Depth (mm) | Operation Condition |
|---|---|---|---|---|---|---|---|---|
| [56] | 2.45 | 99.75 | 520 | −26.4 | 712 (1 g) | skin | 4 | homogeneous |
| [57] | 2.6 | 37 | 400 | −19.7 | 0.7 (10 g) | skin | 4 | homogeneous |
| [58] | 2.45 | 17.15 | 219 | −18.2 | 305 (1 g) | scalp | 3 | homogeneous |
| [59] | 1.42 and 2.42 GHz | 91.4 | 140/240 | −29.4 −21.2 | 500 (1 g) 686 (1 g) | skin | 4 | homogeneous |
| [60] | 2.45 | 2.11 | 152.8 | −24.5 | 233 (1 g) | muscle | 50 | homogeneous |
| Current work | 2.45 | 6.5 | 520, 510, 656, 610, 664 | −24, −26, −25, −31, −30 | 715.7 712.2 788.7 758.2 771.39 | Skin, muscle, brain, liver, kidney | 2 9 11 29 29 | heterogeneous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaki, A.Z.A.; Hamad, E.K.I.; Abouelnaga, T.G.; Elsadek, H.A.; Khaleel, S.A.; Al-Gburi, A.J.A.; Zakaria, Z. Design and Modeling of Ultra-Compact Wideband Implantable Antenna for Wireless ISM Band. Bioengineering 2023, 10, 216. https://doi.org/10.3390/bioengineering10020216
Zaki AZA, Hamad EKI, Abouelnaga TG, Elsadek HA, Khaleel SA, Al-Gburi AJA, Zakaria Z. Design and Modeling of Ultra-Compact Wideband Implantable Antenna for Wireless ISM Band. Bioengineering. 2023; 10(2):216. https://doi.org/10.3390/bioengineering10020216
Chicago/Turabian StyleZaki, Ahmed Z. A., Ehab K. I. Hamad, Tamer Gaber Abouelnaga, Hala A. Elsadek, Sherif A. Khaleel, Ahmed Jamal Abdullah Al-Gburi, and Zahriladha Zakaria. 2023. "Design and Modeling of Ultra-Compact Wideband Implantable Antenna for Wireless ISM Band" Bioengineering 10, no. 2: 216. https://doi.org/10.3390/bioengineering10020216
APA StyleZaki, A. Z. A., Hamad, E. K. I., Abouelnaga, T. G., Elsadek, H. A., Khaleel, S. A., Al-Gburi, A. J. A., & Zakaria, Z. (2023). Design and Modeling of Ultra-Compact Wideband Implantable Antenna for Wireless ISM Band. Bioengineering, 10(2), 216. https://doi.org/10.3390/bioengineering10020216












