Multi-Layered Non-Local Bayes Model for Lung Cancer Early Diagnosis Prediction with the Internet of Medical Things
Abstract
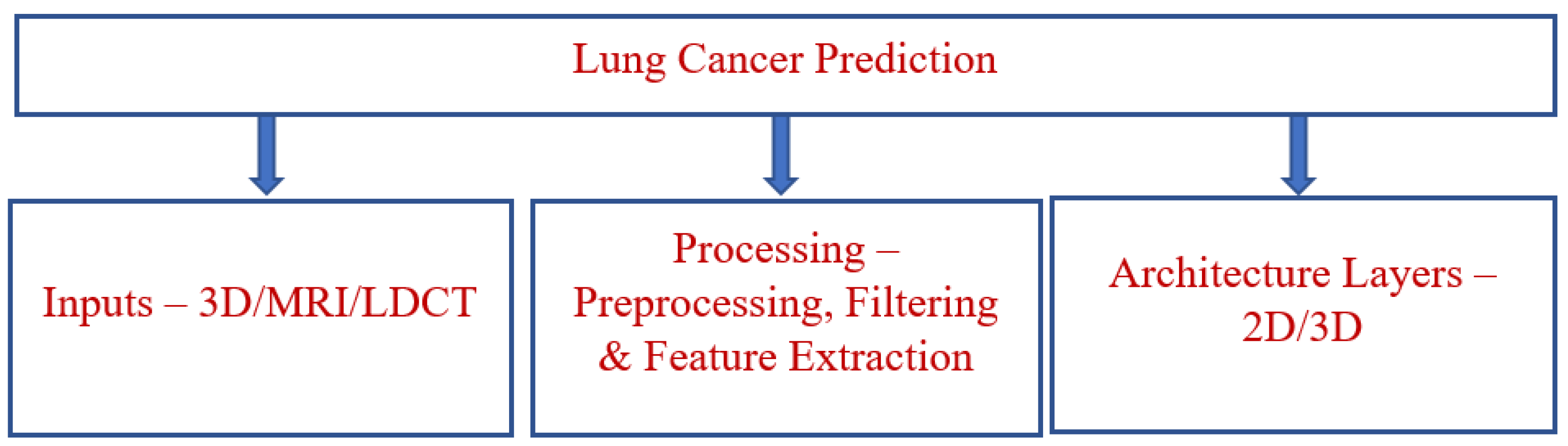
1. Introduction
- i.
- Processing images—removing unwanted data or noise that are present in the image [5].
- ii.
- Extracting images—converting images into specific groups or sectors of input resources.
- iii.
- Choosing features—enhancing the response time and execution time compared with the distinct resultant values of image processing.
2. Literature Survey
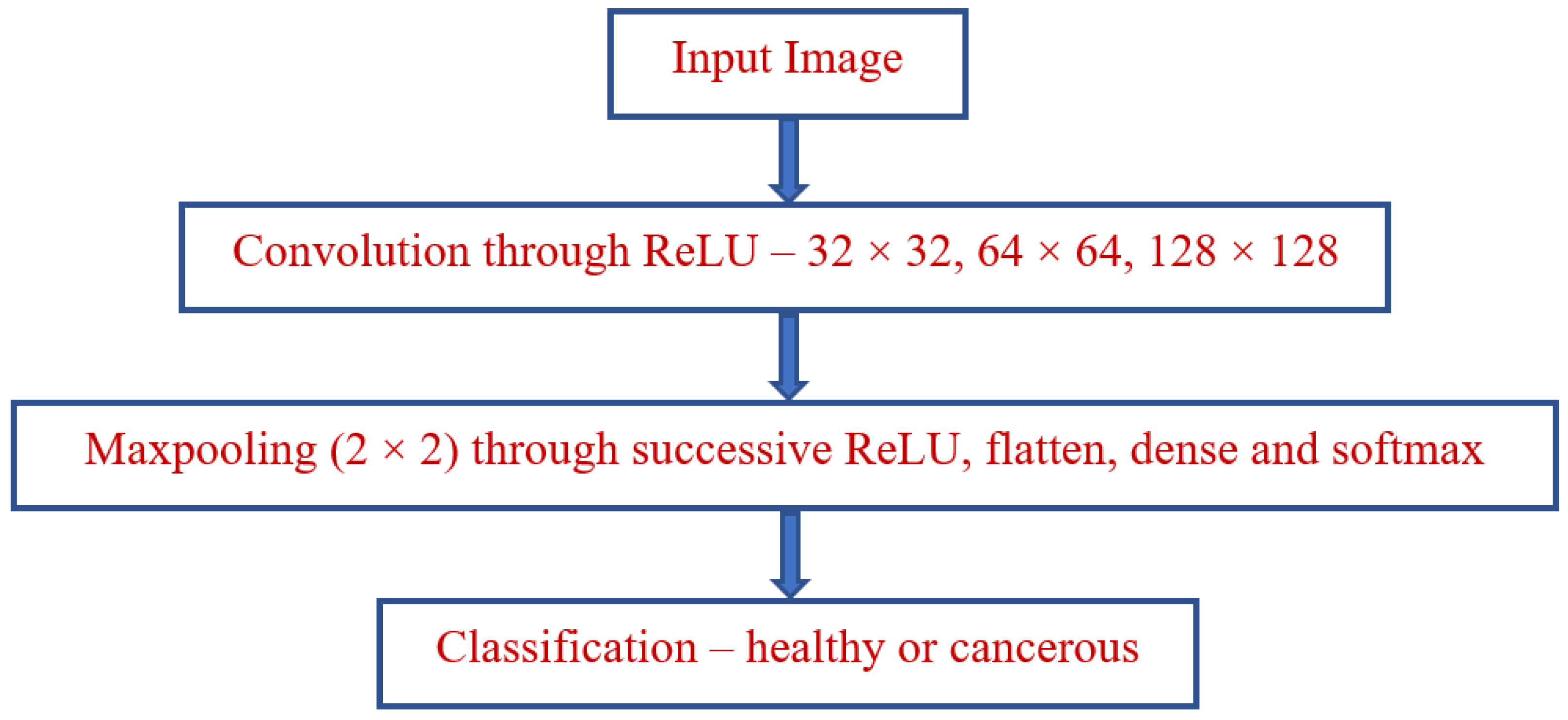
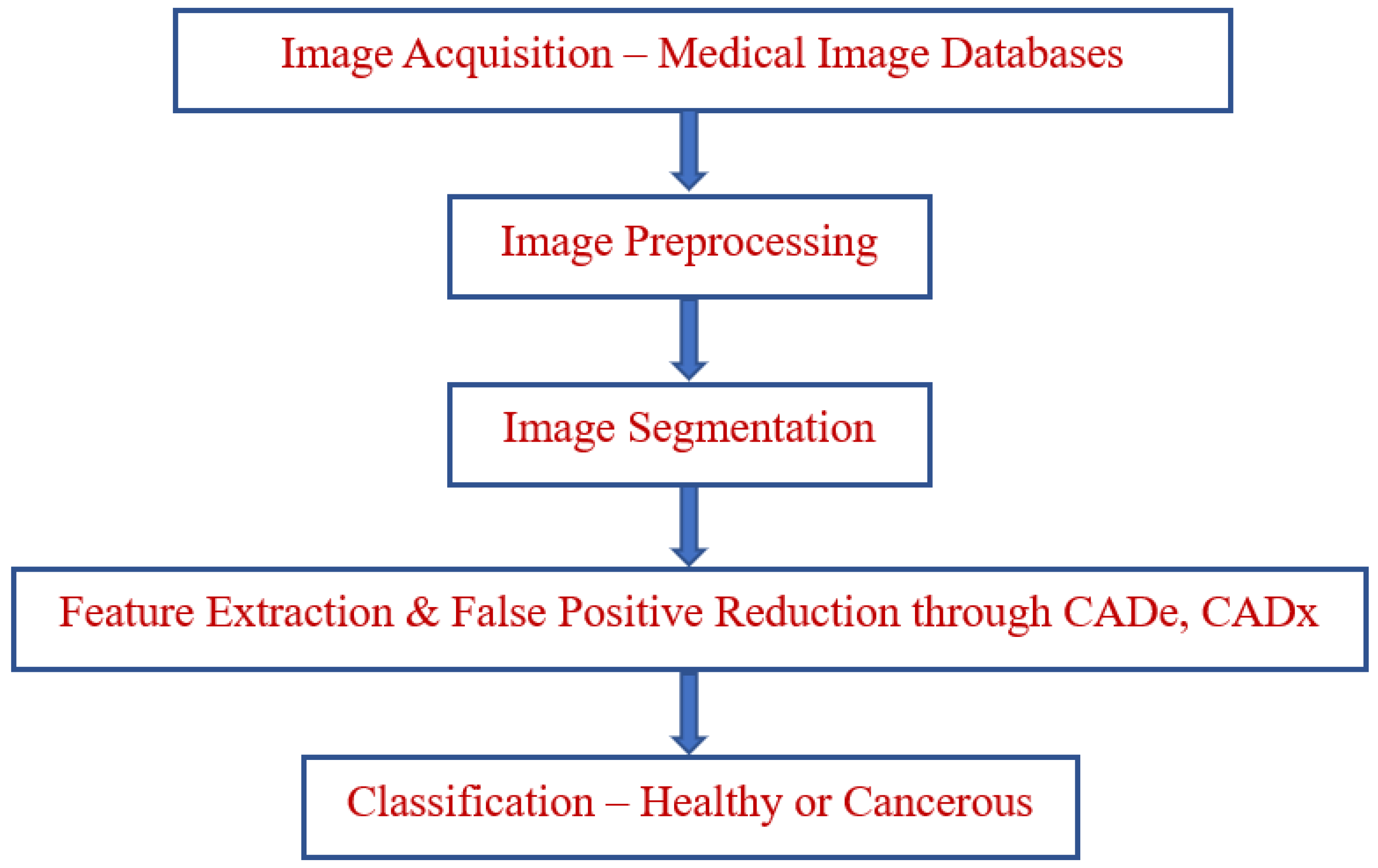
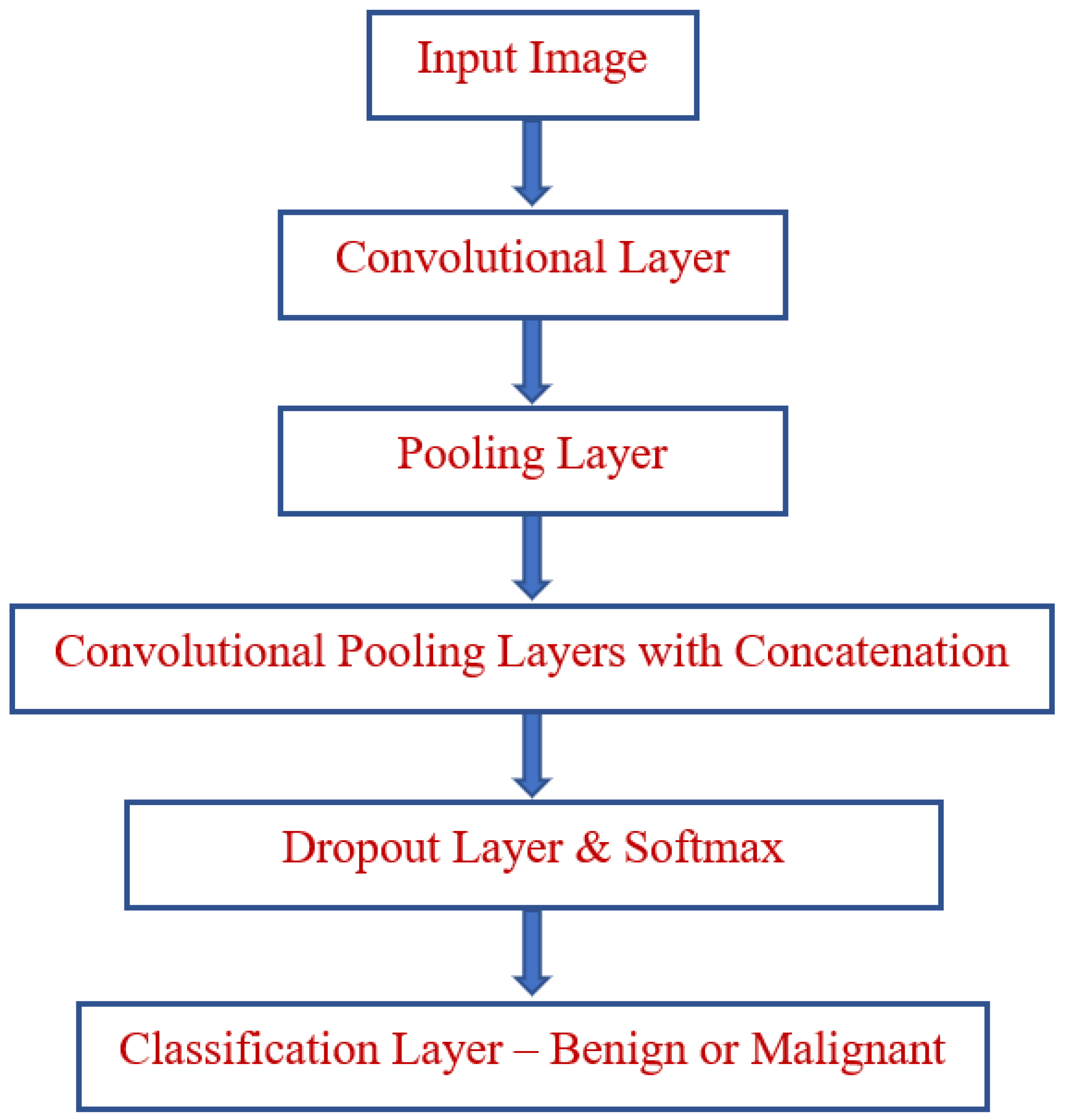
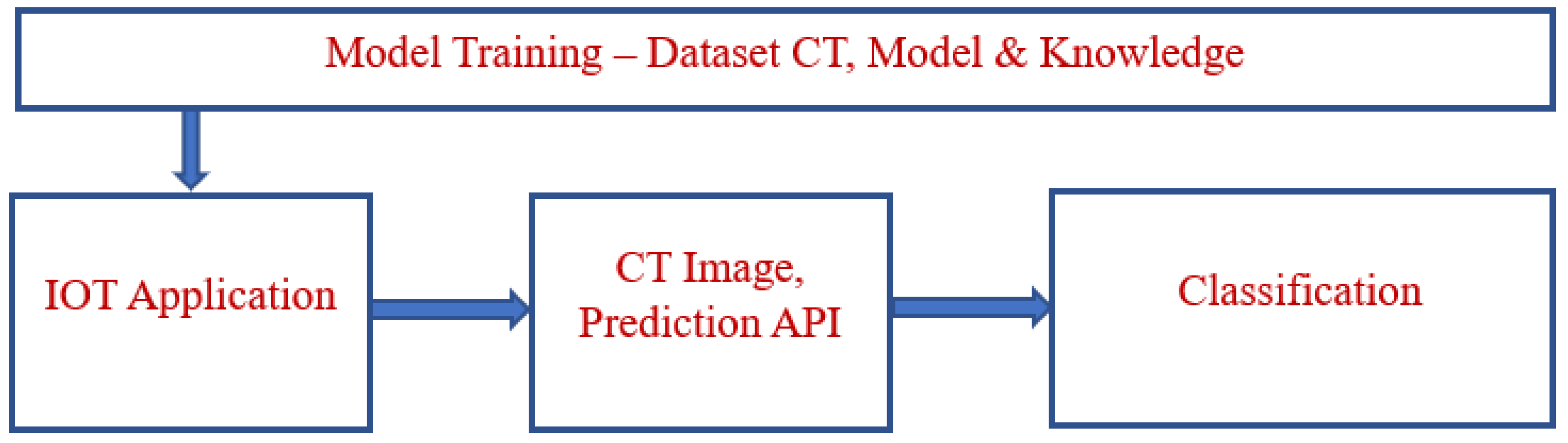
3. Proposed Model
3.1. Preprocessing
3.2. Feature Extraction
3.3. Domain Classification
3.4. Algorithm of Proposed Model
| Algorithm 1: Proposed Model | |||||||||
| 1: Let be an ideal image. Define the noisy image corrupted with Gaussian noise ‘a’, noise factor n, and standard deviation σ using | |||||||||
| (1) | |||||||||
| 2: Evaluate the conditional probability distribution using | |||||||||
| (2) | |||||||||
| 3: Determine the observed noise version Pn given P0, the noiseless patch of a0 using | |||||||||
| where | (3) | ||||||||
| 4: Define the Euclidean norm of as . Compute using the Bayes rule. | |||||||||
| (4) | |||||||||
| 5: For a normalization constant , the initial cluster , iteration t, find the direct path, to construct cluster of Gaussian samples. | |||||||||
| (5) | |||||||||
| 6: Evaluate . | |||||||||
| (6) | |||||||||
| 7: Find the posterior estimation | |||||||||
| (7) | |||||||||
| 8: Determine the maximum posterior estimation using | |||||||||
| (8) | |||||||||
| 9: Evaluate | |||||||||
| (9) | |||||||||
| 10: Update using | |||||||||
| (10) | |||||||||
| 11: Estimate using sampling estimates, neighbor patches . | |||||||||
| (11) | |||||||||
| (12) | |||||||||
| 12: Approximate using the covariance matrix of noisy patches | |||||||||
| (13) | |||||||||
| 13: Apply the Bayes method. | |||||||||
| (14) | |||||||||
| 14: Perform grey level image—denoising process for different values of . | |||||||||
| Type | = 2 | = 5 | = 10 | = 20 | = 30 | = 40 | = 60 | = 80 | = 100 |
| Classic | 46.14 | 40.89 | 35.57 | 32.46 | 30.31 | 27.63 | 26.03 | 26.03 | 25.41 |
| Iteration | 46.19 | 40.12 | 36.62 | 33.60 | 29.67 | 27.51 | 26.24 | 26.24 | 25.72 |
| Patch size: | |||||||||
| (15) | |||||||||
| 15: Find a similar pattern retained: | |||||||||
| (16) | |||||||||
| 16: Compute the size of the search area: | |||||||||
| , | (17) | ||||||||
| 17: Set the threshold as | |||||||||
| (18) | |||||||||
| (19) | |||||||||
| 18: Estimate using | |||||||||
| , | (20) | ||||||||
| (21) | |||||||||
| (22) | |||||||||
| 19: Evaluate the huge set of original patches using minimum mean square estimation | |||||||||
| (23) | |||||||||
| (24) | |||||||||
| (25) | |||||||||
| 20: Evaluate using wavelet neighborhood de-noising. | |||||||||
| (26) | |||||||||
| (27) | |||||||||
| (28) | |||||||||
| = | (29) | ||||||||
| 21: Perform the restoration using | |||||||||
| (30) | |||||||||
| 22: Calculate with index using | |||||||||
| (31) | |||||||||
| (32) | |||||||||
4. Simulation and Analysis
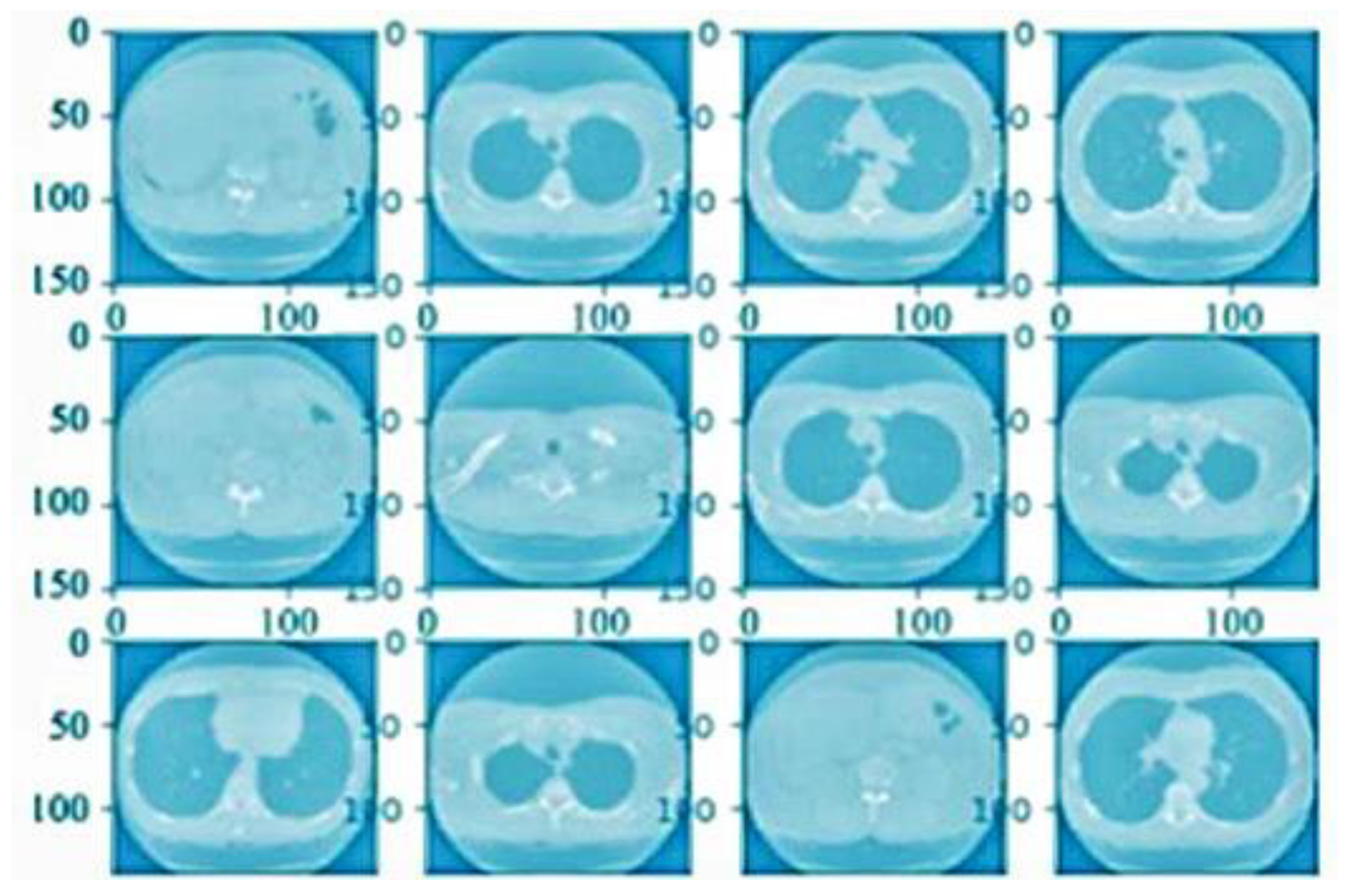
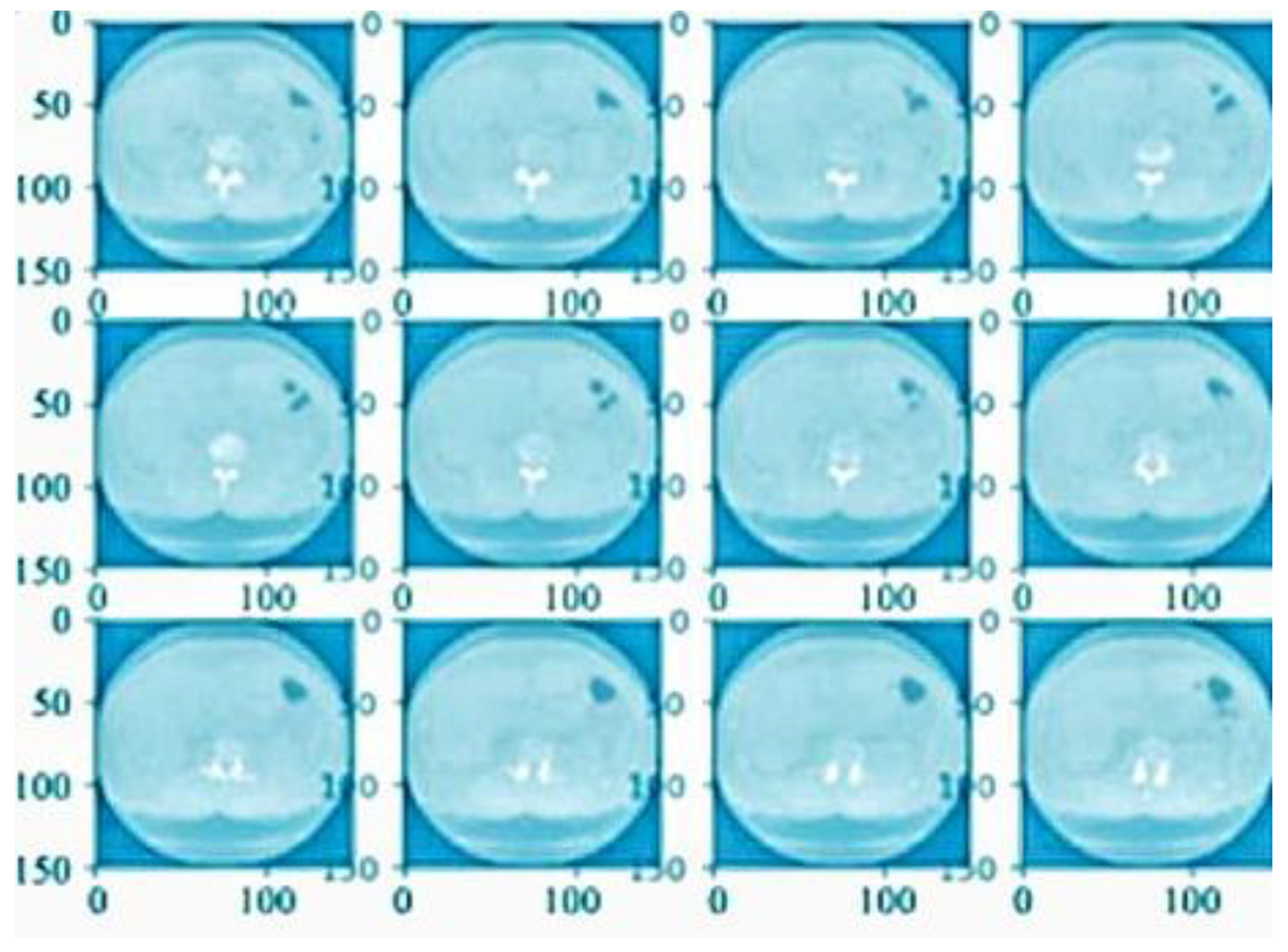
4.1. Preprocessing Analysis
4.2. Image Level Balancing
4.3. Noise Removal Analysis
4.4. Results and Discussion
- ▪
- ▪
- The best typical values of for the gray-level image de-noising process lay in (2, 100).
- ▪
- 87% of the high risk was detected with the highest sensitivity (TP rate) and specificity (TN rate) of 98% compared to the LR models.
- ▪
- The proposed model provided a low error rate (2%, 5%) and an increase in the number of instance values.
- ▪
- The range for smaller patch sizes was randomly defined with three intervals. When the patch size exceeded 2 with three intervals for the considered high-resolution images, variations were observed in restoration and performance measures. Hence, the patch size was set as 2, and the best random intervals were obtained through the simulation.
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Nanehkaran, Y.A.; Fard, M.K. A Method for Optimal Detection of Lung Cancer Based on Deep Learning Optimized by Marine Predators Algorithm. Comput. Intell. Neurosci. 2021, 2021, 3694723. [Google Scholar] [CrossRef]
- Kadir, T.; Gleeson, F. Lung cancer prediction using machine learning and advanced imaging techniques. Transl. Lung Cancer Res. 2018, 7, 304–312. [Google Scholar] [CrossRef]
- Wang, S.; Chen, A.; Yang, L.; Cai, L.; Xie, Y.; Fujimoto, J.; Gazdar, A.; Xiao, G. Comprehensive analysis of lung cancer pathology images to discover tumor shape and boundary features that predict survival outcome. Sci. Rep. 2018, 8, 10393. [Google Scholar] [CrossRef]
- Ausawalaithong, W.; Marukatat, S.; Thirach, A.; Wilaiprasitporn, T. Automatic Lung Cancer Prediction from Chest X-ray Images Using the Deep Learning Approach. In Proceedings of the 2018 11th Biomedical Engineering International Conference (BMEiCON), Chaing Mai, Thailand, 21–24 November 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Baek, S.; He, Y.; Allen, B.G.; Buatti, J.M.; Smith, B.J.; Tong, L.; Sun, Z.; Wu, J.; Diehn, M.; Loo, B.W.; et al. Deep segmentation networks predict survival of non-small cell lung cancer. Sci. Rep. 2019, 9, 17286. [Google Scholar] [CrossRef]
- Liu, M.; Dong, J.; Dong, X.; Yu, H.; Qi, L. Segmentation of Lung Nodule in CT Images Based on Mask R-CNN. In Proceedings of the 2018 9th International Conference on Awareness Science and Technology, Fukuoka, Japan, 19–21 September 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Moitra, D.; Mandal, R.K. Automated AJCC (7th edition) staging of non-small cell lung cancer (NSCLC) using deep convolutional neural network (CNN) and recurrent neural network (RNN). Health Inf. Sci. Syst. 2019, 7, 14. [Google Scholar] [CrossRef]
- Soh, P.W.; Chang, J.W.; Huang, J.W. Adaptive Deep Learning-Based Air Quality Prediction Model Using the Most Relevant Spatial-Temporal Relations. IEEE Access 2018, 6, 38186–38199. [Google Scholar] [CrossRef]
- Sharma, M.; Bhatt, J.S.; Joshi, M.V. Early detection of lung cancer from CT images: Nodule segmentation and classification using deep learning. Int. Conf. Mach. Vis. 2018, 2, 29. [Google Scholar] [CrossRef]
- Chen, C.L.; Chen, C.C.; Yu, W.H.; Chen, S.H.; Chang, Y.C.; Hsu, T.I.; Hsiao, M.; Yeh, C.Y.; Chen, C.Y. An annotation-free whole-slide training approach to pathological classification of lung cancer types using deep learning. Nat. Commun. 2021, 12, 1193. [Google Scholar] [CrossRef]
- Dritsas, E.; Trigka, M. Lung Cancer Risk Prediction with Machine Learning Models. Big Data Cogn. Comput. 2022, 6, 139. [Google Scholar] [CrossRef]
- Capuano, R.; Catini, A.; Paolesse, R.; Di Natale, C. Sensors for Lung Cancer Diagnosis. J. Clin. Med. 2019, 8, 235. [Google Scholar] [CrossRef]
- Causey, J.L.; Zhang, J.; Ma, S.; Jiang, B.; Qualls, J.A.; Politte, D.G.; Prior, F.; Zhang, S.; Huang, X. Highly accurate model for prediction of lung nodule malignancy with CT scans. Sci. Rep. 2018, 8, 9286. [Google Scholar] [CrossRef]
- Gu, Y.; Lu, X.; Yang, L.; Zhang, B.; Yu, D.; Zhao, Y.; Gao, L.; Wu, L.; Zhou, T. Automatic lung nodule detection using a 3D deep convolutional neural network combined with a multi-scale prediction strategy in chest CTs. Comput. Biol. Med. 2018, 103, 220–231. [Google Scholar] [CrossRef]
- Masood, A.; Sheng, B.; Li, P.; Hou, X.; Wei, X.; Qin, J.; Feng, D. Computer-Assisted Decision Support System in Pulmonary Cancer detection and stage classification on CT images. J. Biomed. Inform. 2018, 79, 117–128. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Gan, C.; Lin, H.; Dou, Q.; Tsougenis, E.; Huang, Q.; Cai, M.; Heng, P.A. Weakly Supervised Deep Learning for Whole Slide Lung Cancer Image Analysis. IEEE Trans Cybern. 2020, 50, 3950–3962. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Chao, H.-S.; Chen, Y.-M. Application of Artificial Intelligence in Lung Cancer. Cancers 2022, 14, 1370. [Google Scholar] [CrossRef]
- Dipanjan, M.; Rakesh, K.M. Classification of non-small cell lung cancer using one-dimensional convolutional neural network. 2020, 159, 113564. Expert Syst. Appl. 2020, 159, 113564. [Google Scholar] [CrossRef]
- de Souza, L.; Silva, I.C.L.; Marques, A.G.; Silva, F.H.D.S.; Nunes, V.X.; Hassan, M.M.; Albuquerque, V.H.C.; Filho, P.P.R. Internet of Medical Things: An Effective and Fully Automatic IoT Approach Using Deep Learning and Fine-Tuning to Lung CT Segmentation. Sensors 2020, 20, 6711. [Google Scholar] [CrossRef]
- Paul, R.; Hawkins, S.H.; Schabath, M.B.; Gillies, R.J.; Hall, L.O.; Goldgof, D.B. Predicting malignant nodules by fusing deep features with classical radiomics features. J. Med. Imaging 2018, 5, 011021. [Google Scholar] [CrossRef]
- Murphy, A.; Skalski, M.; Gaillard, F. The utilisation of convolutional neural networks in detecting pulmonary nodules: A review. Br. J. Radiol. 2018, 91, 20180028. [Google Scholar] [CrossRef]
- Tau, N.; Stundzia, A.; Yasufuku, K.; Hussey, D.; Metser, U. Convolutional Neural Networks in Predicting Nodal and Distant Metastatic Potential of Newly Diagnosed Non-Small Cell Lung Cancer on FDG PET Images. AJR Am. J. Roentgenol. 2020, 215, 192–197. [Google Scholar] [CrossRef]
- Hosseini, H.R. Deep Learning Applications for Lung Cancer Diagnosis: A systematic review. arXiv 2022, arXiv:2201.00227. [Google Scholar]
- Faruqui, N.; Yousuf, M.A.; Whaiduzzaman, M.; Azad, A.K.M.; Barros, A.; Moni, M.A. LungNet: A hybrid deep-CNN model for lung cancer diagnosis using CT and wearable sensor-based medical IoT data. Comput. Biol. Med. 2021, 139, 104961. [Google Scholar] [CrossRef]
- Karthikeyani, K.; Selvakumar, T.; Ananthajothi, K. Design of Convolutional Neural Network for Lung Cancer Diagnosis. Ann. Rom. Soc. Cell Biol. 2021, 25, 7630. [Google Scholar]
- Riquelme, D.; Akhloufi, M.A. Deep Learning for Lung Cancer Nodules Detection and Classification in CT Scans. Ai 2020, 1, 28–67. [Google Scholar] [CrossRef]
- Bharati, S.; Podder, P.; Mondal, M.R.H. Hybrid deep learning for detecting lung diseases from X-ray images. Inf. Med. Unlocked 2020, 20, 100391. [Google Scholar] [CrossRef]
- Pragya, C.; Anuj, J.; Meet, V.; Varsha, N. Prediction and Classification of Lung Cancer Using. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1099, 012059. [Google Scholar] [CrossRef]
- Vijh, S.; Gaurav, P.; Pandey, H.M. Hybrid bio-inspired algorithm and convolutional neural network for automatic lung tumor detection. Neural Comput. Appl. 2020, 1–14. [Google Scholar] [CrossRef]
- Cai, L.; Gao, J.; Zhao, D. A review of the application of deep learning in medical image classification and segmentation. Ann. Transl. Med. 2020, 8, 713. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 360–367. [Google Scholar] [CrossRef]
- Marappan, R.; Sethumadhavan, G. Solution to Graph Coloring Using Genetic and Tabu Search Procedures. Arab. J. Sci. Eng. 2018, 43, 525–542. [Google Scholar] [CrossRef]
- Marappan, R.; Sethumadhavan, G. Complexity Analysis and Stochastic Convergence of Some Well-known Evolutionary Operators for Solving Graph Coloring Problem. Mathematics 2020, 8, 303. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Marappan, R. Design and analysis of an efficient machine learning based hybrid recommendation system with enhanced density-based spatial clustering for digital e-learning applications. Complex Intell. Syst. 2021, 1–17. [Google Scholar] [CrossRef]
- Marappan, R.; Sethumadhavan, G. A New Genetic Algorithm for Graph Coloring. In Proceedings of the 2013 Fifth International Conference on Computational Intelligence, Modelling and Simulation, Seoul, Republic of Korea, 24–25 September 2013; pp. 49–54. [Google Scholar] [CrossRef]
- Sethumadhavan, G.; Marappan, R. A genetic algorithm for graph coloring using single parent conflict gene crossover and mutation with conflict gene removal procedure. In Proceedings of the 2013 IEEE International Conference on Computational Intelligence and Computing Research, Enathi, India, 26–28 December 2013; pp. 1–6. [Google Scholar] [CrossRef]
- Marappan, R.; Sethumadhavan, G. Divide and conquer based genetic method for solving channel allocation. In Proceedings of the 2016 International Conference on Information Communication and Embedded Systems (ICICES), Chennai, India, 25–26 February 2016; pp. 1–5. [Google Scholar] [CrossRef]
- Yao, Y.; Xia, C.; Li, J.; Li, Q. Head CT Image Convolution Feature Segmentation and Morphological Filtering for Densely Matching Points of IoTs. IEEE Access 2020, 8, 12521–12532. [Google Scholar] [CrossRef]
- Dourado, C.M., Jr.; da Silva, S.P.P.; da Nobrega, R.V.M.; Barros, A.C.D.S.; Reboucas Filho, P.P.; de Albuquerque, V.H.C. Deep learning IoT system for online stroke detection in skull computed tomography images. Comput. Netw. 2019, 152, 25–39. [Google Scholar] [CrossRef]
- Souza, L.F.D.F.; Holanda, G.B.; Silva, F.H.D.S.; Alves, S.S. Automatic lung segmentation in CT images using mask R-CNN for mapping the feature extraction in supervised methods of machine learning using transfer learning. Int. J. Hybrid Intell. Syst. 2020, 16, 189–205. [Google Scholar] [CrossRef]
- Han, T.; Nunes, V.X.; Souza, L.F.D.F.; Marques, A.G.; Silva, I.C.L.; Junior, M.A.A.F.; Sun, J.; Rebouças Filho, P.P. Internet of Medical Things—Based on Deep Learning Techniques for Segmentation of Lung and Stroke Regions in CT Scans. IEEE Access 2020, 8, 71117–71135. [Google Scholar] [CrossRef]
- Bajwa, M.N.; Muta, K.; Malik, M.I.; Siddiqui, S.A.; Braun, S.A.; Homey, B.; Dengel, A.; Ahmed, S. Computer-Aided Diagnosis of Skin Diseases Using Deep Neural Networks. Appl. Sci. 2020, 10, 2488. [Google Scholar] [CrossRef]


| Types | Feature Name |
|---|---|
| Texture features | Correlation, Contrast, Homogeneity, Sum of square variance, Spectral, Spatial, and Entropy |
| Shape features | Area, Irregularity, Roundness, Perimeter, Circularity |
| Intensity features | Intensity, Mean, Standard Variance, Kurtosis, Skewness, Median |
| Geometric features | Eccentricity, Compactness, Roughness, Local Area Integral Invariant, Radial Distance Signatures |
| Parameters | Other Models | Proposed Model |
|---|---|---|
| Accuracy | 84.02% | 92.2% |
| Specificity | 85.34% | 98.8% |
| Sensitivity | 82.71% | 99.4% |
| Parameters | Other Models | Proposed Model |
|---|---|---|
| Accuracy | (58%, 72%) | (81%, 95%) |
| Specificity | (60%, 85%) | (83%, 95%) |
| Sensitivity | (24%, 67%) | (80%, 95%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain Ali, Y.; Chinnaperumal, S.; Marappan, R.; Raju, S.K.; Sadiq, A.T.; Farhan, A.K.; Srinivasan, P. Multi-Layered Non-Local Bayes Model for Lung Cancer Early Diagnosis Prediction with the Internet of Medical Things. Bioengineering 2023, 10, 138. https://doi.org/10.3390/bioengineering10020138
Hussain Ali Y, Chinnaperumal S, Marappan R, Raju SK, Sadiq AT, Farhan AK, Srinivasan P. Multi-Layered Non-Local Bayes Model for Lung Cancer Early Diagnosis Prediction with the Internet of Medical Things. Bioengineering. 2023; 10(2):138. https://doi.org/10.3390/bioengineering10020138
Chicago/Turabian StyleHussain Ali, Yossra, Seelammal Chinnaperumal, Raja Marappan, Sekar Kidambi Raju, Ahmed T. Sadiq, Alaa K. Farhan, and Palanivel Srinivasan. 2023. "Multi-Layered Non-Local Bayes Model for Lung Cancer Early Diagnosis Prediction with the Internet of Medical Things" Bioengineering 10, no. 2: 138. https://doi.org/10.3390/bioengineering10020138
APA StyleHussain Ali, Y., Chinnaperumal, S., Marappan, R., Raju, S. K., Sadiq, A. T., Farhan, A. K., & Srinivasan, P. (2023). Multi-Layered Non-Local Bayes Model for Lung Cancer Early Diagnosis Prediction with the Internet of Medical Things. Bioengineering, 10(2), 138. https://doi.org/10.3390/bioengineering10020138









