Early Osteogenic-Induced Adipose-Derived Stem Cells and Canine Bone Regeneration Potential Analyzed Using Biodegradable Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Primary Cells and Cultures
2.3. Cell Characterization
2.4. Osteogenic Differentiation and Analysis
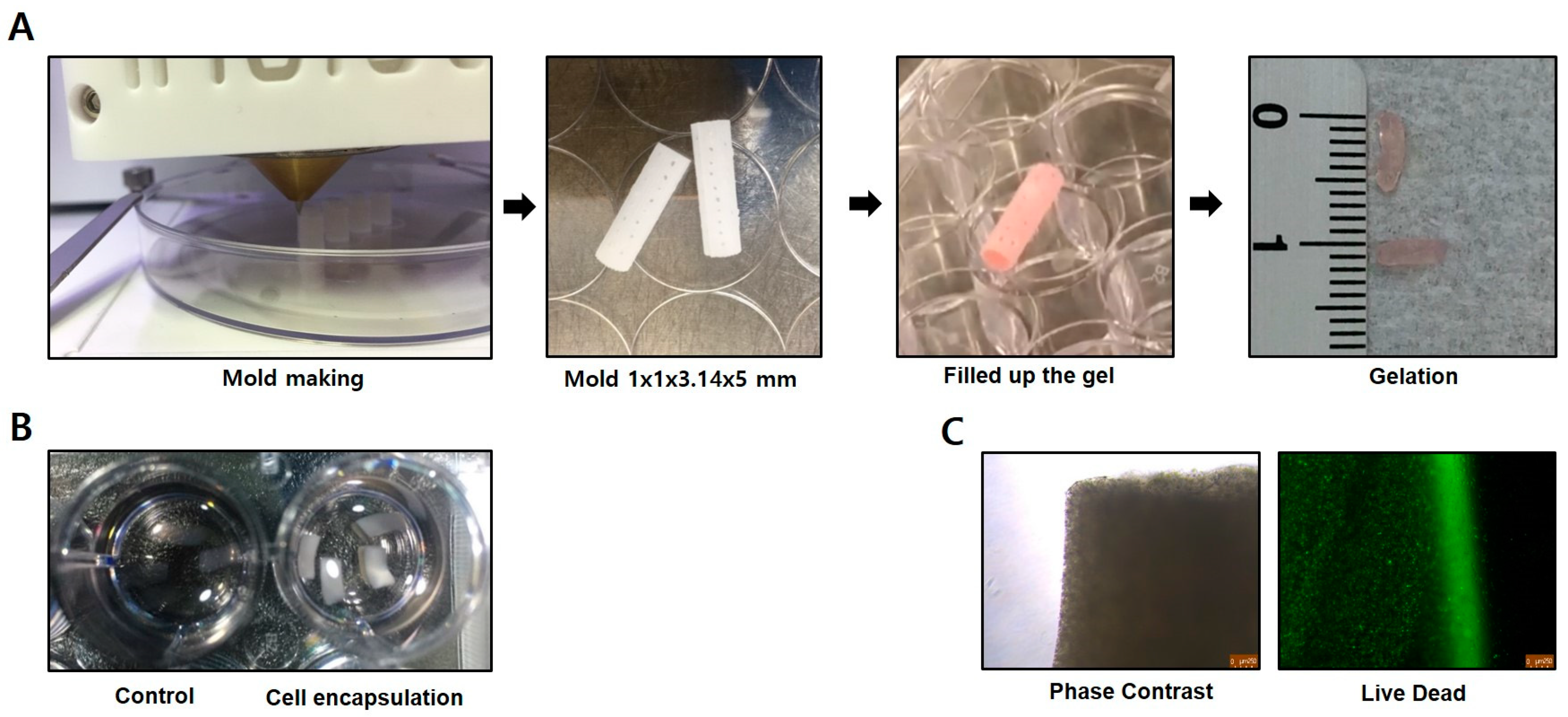
2.5. Cell Preparation for Transplantation
2.6. Animals
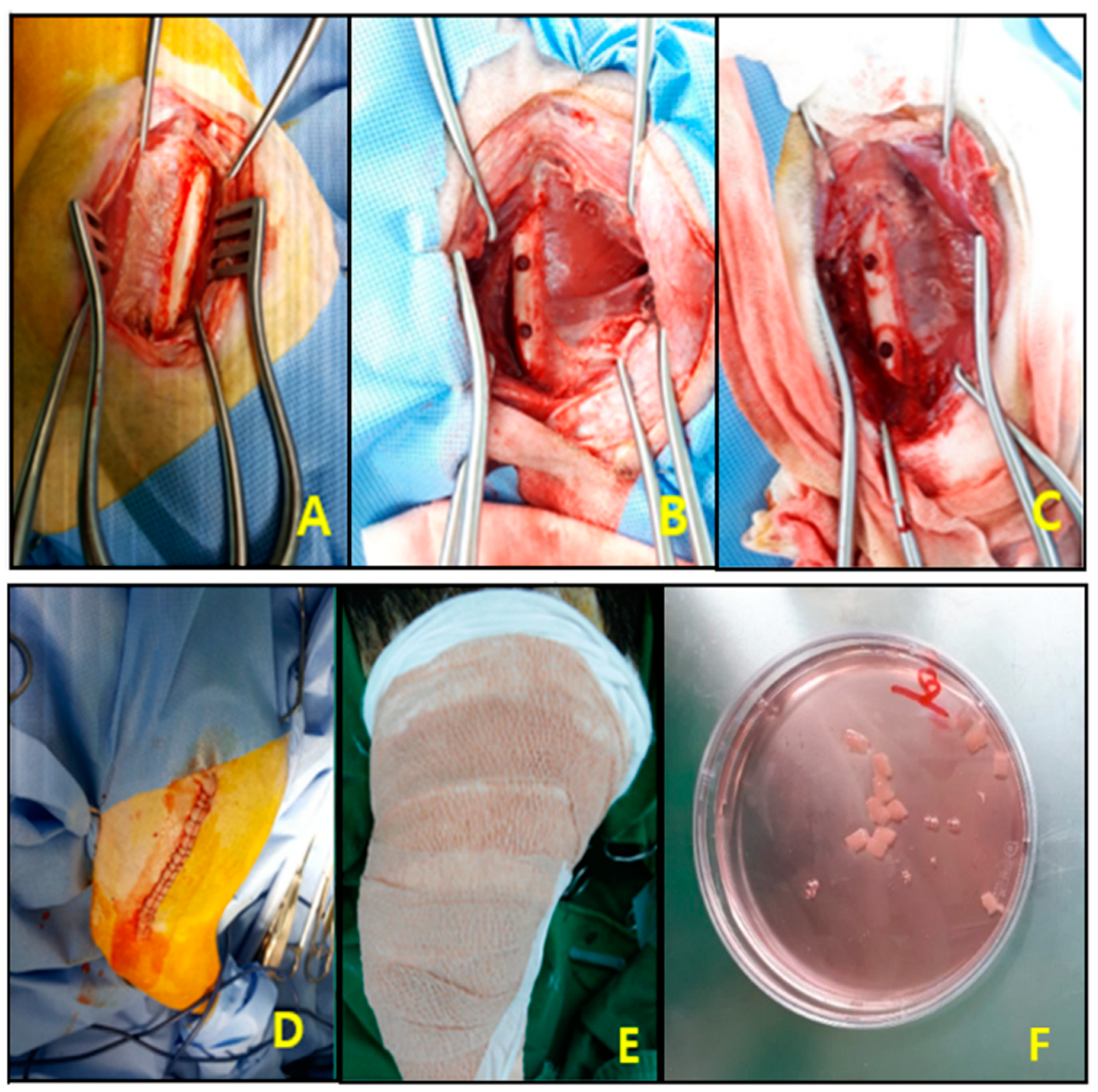
2.7. Surgical Procedures
2.8. Serum Analysis
2.9. Imaging Analysis
2.10. Histopathology
2.11. Statistical Analysis
3. Results
3.1. Analysis of Cell Surface Proteins and Osteogenic Differentiation in the cADMSCs
3.2. Body Weight and Serum Analysis
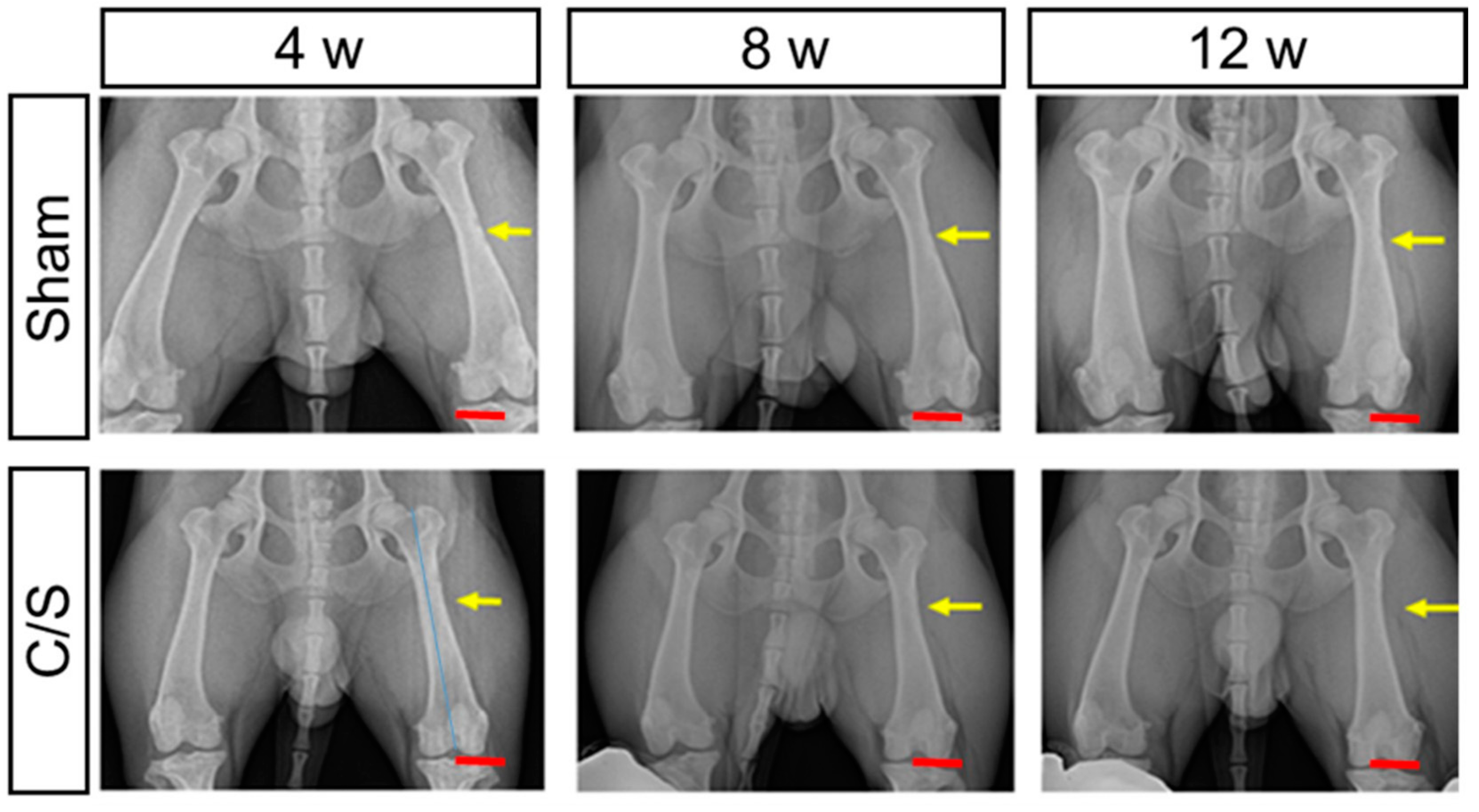
3.3. X-ray
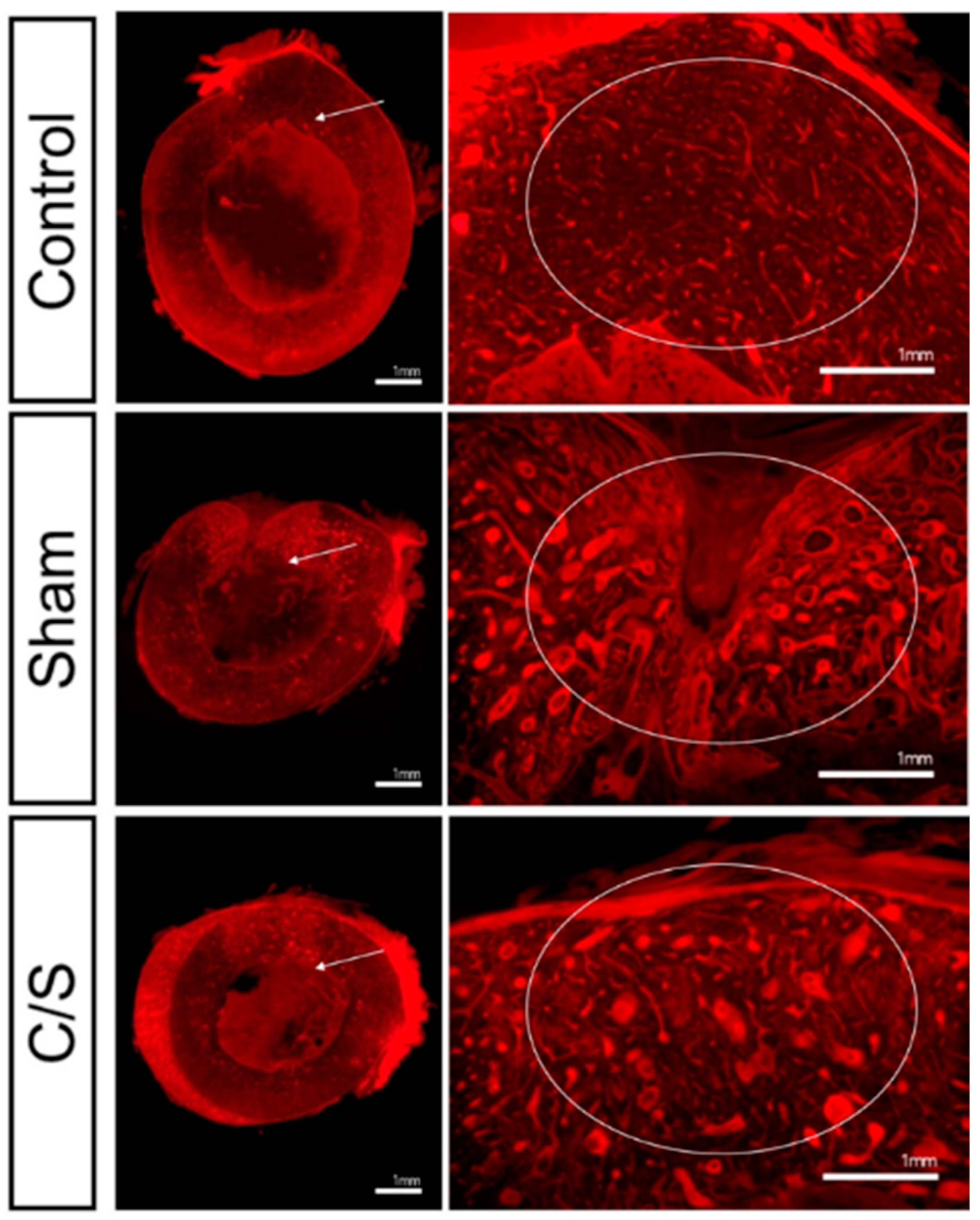
3.4. Morphology
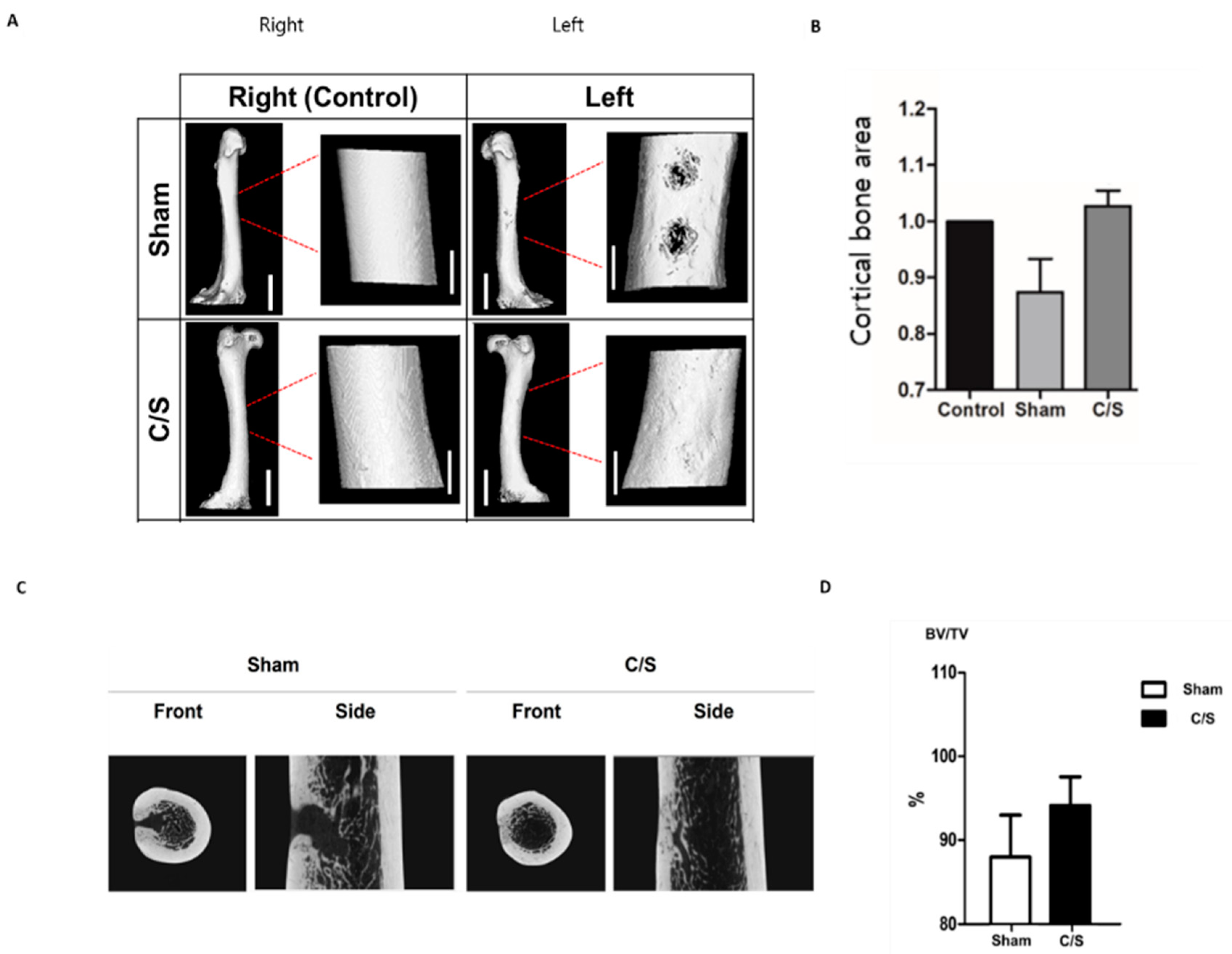
3.5. Micro-CT
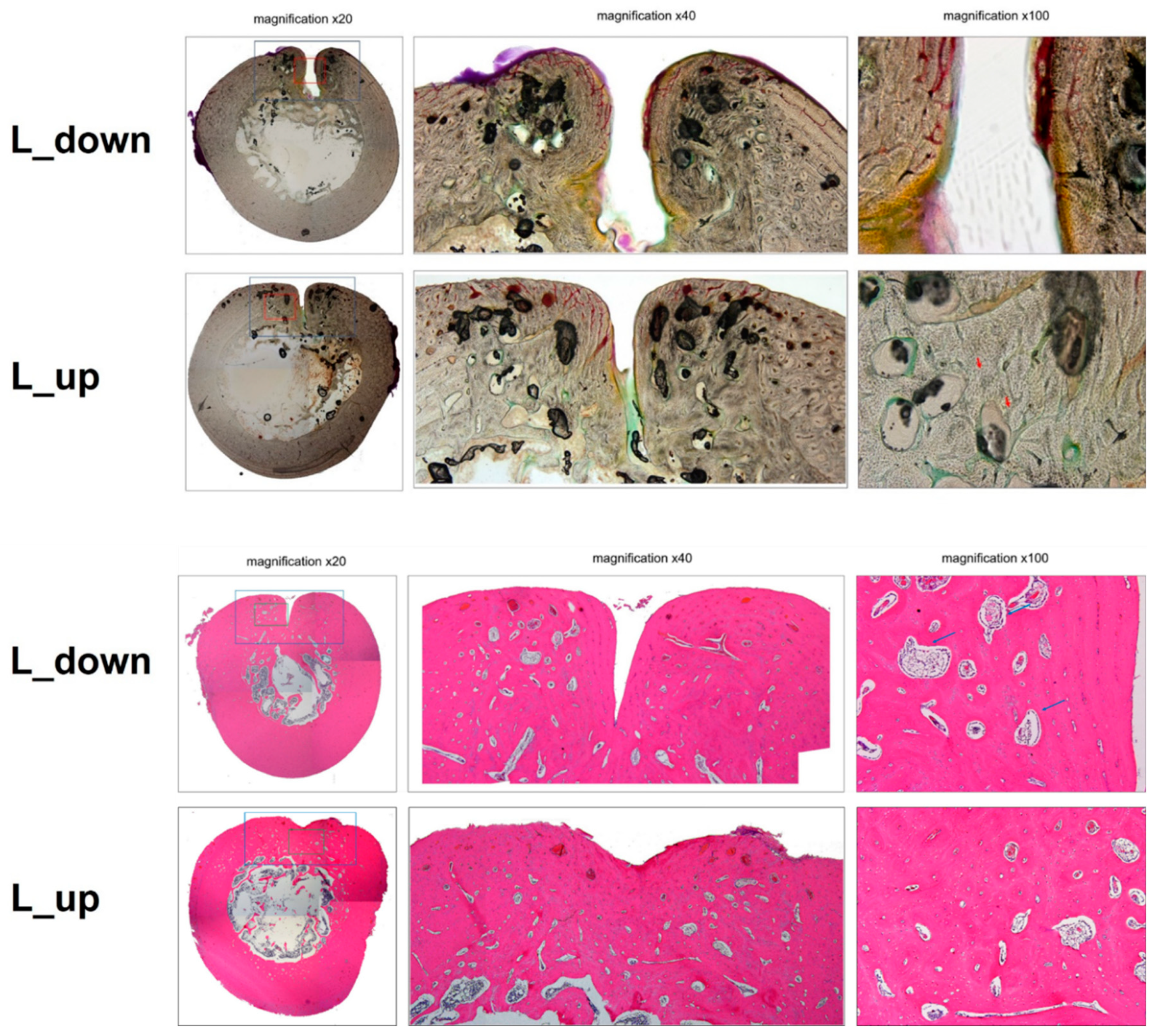
3.6. Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lohmann, H.; Grass, G.; Rangger, C. Economic impact of cancellous bone grafting in trauma surgery. Arch. Orthop. Trauma. Surg. 2007, 127, 345–348. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Drosse, I.; Volkmer, E.; Capanna, R.; De Biase, P.; Mutschler, W.; Schieker, M. Tissue engineering for bone defect healing: An update on a multi-component approach. Injury 2008, 39, S9–S20. [Google Scholar] [CrossRef]
- Aho, A.J.; Ekfors, T.; Dean, P.B.; Aro, H.T.; Ahonen, A.; Nikkanen, V. Incorporation and clinical results of large allografts of the extremities and pelvis. Clin. Orthop. Relat. Res. 1994, 307, 200–213. [Google Scholar]
- Ehrler, D.M.; Vaccaro, A.R. The Use of Allograft Bone in Lumbar Spine Surgery. Clin. Orthop. Relat. Res. 2000, 371, 38–45. [Google Scholar] [CrossRef]
- Head, W.C.; Emerson, R.H.J.; Malinin, T.I. Structural Bone Grafting for Femoral Reconstruction. Clin. Orthop. Relat. Res. 1999, 369, 223–229. [Google Scholar] [CrossRef]
- Sahar, D.E.; Walker, J.A.; Wang, H.T.; Stephenson, S.M.; Shah, A.R.; Krishnegowda, N.K.; Wenke, J.C. Effect of Endothelial Differentiated Adipose-Derived Stem Cells on Vascularity and Osteogenesis in Poly (D, L-Lactide) Scaffolds In Vivo. J. Craniofacial Surg. 2012, 23, 913–918. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Chung, M.-J.; Son, J.Y.; Park, S.Y.; Hur, K.; Lee, S.H.; Lee, E.J.; Park, J.K.; Hong, I.H.; Kim, T.H.; Jeong, K.S. Mesenchymal Stem Cell and MicroRNA Therapy of Musculoskeletal Diseases. Int. J. Stem Cells 2021, 14, 150–167. [Google Scholar] [CrossRef]
- Chung, M.-J.; Park, S.Y.; Son, J.Y.; Lee, J.Y.; Yun, H.H.; Lee, E.J.; Lee, E.M.; Cho, G.J.; Lee, S.; Park, H.S.; et al. Differentiation of equine induced pluripotent stem cells into mesenchymal lineage for therapeutic use. Cell Cycle 2019, 18, 2954–2971. [Google Scholar] [CrossRef]
- Van, R.L.R.; Roncari, D.A.K. Cell and Tissue Isolation of Fat Cell Precursors from Adult Rat Adipose Tissue. Cell 1977, 203, 197–203. [Google Scholar]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Peter, A.; Daniel, A.D.U.; Jerry, I.H.; Hiroshi, M.; Zeni, C.A.; John, K.F.; Prosper, B.; Marc, H.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef]
- Yoon, E.; Dhar, S.; Chun, D.E.; Gharibjanian, N.A.; Evans, G.R.D. In Vivo Osteogenic Potential of Human Adipose-Derived Stem Cells/Poly Lactide-Co-Glycolic Acid Constructs for Bone Regeneration in a Rat Critical-Sized Calvarial Defect Model. Tissue Eng. 2007, 13, 619–627. [Google Scholar] [CrossRef]
- Cornejo, A.; Agustin, C.; David, E.S.; Stacy, M.S.; Shiliang, C.; Son, N.T.G.; Joseph, C.W.; Amanda, V.; Joel, E.M.; Ramaswamy, S.; et al. Effect of Adipose Tissue-Derived Osteogenic and Endothelial Cells on Bone Allograft Osteogenesis and Vascularization in Critical-Sized Calvarial Defects. Tissue Eng. Part A 2012, 18, 1552–1561. [Google Scholar] [CrossRef]
- Vieira, N.M.; Brandalise, V.; Zucconi, E.; Secco, M.; Strauss, B.E.; Zatz, M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010, 19, 279–289. [Google Scholar] [CrossRef]
- Dominici, M.; Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; Díaz-Payno, P.J.; Sheehy, E.J.; Critchley, S.E.; Almeida, H.V.; Pitacco, P.; Carroll, S.F.; Mahon, O.R.; Dunne, A.; Levingstone, T.J.; et al. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials 2019, 188, 63–73. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Kikionis, S.; Ioannou, E.; Aggelidou, E.; Tziveleka, L.A.; Demiri, E.; Bakopoulou, A.; Zinelis, S.; Kritis, A.; Roussis, V. The Marine Polysaccharide Ulvan Confers Potent Osteoinductive Capacity to PCL-Based Scaffolds for Bone Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 3086. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Atwood, R.C.; Poologasundarampillai, G.; Yue, S.; Lee, P.D. Quantifying the 3D macrostructure of tissue scaffolds. J. Mater. Sci. Mater. Med. 2009, 20, 463–471. [Google Scholar] [CrossRef]
- Neiva, R.; Pagni, G.; Duarte, F.; Park, C.H.; Yi, E.; Holman, L.A.; Giannobile, W.V. Analysis of tissue neogenesis in extraction sockets treated with guided bone regeneration: Clinical, histologic, and micro-CT results. Int. J. Periodontics Restor. Dent. 2011, 31, 457–469. [Google Scholar]
- Vasquez, S.X.; Shah, N.; Hoberman, A.M. Small animal imaging and examination by micro-CT. Methods Mol. Biol. 2013, 947, 223–231. [Google Scholar] [CrossRef]
- Arinzeh, T.L.; Peter, S.J.; Archambault, M.P.; Bos, C.; Gordon, S.; Kraus, K.; Smith, A.; Kadiyala, S. Allogeneic Mesenchymal Stem Cells Regenerate Bone in a Critical-Sized Canine Segmental Defect. J. Bone Jt. Surg. Am. 2003, 85, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-R.V.; Hwang, Y.S.; Phadke, A.; Kang, H.M.; Hwang, N.S.; Eduardo Caro, J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.C.; et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990–995. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Kim, J.M.; Kim, J.R.; Kim, K.J.; Kim, Y.J.; Park, S.I.; Jeong, J.H.; Moon, Y.; Lim, H.S.; et al. Systemic transplantation of human adipose-derived stem cells stimulates bone repair by promoting osteoblast and osteoclast function. J. Cell. Mol. Med. 2011, 15, 2082–2094. [Google Scholar] [CrossRef]
- Yeatts, A.B.; Geibel, E.M.; Fears, F.F.; Fisher, J.P. Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnol. Bioeng. 2012, 109, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.S.; Tran, M.C.; Wong, V.W.; Chung, M.T.; Lo, D.D.; Montoro, D.T.; Wan, D.C.; Longaker, M.T. Enhancing stem cell survival in vivo for tissue repair. Biotechnol. Adv. 2013, 31, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, H.; Kalarestaghi, H. Adipose-derived stem cells: An appropriate selection for osteogenic differentiation. J. Cell. Physiol. 2020, 235, 8371–8386. [Google Scholar] [CrossRef] [PubMed]
- Clokie, C.M.L.; Moghadam, H.; Jackson, M.T.; Sandor, G.K.B. Closure of Critical Sized Defects with Allogenic and Alloplastic Bone Substitutes. J. Craniofacial Surg. 2002, 13, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ueki, K.; Takazakura, D.; Marukawa, K.; Shimada, M.; Nakagawa, K.; Takatsuka, S.; Yamamoto, E. The use of polylactic acid/polyglycolic acid copolymer and gelatin sponge complex containing human recombinant bone morphogenetic protein-2 following condylectomy in rabbits. J. Cranio-Maxillofac. Surg. 2003, 31, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.H.; Chen, C.H.; Chen, C.T. Osteogenic potential of induced pluripotent stem cells from human adipose-derived stem cells. Stem Cell Res. Ther. 2019, 10, 303. [Google Scholar] [CrossRef]
- Byeon, Y.-E.; Ryu, H.H.; Park, S.S.; Koyama, Y.; Kikuchi, M.; Kim, W.H.; Kang, K.S.; Kweon, O.K. Paracrine effect of canine allogenic umbilical cord blood-derived mesenchymal stromal cells mixed with beta-tricalcium phosphate on bone regeneration in ectopic implantations. Cytotherapy 2010, 12, 626–636. [Google Scholar] [CrossRef]
- Bi, L.X.; Simmons, D.J.; Mainous, E. Expression of BMP-2 by Rat Bone Marrow Stromal Cells in Culture. Calcif. Tissue Int. 1999, 64, 63–68. [Google Scholar] [CrossRef]
- Frank, O.; Manuel, H.; Marcel, J.; Andrea, B.; Dirk, S.; Igor, B.; Walter, D.; Michael, H.; Ivan, M. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J. Cell. Biochem. 2002, 85, 737–746. [Google Scholar] [CrossRef]
- Harris, S.E.; Sabatini, M.; Harris, M.A.; Feng, J.Q.; Wozney, J.; Mundy, G.R. Expression of bone morphogenetic protein messenger RNA in prolonged cultures of fetal rat calvarial cells. J. Bone Miner. Res. 1994, 9, 389–394. [Google Scholar] [CrossRef]
- Kang, B.-J.; Hak, H.R.; Sung, S.P.; Yoshihisa, K.; Masanori, K.; Heung, M.W.; Wan, H.K.; Oh, K.K. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton’s jelly for treating bone defects. J. Vet. Sci. 2012, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Hajime, I.; Tomohiko, A.; Tatsuya, K.; Shinichiro, O.; Yusuke, S.; Toshiyuki, A.; Akihiko, S.; Masaaki, H.; Mitsuhisa, T.; et al. An engineered cell sheet composed of human islets and human fibroblast, bone marrow–derived mesenchymal stem cells, or adipose–derived mesenchymal stem cells: An in vitro comparison study. Islets 2018, 10, e1445948. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Kim, A.Y.; Lee, E.J.; Park, J.K.; Lee, M.M.; Hwang, M.; Kim, C.Y.; Kim, S.Y.; Jeong, K.S. Therapeutic effects of mouse adipose-derived stem cells and losartan in the skeletal muscle of injured mdx mice. Cell Transplant. 2015, 24, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, M.; Kim, Y.D.; Chung, M.J.; Elfadl, A.; Ulah, H.M.A.; Park, D.; Lee, S.; Park, H.S.; Kim, T.H.; et al. Establishment of stably expandable induced myogenic stem cells by four transcription factors. Cell Death Dis. 2018, 9, 1092. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact. Mater. 2021, 6, 2467–2478. [Google Scholar] [CrossRef]
- Stamnitz, S.; Klimczak, A. Mesenchymal stem cells, bioactive factors, and scaffolds in bone repair: From research perspectives to clinical practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell. Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef]
- Mende, W.; Götzl, R.; Kubo, Y.; Pufe, T.; Ruhl, T.; Beier, J.P. The role of adipose stem cells in bone regeneration and bone tissue engineering. Cells 2021, 10, 975. [Google Scholar] [CrossRef]


| Animal | Time | Na | K | Cl | TP | ALB | BUN | CREA | GLU | TBIL | Ca | PHOS | TCHOL | TG | AST | ALT | ALP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mmol/L) | (mmol/L) | (mmol/L) | (g/dL) | (g/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (U/L) | (U/L) | (U/L) | ||
| Sham | Pre-operation | 148.0 | 4.4 | 109.9 | 6.2 | 3.2 | 10.8 | 0.7 | 88 | 0 | 10.6 | 6.0 | 162 | 25 | 28 | 34 | 132 |
| 2 Weeks | 146.8 | 4.6 | 109.3 | 5.7 | 3.0 | 15.1 | 0.6 | 78 | 0 | 10.5 | 6.7 | 169 | 37 | 26 | 20 | 158 | |
| 4 Weeks | 147.6 | 4.6 | 109.7 | 5.8 | 3.1 | 17.1 | 0.6 | 77 | 0 | 10.6 | 5.9 | 176 | 54 | 26 | 23 | 139 | |
| 8 Weeks | 147.5 | 4.6 | 110.6 | 6.0 | 3.3 | 18.2 | 0.8 | 79 | 0 | 10.4 | 6.4 | 175 | 33 | 27 | 32 | 119 | |
| 16 Weeks | 146.8 | 5.3 | 111.8 | 6.3 | 3.3 | 27.3 | 0.8 | 64 | 0 | 10.7 | 4.4 | 183 | 59 | 29 | 32 | 107 | |
| C/S | Pre-operation | 148.2 | 5.2 | 109.2 | 5.7 | 3.1 | 11.6 | 0.7 | 94 | 0 | 10.4 | 5.7 | 185 | 28 | 27 | 33 | 139 |
| 2 Weeks | 147.6 | 5.2 | 108.9 | 6.0 | 3.1 | 17.0 | 0.7 | 93 | 0 | 10.5 | 6.1 | 209 | 76 | 29 | 22 | 237 | |
| 4 Weeks | 147.0 | 5.2 | 107.1 | 6.2 | 3.3 | 18.5 | 0.7 | 75 | 0 | 10.7 | 5.6 | 211 | 95 | 31 | 27 | 172 | |
| 8 Weeks | 147.9 | 5.0 | 107.7 | 5.9 | 3.1 | 13.3 | 0.7 | 67 | 0 | 10.1 | 5.1 | 200 | 37 | 28 | 31 | 141 | |
| 16 Weeks | 147.4 | 5.4 | 108.6 | 6.4 | 3.1 | 24.7 | 0.9 | 89 | 0 | 10.4 | 5.3 | 221 | 98 | 26 | 40 | 157 |
| Group | Control/Sham | C/S | ||
|---|---|---|---|---|
| Location | Right | Left | Right | Left |
| Bone mineral density (mg/cc) | 1347.36 | 1301.57 | 1361.17 | 1352.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-H.; Kim, S.-G.; Park, S.-I.; Jo, W.; Kang, K.-K.; Lee, E.-J.; Kim, D.-K.; Jung, H.-S.; Son, J.-Y.; Park, J.-M.; et al. Early Osteogenic-Induced Adipose-Derived Stem Cells and Canine Bone Regeneration Potential Analyzed Using Biodegradable Scaffolds. Bioengineering 2023, 10, 1311. https://doi.org/10.3390/bioengineering10111311
Yun H-H, Kim S-G, Park S-I, Jo W, Kang K-K, Lee E-J, Kim D-K, Jung H-S, Son J-Y, Park J-M, et al. Early Osteogenic-Induced Adipose-Derived Stem Cells and Canine Bone Regeneration Potential Analyzed Using Biodegradable Scaffolds. Bioengineering. 2023; 10(11):1311. https://doi.org/10.3390/bioengineering10111311
Chicago/Turabian StyleYun, Hyun-Ho, Seong-Gon Kim, Se-Il Park, Woori Jo, Kyung-Ku Kang, Eun-Joo Lee, Dong-Kyu Kim, Hoe-Su Jung, Ji-Yoon Son, Jae-Min Park, and et al. 2023. "Early Osteogenic-Induced Adipose-Derived Stem Cells and Canine Bone Regeneration Potential Analyzed Using Biodegradable Scaffolds" Bioengineering 10, no. 11: 1311. https://doi.org/10.3390/bioengineering10111311
APA StyleYun, H.-H., Kim, S.-G., Park, S.-I., Jo, W., Kang, K.-K., Lee, E.-J., Kim, D.-K., Jung, H.-S., Son, J.-Y., Park, J.-M., Park, H.-S., Lee, S., Shin, H.-I., Hong, I.-H., & Jeong, K.-S. (2023). Early Osteogenic-Induced Adipose-Derived Stem Cells and Canine Bone Regeneration Potential Analyzed Using Biodegradable Scaffolds. Bioengineering, 10(11), 1311. https://doi.org/10.3390/bioengineering10111311







