Prediction of Spasticity through Upper Limb Active Range of Motion in Stroke Survivors: A Generalized Estimating Equation Model
Abstract
:1. Introduction
2. Materials and Methods
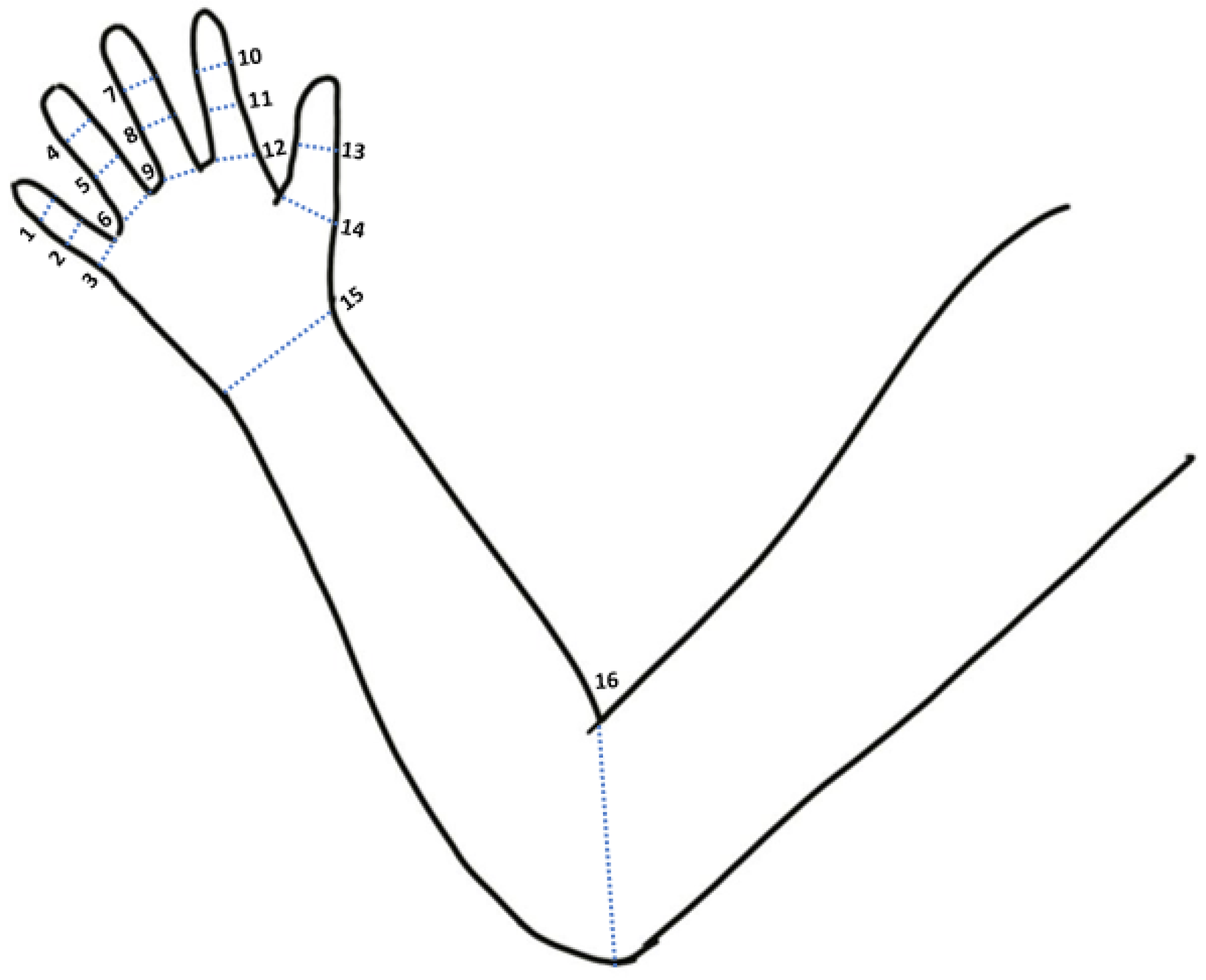
2.1. Wearable System and Joint Angle Measurement
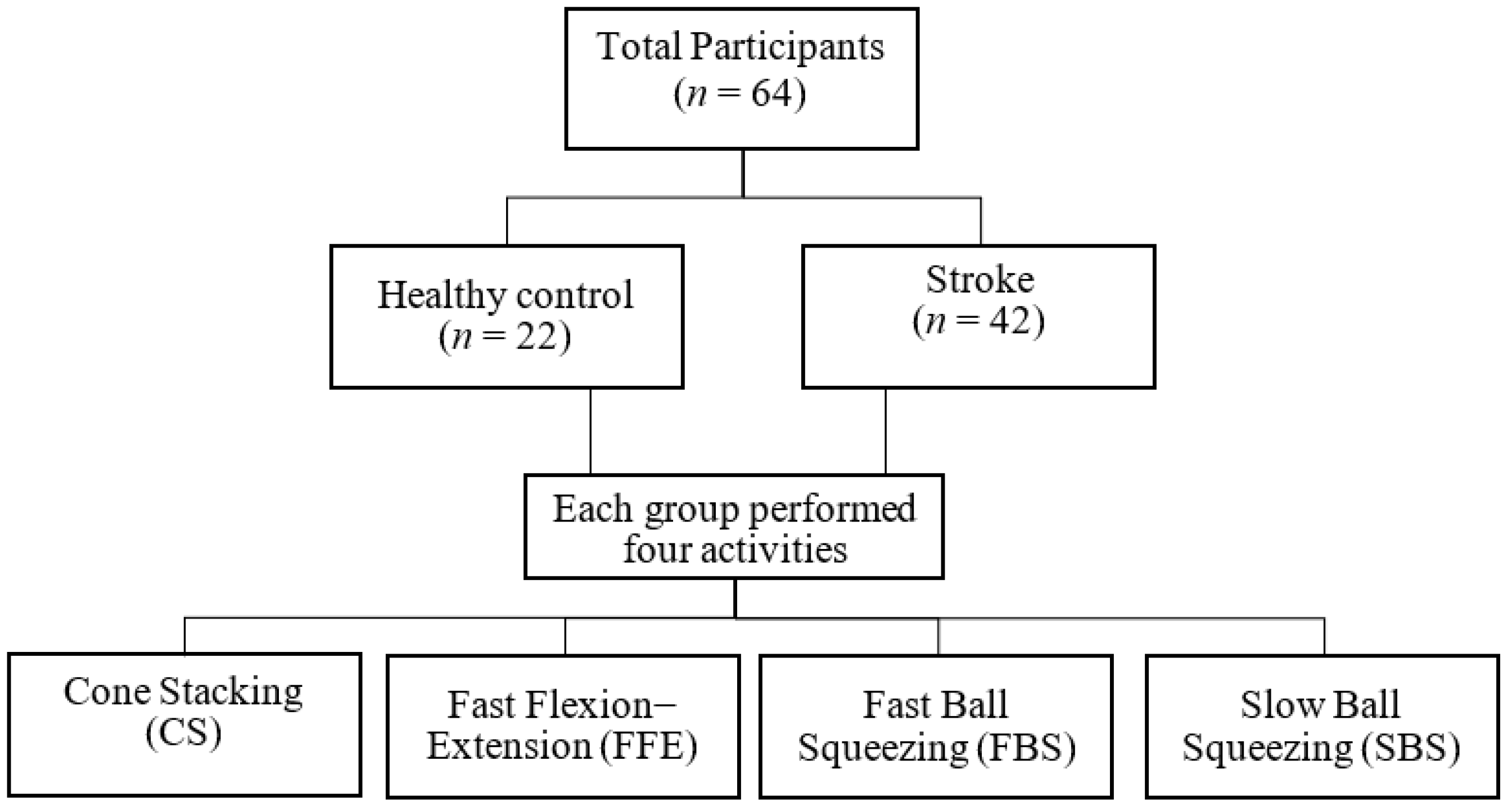
2.2. Data Acquisition Protocol
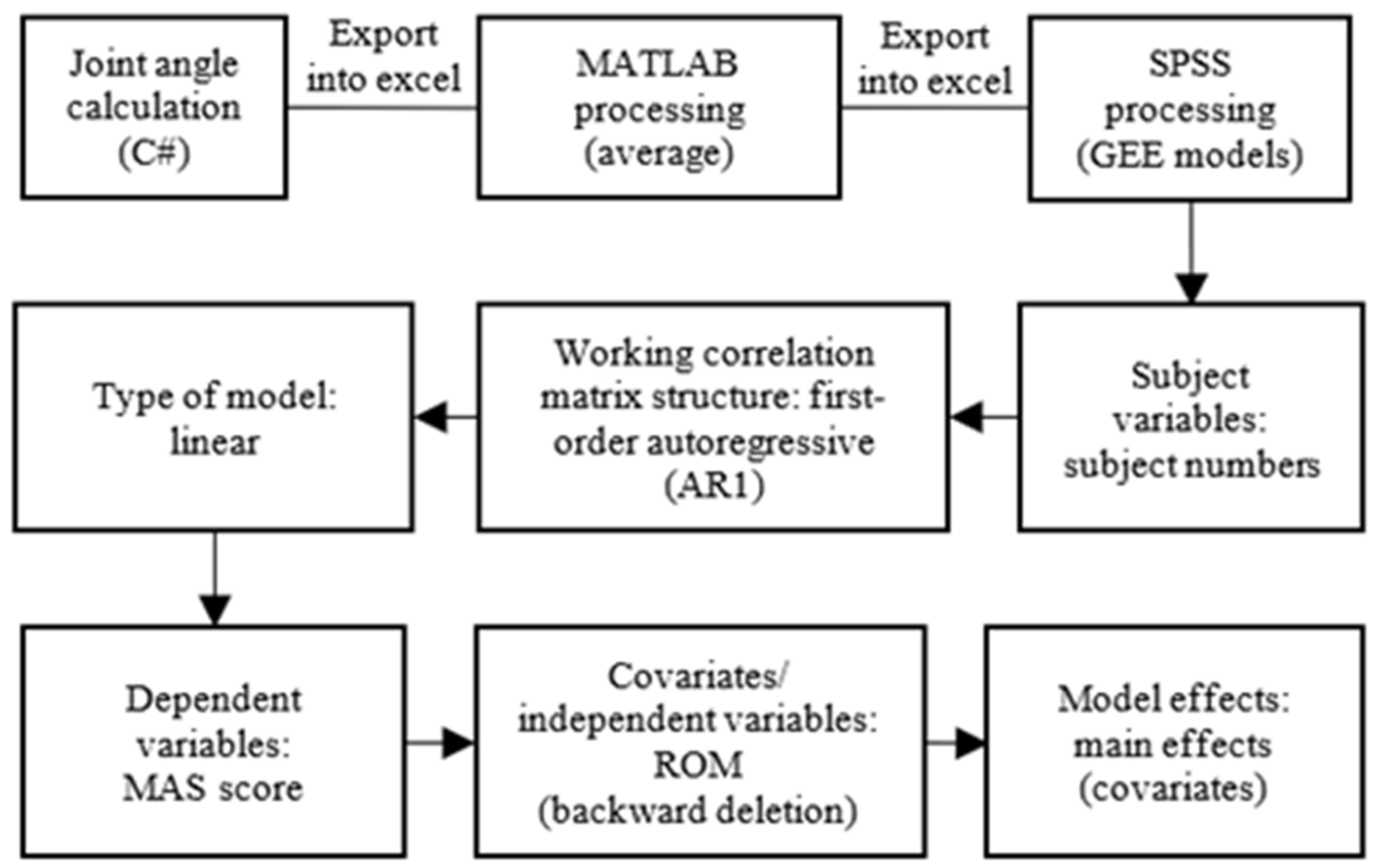
2.3. Model for Spasticity Assessment
3. Results
3.1. GEE Models of Spasticity for the Affected Sides
3.1.1. Right-Dominant Right-Affected Side Models (n = 17)
3.1.2. Right-Dominant Left-Affected Side Models (n = 19)
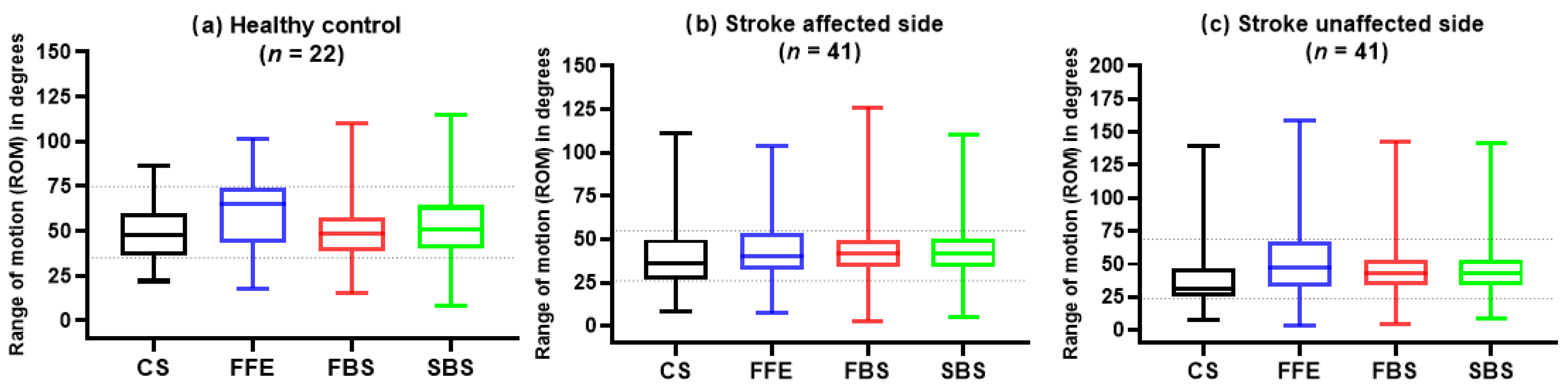
3.2. ROMs of Healthy Control and Stroke Survivors
3.3. Average AROMs of the Upper Limb Joints in Stroke Patients
4. Discussion
5. Conclusions
5.1. Limitations
5.2. Future Directions
5.3. Contributions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sommerfeld, D.K.; Gripenstedt, U.; Welmer, A.K. Spasticity after stroke: An overview of prevalence, test instruments, and treatments. Am. J. Phys. Med. Rehabil. 2012, 91, 814–820. [Google Scholar] [CrossRef]
- Santos, C.M.d.; Ferreira, G.; Malacco, P.L.; Sabino, G.S.; Moraes, G.F.d.S.; Felício, D.C. Intra and inter examiner reliability and measurement error of goniometer and digital inclinometer use. Rev. Bras. Med. Esporte 2012, 18, 38–41. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, S.B.; Lee, J.H.; Lee, S.J.; Yoo, S.W. Effect of upper extremity robot-assisted exercise on spasticity in stroke patients. Ann. Rehabil. Med. 2016, 40, 961–971. [Google Scholar] [CrossRef]
- Dietz, V.; Trippel, M.; Berger, W. Reflex activity and muscle tone during elbow movements in patients with spastic paresis. Ann. Neurol. 1991, 30, 767–779. [Google Scholar] [CrossRef]
- Ada, L.; O’Dwyer, N.; Ada, L.; O’Dwyer, N.; O’Neill, E. Relation between spasticity, weakness and contracture of the elbow flexors and upper limb activity after stroke: An observational study. Disabil. Rehabil. 2006, 28, 891–897. [Google Scholar] [CrossRef]
- Plantin, J.; Pennati, G.V.; Roca, P.; Baron, J.C.; Laurencikas, E.; Weber, K.; Godbolt, A.K.; Borg, J.; Lindberg, P.G. Quantitative Assessment of Hand Spasticity After Stroke: Imaging Correlates and Impact on Motor Recovery. Front. Neurol. 2019, 10, 836. [Google Scholar] [CrossRef]
- Craven, B.C.; Morris, A.R. Modified Ashworth scale reliability for measurement of lower extremity spasticity among patients with SCI. Spinal Cord 2010, 48, 207–213. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Arab, T.K.; Jalaie, S. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: Limb and muscle group effect. NeuroRehabilitation 2008, 23, 231–237. [Google Scholar] [CrossRef]
- Ang, W.S.; Geyer, H.; Chen, I.M.; Ang, W.T. Objective Assessment of Spasticity With a Method Based on a Human Upper Limb Model. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1414–1423. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, G.; Lee, S.A.; Nam, Y. Analysis of machine learning-based assessment for elbow spasticity using inertial sensors. Sensors 2020, 20, 1622. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Zhu, X.; Gao, X.; Chen, X.; Chen, X. A regression-based framework for quantitative assessment of muscle spasticity using combined EMG and Inertial data from wearable sensors. Front. Neurosci. 2019, 13, 398. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Pepper, M.G.; Yan, Y.; Phillips, M.; Sakel, M. Quantitative measurement of upper limb motion pre-and post-treatment with Botulinum Toxin. Measurement 2021, 168, 108304. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, S.; Cai, Q.; Huang, S.; Ma, K.; Zheng, H.; Xie, L. A spasticity assessment method for voluntary movement using data fusion and machine learning. Biomed. Signal Process. Control 2021, 65, 102353. [Google Scholar] [CrossRef]
- Lin, B.-S.; Lee, I.; Hsiao, P.-C.; Yang, S.-Y.; Chen, C.-Y.; Lee, S.-H.; Huang, Y.-F.; Yen, M.-H.; Hu, Y.-H. Design of a multi-sensor system for exploring the relation between finger spasticity and voluntary movement in patients with stroke. Sensors 2022, 22, 7212. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, J.; Damiano, D.L. Development of a haptic elbow spasticity simulator (HESS) for improving accuracy and reliability of clinical assessment of spasticity. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 361–370. [Google Scholar] [CrossRef]
- Haaland, K.Y.; Prestopnik, J.L.; Knight, R.T.; Lee, R.R. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain A J. Neurol. 2004, 127, 1145–1158. [Google Scholar] [CrossRef]
- Sainburg, R.L.; Duff, S.V. Does motor lateralization have implications for stroke rehabilitation? J. Rehabil. Res. Dev. 2006, 43, 311–322. [Google Scholar] [CrossRef]
- Wetter, S.; Poole, J.L.; Haaland, K.Y. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch. Phys. Med. Rehabil. 2005, 86, 776–781. [Google Scholar] [CrossRef]
- Yarosh, C.A.; Hoffman, D.S.; Strick, P.L. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J. Neurophysiol. 2004, 92, 3276–3285. [Google Scholar] [CrossRef]
- Sunderland, A. Recovery of ipsilateral dexterity after stroke. Stroke 2000, 31, 430–433. [Google Scholar] [CrossRef]
- Lin, B.-S.; Lee, I.-J.; Chen, J.-L. Novel assembled sensorized glove platform for comprehensive hand function assessment by using inertial sensors and force sensing resistors. IEEE Sens. J. 2019, 20, 3379–3389. [Google Scholar] [CrossRef]
- Madgwick, S.O.; Harrison, A.J.; Vaidyanathan, A. Estimation of IMU and MARG orientation using a gradient descent algorithm. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–7. [Google Scholar] [CrossRef]
- Burton, P.; Gurrin, L.; Sly, P. Extending the simple linear regression model to account for correlated responses: An introduction to Generalized Estimating Equations and multi-level mixed modelling. Stat. Med. 1998, 17, 1261–1291. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: Oxford, UK, 1989. [Google Scholar]
- Aitkin, M.; Anderson, D.; Francis, B.; Hinde, J. Statistical Modelling in GLIM; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Zeger, S.L.; Liang, K.Y. An overview of methods for the analysis of longitudinal data. Stat. Med. 1992, 11, 1825–1839. [Google Scholar] [CrossRef] [PubMed]
- Piggott, B.; Müller, S.; Chivers, P.; Cripps, A.; Hoyne, G. Interdisciplinary sport research can better predict competition performance, identify individual differences, and quantify task representation. Front. Sports Act. Living 2020, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Tan, S.B. The correct interpretation of confidence intervals. Proc. Singap. Healthc. 2010, 19, 276–278. [Google Scholar] [CrossRef]
- Pan, W. Akaike’s information criterion in Generalized Estimating Equations. Biometrics 2001, 57, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, A.; Bowers, M.P.; Sluman, S.M.; Wilcock, D.J.; Ardron, M.E. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke 1999, 30, 949–955. [Google Scholar] [CrossRef]

| Our Study | Lin et al. [14] | Park et al. [15] | Zhang et al. [11] | Kim et al. [10] | Chen et al. [13] | |
|---|---|---|---|---|---|---|
| Type of tasks | V | V | P | P | P | V |
| Assessment scale | MAS | MAS | MAS | MAS | MAS | MAS |
| Included joints | E, W, T, F | E, W, T, F | E | E | E | E |
| Analysis of right/left-affected side | Yes | No | No | No | No | No |
| Analysis of each finger joint | Yes | No | No | No | No | No |
| Correlation (r) | N/A | E: 0.93 W: 0.94 T: 0.92 F: 0.92 | 0.83 | 0.93 | N/A | N/A |
| p-value | <0.05 * | <0.05 * | N/A | <0.05 * | N/A | <0.05 * |
| Healthy Control (n = 22) | Stroke (n = 42) | |
|---|---|---|
| Gender (male/female) | 12/10 | 32/10 |
| Age (years) | 54.68 ± 9.63 | 56.83 ± 11.74 |
| Dominant side (right/left) | 21/1 | 37/5 |
| Affected side (right/left/both) | 19/22/1 | |
| Type of injury (hemorrhagic/ischemic) | 11/31 | |
| Time since stroke (months) | 38.52 ± 48.22 | |
| MAS elbow (healthy/mild/moderate) | 7/15/20 | |
| MAS wrist (healthy/mild/moderate) | 19/9/14 | |
| MAS thumb (healthy/mild/moderate) | 23/16/3 | |
| MAS finger (healthy/mild/moderate) | 20/13/9 |
| Finger | Variable | Estimate (β) | SE | 95% CI (Lower~Upper) | p |
|---|---|---|---|---|---|
| Finger 2 (QIC = 22) | Intercept | 12.76 | 0.44 | 11.89~13.63 | 0.0001 *** |
| CS_DIP2 | −0.04 | 0.00 | −0.05~−0.03 | 0.0001 *** | |
| CS_PIP2 | −0.02 | 0.00 | −0.02~−0.01 | 0.0001 *** | |
| CS_MP2 | 0.39 | 0.01 | 0.37~0.41 | 0.0001 *** | |
| FFE_DIP2 | −0.26 | 0.01 | −0.27~−0.24 | 0.0001 *** | |
| FFE_PIP2 | 0.01 | 0.00 | 0.01~0.02 | 0.002 * | |
| FFE_MP2 | −0.09 | 0.00 | −0.09~−0.08 | 0.0001 *** | |
| FBS_DIP2 | −0.08 | 0.01 | −0.09~−0.06 | 0.0001 *** | |
| FBS_PIP2 | −0.08 | 0.01 | −0.10~−0.06 | 0.0001 *** | |
| FBS_MP2 | 0.06 | 0.01 | 0.05~0.07 | 0.0001 *** | |
| SBS_DIP2 | 0.20 | 0.01 | 0.19~0.22 | 0.0001 *** | |
| SBS_PIP2 | −0.03 | 0.01 | −0.05~−0.01 | 0.001 ** | |
| SBS_MP2 | −0.26 | 0.01 | −0.28~−0.23 | 0.0001 *** | |
| Finger 3 (QIC = 19) | Intercept | −1.97 | 1.73 | −5.36~1.42 | 0.255 |
| CS_DIP3 | −0.09 | 0.01 | −0.11~−0.06 | 0.0001 *** | |
| CS_PIP3 | 0.04 | 0.01 | 0.02~0.06 | 0.0001 *** | |
| FFE_PIP3 | 0.05 | 0.01 | 0.03~0.06 | 0.0001 *** | |
| FFE_MP3 | 0.07 | 0.01 | 0.05~0.09 | 0.0001 *** | |
| FBS_DIP3 | −0.08 | 0.03 | −0.14~−0.02 | 0.007 * | |
| FBS_PIP3 | -0.16 | 0.03 | −0.21~−0.10 | 0.0001 *** | |
| FBS_MP3 | −0.09 | 0.01 | −0.11~−0.08 | 0.0001 *** | |
| SBS_DIP3 | 0.08 | 0.03 | 0.03~0.14 | 0.004 * | |
| SBS_PIP3 | 0.18 | 0.03 | 0.12~0.23 | 0.0001 *** | |
| Finger 4 (QIC = 22) | Intercept | −1.59 | 0.41 | −2.39~−0.80 | 0.0001 *** |
| CS_DIP4 | −0.05 | 0.01 | −0.06~−0.04 | 0.0001 *** | |
| CS_PIP4 | −0.02 | 0.00 | −0.02~−0.01 | 0.0001 *** | |
| CS_MP4 | −0.02 | 0.01 | −0.03~−0.00 | 0.026 * | |
| FFE_DIP4 | −0.02 | 0.00 | −0.02~−0.01 | 0.0001 *** | |
| FFE_PIP4 | 0.03 | 0.00 | 0.02~0.03 | 0.0001 *** | |
| FFE_MP4 | 0.08 | 0.01 | 0.06~0.10 | 0.0001 *** | |
| FBS_DIP4 | −0.06 | 0.01 | −0.07~−0.05 | 0.0001 *** | |
| FBS_MP4 | −0.11 | 0.01 | −0.12~−0.09 | 0.0001 *** | |
| SBS_DIP4 | 0.08 | 0.01 | 0.07~0.09 | 0.0001 *** | |
| SBS_PIP4 | 0.03 | 0.01 | 0.02~0.04 | 0.0001 *** | |
| SBS_MP4 | 0.05 | 0.01 | 0.03~0.06 | 0.0001 *** | |
| Finger 5 (QIC = 12) | Intercept | −0.40 | 0.49 | −1.37~0.56 | 0.414 |
| FBS_PIP5 | −0.02 | 0.01 | −0.03~−0.01 | 0.0001 *** | |
| SBS_PIP5 | 0.04 | 0.01 | 0.02~0.06 | 0.0001 *** |
| Joint | Variable | Estimate (β) | SE | 95% CI (Lower~Upper) | p |
|---|---|---|---|---|---|
| Elbow (QIC 9) | Intercept | 0.26 | 0.30 | −0.33~0.86 | 0.387 |
| FBS | 0.02 | 0.01 | 0.01~0.03 | 0.0001 *** | |
| Thumb (QIC 8) | Intercept | −0.17 | 0.16 | −0.49~0.14 | 0.284 |
| FFE_IP | 0.01 | 0.00 | 0.01~0.02 | 0.0001 *** | |
| Finger 2 (QIC 15) | Intercept | −1.65 | 0.42 | −2.47~−0.83 | 0.0001 *** |
| CS_DIP2 | −0.02 | 0.01 | −0.03~−0.00 | 0.023 * | |
| FFE_DIP2 | 0.01 | 0.03 | 0.01~0.02 | 0.0001 *** | |
| FFE_MP2 | 0.05 | 0.01 | 0.04~0.06 | 0.0001 *** | |
| FBS_MP2 | −0.04 | 0.01 | −0.05~−0.02 | 0.0001 *** | |
| SBS_PIP2 | 0.02 | 0.01 | 0.00~0.03 | 0.030 * | |
| SBS_MP2 | 0.03 | 0.01 | 0.01~0.05 | 0.016 * | |
| Finger 3 (QIC 14) | Intercept | −1.20 | 0.33 | −1.84~−0.56 | 0.0001 *** |
| CS_DIP3 | 0.01 | 0.00 | 0.00~0.01 | 0.037 * | |
| CS_PIP3 | 0.02 | 0.01 | 0.01~0.04 | 0.0001 *** | |
| FFE_DIP3 | 0.01 | 0.00 | 0.00~0.02 | 0.004 * | |
| FBS_DIP3 | −0.01 | 0.0 | −0.02~−0.00 | 0.002 * | |
| FBS_PIP3 | 0.03 | 0.01 | 0.02~0.05 | 0.0001 *** | |
| SBS_PIP3 | −0.03 | 0.01 | −0.04~−0.01 | 0.0001 *** | |
| Finger 4 (QIC 11) | Intercept | −1.25 | 0.34 | −1.92~−0.59 | 0.0001 *** |
| FFE_PIP4 | 0.01 | 0.00 | 0.00~0.02 | 0.001 ** | |
| FFE_MP4 | 0.03 | 0.01 | 0.01~0.04 | 0.0001 *** | |
| FBS_DIP4 | 0.02 | 0.05 | 0.01~0.02 | 0.002 * | |
| FBS_MP4 | −0.01 | 0.00 | −0.02~−0.01 | 0.0001 *** | |
| SBS_DIP4 | −0.02 | 0.01 | −0.03~−0.01 | 0.0001 *** | |
| SBS_PIP4 | 0.01 | 0.00 | 0.01~0.01 | 0.0001 *** |
| CS | FFE | FBS | SBS |
|---|---|---|---|
| Elbow_R | Elbow_R | MP1_R | IP_R |
| Wrist_L | MP3_R | PIP2_R | MP2_R |
| MP1_L | PIP3_R | MP5_R | DIP2_R |
| DIP3_L | DIP3_R | PIP5_R | PIP3_R |
| PIP4_L | MP4_R | Wrist_L | PIP4_R |
| MP5_L | DIP4_R | IP_L | PIP5_R |
| DIP5_R | PIP2_L | Elbow_L | |
| Wrist_L | MP3_L | Wrist_L | |
| DIP2_L | DIP4_L | MP2_L | |
| PIP5_L | PIP2_L | ||
| DIP5_L | PIP3_L | ||
| MP4_L |
| Joints | Spasticity | MAS_0 | MAS_1 | MAS_2 | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | p-Value | Mean (SD) | p-Value | Mean (SD) | p-Value | ||
| Elbow | Un_affected | 45.74 (30.98) | 0.450 | 43.32 (31.94) | 0.915 | 54.53 (30.71) | 0.738 |
| Affected | 31.93 (17.27) | 44.36 (16.98) | 51.26 (21.22) | ||||
| Wrist | Un_affected | 40.23 (25.13) | 0.052 | 49.55 (29.26) | 0.185 | 32.86 (9.51) | 0.704 |
| Affected | 29.81 (12.29) | 32.39 (15.11) | 27.60 (10.71) | ||||
| Thumb | Un_affected | 35.53 (13.00) | 0.235 | 36.09 (7.48) | 0.330 | 34.88 (8.39) | 0.241 |
| Affected | 37.88 (10.51) | 39.20 (9.95) | 61.73 (23.79) | ||||
| Finger_2 | Un_affected | 46.44 (9.82) | 0.126 | 50.16 (10.45) | 0.294 | 41.79 (5.36) | 0.032 |
| Affected | 42.09 (8.88) | 46.87 (9.35) | 48.12 (7.17) | ||||
| Finger_3 | Un_affected | 50.29 (12.72) | 0.079 | 56.41 (14.81) | 0.270 | 46.00 (6.13) | 0.237 |
| Affected | 44.89 (7.70) | 50.74 (16.70) | 49.34 (10.88) | ||||
| Finger_4 | Un_affected | 48.49 (11.38) | 0.087 | 57.32 (18.08) | 0.245 | 48.42 (10.58) | 0.663 |
| Affected | 43.48 (7.05) | 49.60 (14.13) | 50.92 (11.96) | ||||
| Finger_5 | Un_affected | 43.40 (9.71) | 0.909 | 51.76 (15.39) | 0.416 | 43.04 (9.19) | 0.389 |
| Affected | 43.11 (10.98) | 47.45 (14.86) | 46.15 (8.48) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeel, M.; Peng, C.-W.; Lee, I.-J.; Lin, B.-S. Prediction of Spasticity through Upper Limb Active Range of Motion in Stroke Survivors: A Generalized Estimating Equation Model. Bioengineering 2023, 10, 1273. https://doi.org/10.3390/bioengineering10111273
Adeel M, Peng C-W, Lee I-J, Lin B-S. Prediction of Spasticity through Upper Limb Active Range of Motion in Stroke Survivors: A Generalized Estimating Equation Model. Bioengineering. 2023; 10(11):1273. https://doi.org/10.3390/bioengineering10111273
Chicago/Turabian StyleAdeel, Muhammad, Chih-Wei Peng, I-Jung Lee, and Bor-Shing Lin. 2023. "Prediction of Spasticity through Upper Limb Active Range of Motion in Stroke Survivors: A Generalized Estimating Equation Model" Bioengineering 10, no. 11: 1273. https://doi.org/10.3390/bioengineering10111273
APA StyleAdeel, M., Peng, C.-W., Lee, I.-J., & Lin, B.-S. (2023). Prediction of Spasticity through Upper Limb Active Range of Motion in Stroke Survivors: A Generalized Estimating Equation Model. Bioengineering, 10(11), 1273. https://doi.org/10.3390/bioengineering10111273








