Biological Prosthesis (Hollow 3D-Printed Titanium Custom-Made Prosthesis and Bone Graft) for Humeral Reconstruction in Pediatric Oncologic Patients: Surgical Indications and Results
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dome, J.S.; Rodriguez-Galindo, C.; Spunt, S.L.; Santana, V.M. 92—Pediatric Solid Tumors. In Abeloff’s Clinical Oncology, 6th ed.; Niederhuber, J.E., Armitage, J.O., Kastan, M.B., Doroshow, J.H., Tepper, J.E., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 1703–1747.e1711. [Google Scholar] [CrossRef]
- Abed, R.; Grimer, R. Surgical modalities in the treatment of bone sarcoma in children. Cancer Treat. Rev. 2010, 36, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. BioMed. Eng. OnLine 2021, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Dürr, H.R.; Bakhshai, Y.; Rechl, H.; Tunn, P.U. Resection margins in bone tumors: What is adequate? Unfallchirurg 2014, 117, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ogink, P.T.; Teunissen, F.R.; Massier, J.R.; Raskin, K.A.; Schwab, J.H.; Lozano-Calderon, S.A. Allograft reconstruction of the humerus: Complications and revision surgery. J. Surg. Oncol. 2019, 119, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Barbier, D.; De Billy, B.; Gicquel, P.; Bourelle, S.; Journeau, P. Is the clavicula pro humero technique of value for reconstruction after resection of the proximal humerus in children? Clin. Orthop. Relat. Res. 2017, 475, 2550–2561. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Delcroix, L.; Manfrini, M.; Ceruso, M.; Capanna, R. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J. Bone Jt. Surg. Am. 2004, 86, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Bus, M.P.; van de Sande, M.A.; Taminiau, A.H.; Dijkstra, P.D. Is there still a role for osteoarticular allograft reconstruction in musculoskeletal tumour surgery? A long-term follow-up study of 38 patients and systematic review of the literature. Bone Jt. J. 2017, 99-b, 522–530. [Google Scholar] [CrossRef]
- Hopyan, S. Reconstruction for bone tumours of the shoulder and humerus in children and adolescents. J. Child. Orthop. 2021, 15, 358–365. [Google Scholar] [CrossRef]
- Tsuda, Y.; Fujiwara, T.; Stevenson, J.D.; Parry, M.C.; Tillman, R.; Abudu, A. The long-term results of extendable endoprostheses of the humerus in children after the resection of a bone sarcoma. Bone Jt. J. 2020, 102-b, 64–71. [Google Scholar] [CrossRef]
- Wafa, H.; Reddy, K.; Grimer, R.; Abudu, A.; Jeys, L.; Carter, S.; Tillman, R. Does total humeral endoprosthetic replacement provide reliable reconstruction with preservation of a useful extremity? Clin. Orthop. Relat. Res. 2015, 473, 917–925. [Google Scholar] [CrossRef]
- Sanchez-Sotelo, J.; Wagner, E.R.; Sim, F.H.; Houdek, M.T. Allograft-prosthetic composite reconstruction for massive proximal humeral bone loss in reverse shoulder arthroplasty. J. Bone Jt. Surg. Am. 2017, 99, 2069–2076. [Google Scholar] [CrossRef]
- Beltrami, G.; Ristori, G.; Nucci, A.M.; Galeotti, A.; Tamburini, A.; Scoccianti, G.; Campanacci, D.; Innocenti, M.; Capanna, R. Custom-made 3D-printed implants as novel approach to reconstructive surgery after oncologic resection in pediatric patients. J. Clin. Med. 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure mode classification for tumor endoprostheses: Retrospective review of five institutions and a literature review. J. Bone Jt. Surg. Am. 2011, 93, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Gautam, D.; Arora, N.; Gupta, S.; George, J.; Malhotra, R. Megaprosthesis Versus Allograft Prosthesis Composite for the Management of Massive Skeletal Defects: A Meta-Analysis of Comparative Studies. Curr. Rev. Musculoskelet. Med. 2021, 14, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Malhotra, R. Megaprosthesis versus allograft prosthesis composite for massive skeletal defects. J. Clin. Orthop. Trauma 2018, 9, 63–80. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Dickinson, I.C. Functional outcome of arthrodesis with a vascularized fibular graft and a rotational latissimus dorsi flap after proximal humerus sarcoma resection. Ann. Surg. Oncol. 2011, 18, 1852–1859. [Google Scholar] [CrossRef]
- Innocenti, M.; Ceruso, M.; Manfrini, M.; Angeloni, R.; Lauri, G.; Capanna, R.; Bufalini, C. Free vascularized growth-plate transfer after bone tumor resection in children. J. Reconstr. Microsurg. 1998, 14, 137–143. [Google Scholar] [CrossRef]
- Stevenson, J.D.; Doxey, R.; Abudu, A.; Parry, M.; Evans, S.; Peart, F.; Jeys, L. Vascularized fibular epiphyseal transfer for proximal humeral reconstruction in children with a primary sarcoma of bone. Bone Jt. J. 2018, 100-b, 535–541. [Google Scholar] [CrossRef]
- Puri, A.; Gulia, A.; Agarwal, M.; Jambhekar, N.; Laskar, S. Extracorporeal irradiated tumor bone: A reconstruction option in diaphyseal Ewing’s sarcomas. Indian J. Orthop. 2010, 44, 390–396. [Google Scholar] [CrossRef]
- Gupta, S.; Kafchinski, L.A.; Gundle, K.R.; Saidi, K.; Griffin, A.M.; Wunder, J.S.; Ferguson, P.C. Intercalary allograft augmented with intramedullary cement and plate fixation is a reliable solution after resection of a diaphyseal tumour. Bone Jt. J. 2017, 99-b, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Pazourek, L.; Tomáš, T.; Mahdal, M.; Janíček, P.; Černý, J.; Ondrůšek, Š. Use of Solid Intercalary Allografts for Reconstruction Following the Resection of Primary Bone Tumors. Acta Chir. Orthop. Traumatol. Cechoslov. 2018, 85, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Goulding, K.A.; Schwartz, A.; Hattrup, S.J.; Randall, R.L.; Lee, D.; Rispoli, D.M.; Lerman, D.M.; Beauchamp, C. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: A viable option in the upper extremity. Clin. Orthop. Relat. Res. 2017, 475, 1702–1711. [Google Scholar] [CrossRef]
- Henrichs, M.P.; Liem, D.; Gosheger, G.; Streitbuerger, A.; Nottrott, M.; Andreou, D.; Hardes, J. Megaprosthetic replacement of the distal humerus: Still a challenge in limb salvage. J. Shoulder Elbow Surg. 2019, 28, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, G. Custom 3D-printed finger proximal phalanx as salvage of limb function after aggressive recurrence of giant cell tumour. BMJ Case Rep. 2018, 2018, bcr2018226007. [Google Scholar] [CrossRef]
- Beltrami, G.; Ristori, G.; Scoccianti, G.; Tamburini, A.; Capanna, R.; Campanacci, D.; Innocenti, M. Latissimus dorsi rotational flap combined with a custom-made scapular prosthesis after oncological surgical resection: A report of two patients. BMC Cancer 2018, 18, 1003. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Fu, J.; Li, X.; Pei, Y.; Li, X.; Pei, G.; Guo, Z. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: A case series and review of the literature. World J. Surg. Oncol. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, G.; Ristori, G.; Galeotti, A.; Scoccianti, G.; Tamburini, A.; Campanacci, D.; Capanna, R.; Innocenti, M. A hollow, custom-made prosthesis combined with a vascularized flap and bone graft for skeletal reconstruction after bone tumour resection. Surg. Oncol. 2021, 36, 56–60. [Google Scholar] [CrossRef]
- Beltrami, G.; Nucci, A.M.; Tamburini, A.; Innocenti, M. Custom-made 3D-printed prosthesis and free vascularised fibula for humeral reconstruction after osteosarcoma resection in a 13-year-old patient. BMJ Case Rep. 2021, 14, e240726. [Google Scholar] [CrossRef]
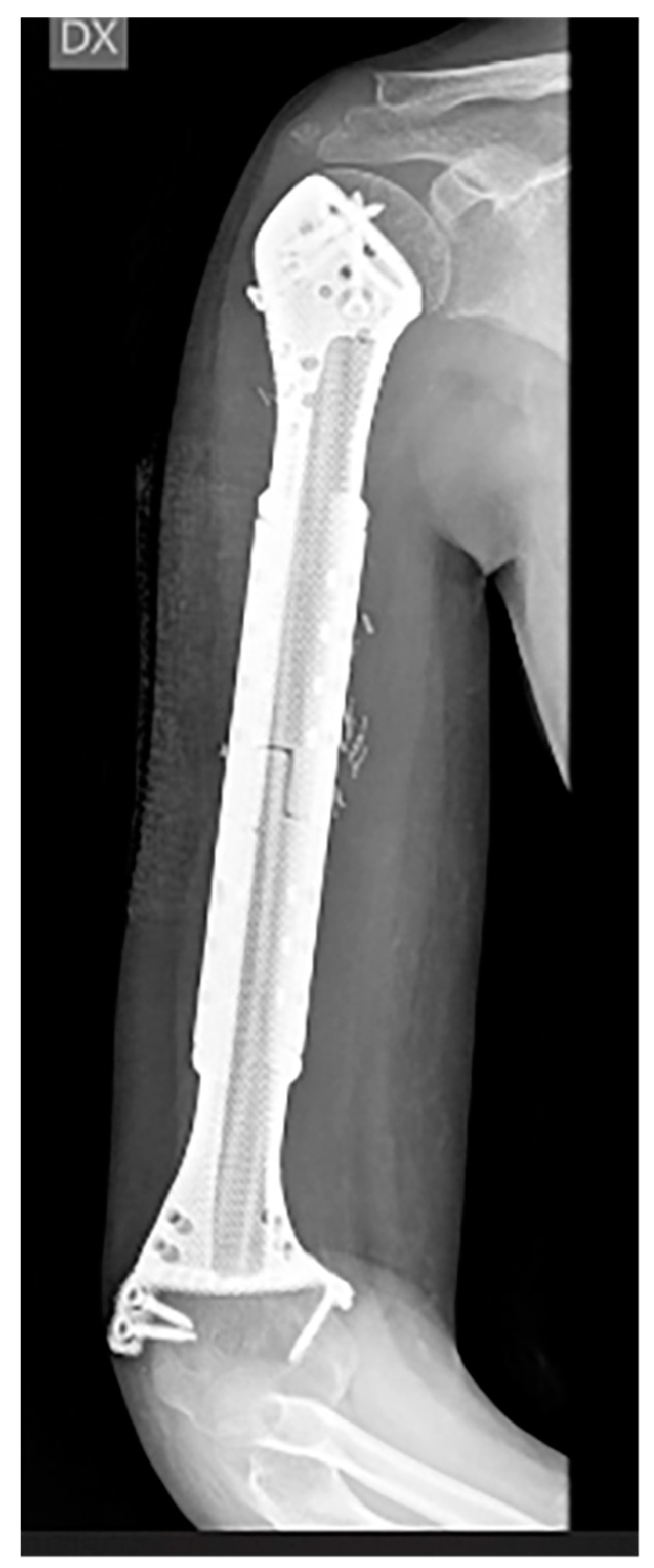
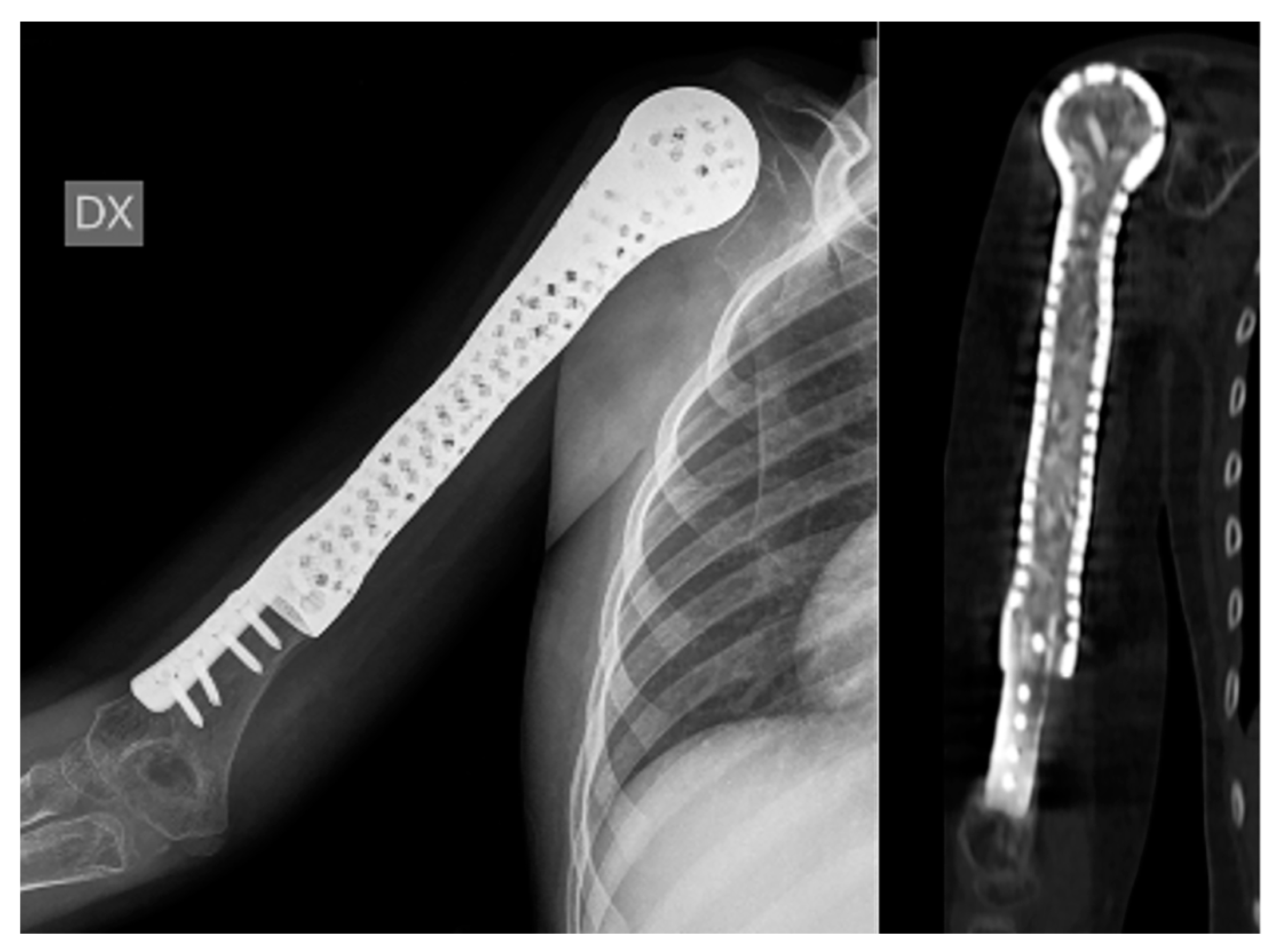

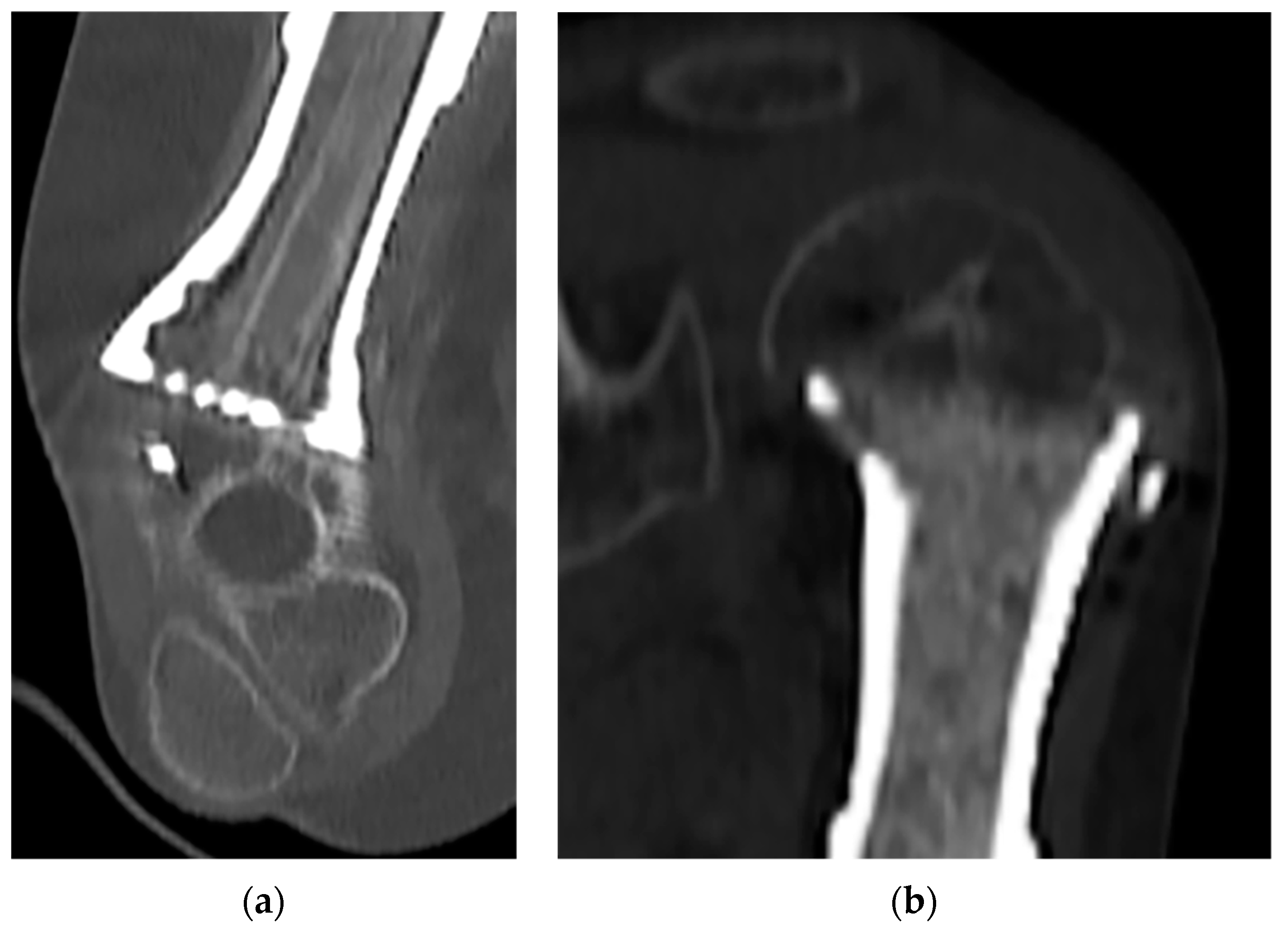
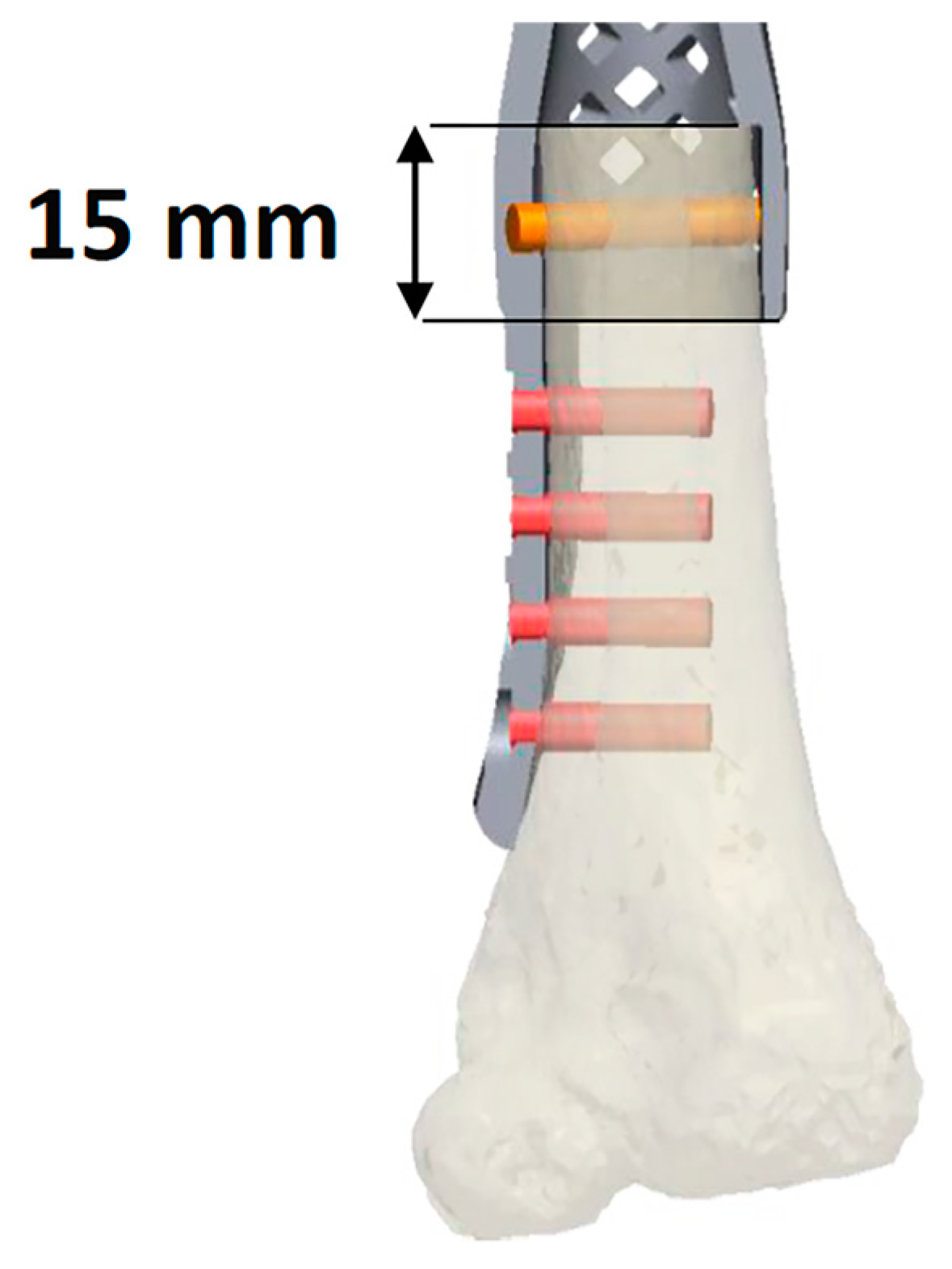

| Patient | Sex | Age | Anatomical Site | Histology | TNM Staging | Pathologic Fracture at Diagnosis | Mets at Diagnosis | Concomitant Therapy | Surgical Time (Min) | Quality of the Surgical Margins | Bone/Spacer |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 14 | Diaphysis | OS | T2 M0 N0 | Yes | No | OS2 PGP | 600 | R0 | VFG |
| 2 | F | 17 | Meta-physis prox | Ewing | T2 M0 N0 | Yes | No | ISG/AIEOP EW-1 | 240 | R0 | CBF |
| 3 | F | 7 | Diaphysis | Ewing | T2 M0 N0 | No | No | ISG/AIEOP EW-1 | 300 | R0 | CBF |
| 4 | F | 10 | Metaphysis prox | Ewing | T2 M1 N0 | Yes | Yes | ISG/AIEOP EW-1 | 540 | R0 | VFG |
| 5 | F | 8 | Metaphysis dist | Ewing | T2 M0 N0 | No | No | ISG/AIEOP EW-1 | 280 | R0 | CBF |
| Patient | Follow-Up | Age | Autologous Bone Sparing | Prosthetic Surface | MSTS | Delta Limb Length (cm) | Final Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 68 | 14 | Proximal humerus epiphysis | Distal humerus osteoarticular | 93% | 0.8 | NED |
| 2 | 30 | 17 | Diaphysis and distal humerus | Entire shoulder | 66% | 0 | NED |
| 3 | 27 | 7 | Distal humerus | Proximal humerus osteoarticular | 73% | 4 | NED |
| 4 | 22 | 10 | Proximal epiphysis and distal humerus metaphysis | Native proximal | 83% | 2 | AWD |
| and distal articular surfaces | |||||||
| 5 | 14 | 8 | Proximal humerus metaphysis | Distal humerus osteoarticular | 93% | 3 | DOD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrami, G.; Rajan, S.; Nucci, A.M.; Galeotti, A.; Guido, D.; Campanacci, D.; Innocenti, M. Biological Prosthesis (Hollow 3D-Printed Titanium Custom-Made Prosthesis and Bone Graft) for Humeral Reconstruction in Pediatric Oncologic Patients: Surgical Indications and Results. Bioengineering 2023, 10, 1371. https://doi.org/10.3390/bioengineering10121371
Beltrami G, Rajan S, Nucci AM, Galeotti A, Guido D, Campanacci D, Innocenti M. Biological Prosthesis (Hollow 3D-Printed Titanium Custom-Made Prosthesis and Bone Graft) for Humeral Reconstruction in Pediatric Oncologic Patients: Surgical Indications and Results. Bioengineering. 2023; 10(12):1371. https://doi.org/10.3390/bioengineering10121371
Chicago/Turabian StyleBeltrami, Giovanni, Sreeraj Rajan, Anna Maria Nucci, Alberto Galeotti, Davide Guido, Domenico Campanacci, and Marco Innocenti. 2023. "Biological Prosthesis (Hollow 3D-Printed Titanium Custom-Made Prosthesis and Bone Graft) for Humeral Reconstruction in Pediatric Oncologic Patients: Surgical Indications and Results" Bioengineering 10, no. 12: 1371. https://doi.org/10.3390/bioengineering10121371
APA StyleBeltrami, G., Rajan, S., Nucci, A. M., Galeotti, A., Guido, D., Campanacci, D., & Innocenti, M. (2023). Biological Prosthesis (Hollow 3D-Printed Titanium Custom-Made Prosthesis and Bone Graft) for Humeral Reconstruction in Pediatric Oncologic Patients: Surgical Indications and Results. Bioengineering, 10(12), 1371. https://doi.org/10.3390/bioengineering10121371








